Review Article | Open Access
Using Artificial Intelligence to Advance Renal Cancer Diagnosis, Treatment, and Precision Medicine
Darragh Walsh1, Callum Hayes11School of Biomolecular and Biomedical Sciences, University College Dublin, Belfield, D04V1W8 Dublin 4, Ireland.
Correspondence: Callum Hayes ( School of Biomolecular and Biomedical Sciences, University College Dublin, Belfield, D04V1W8 Dublin 4, Ireland; E-mail: hbutt8608@gmail.com).
Annals of Urologic Oncology 2025, 8(2): 59-67. https://doi.org/10.32948/auo.2025.08.20
Received: 15 May 2025 | Accepted: 18 Jul 2025 | Published online: 02 Sep 2025
Key words renal cancer, artificial intelligence, machine learning, deep learning, treatment
AI has transformed medical research and clinical practice, significantly improving the diagnosis, management, and prevention of various cancers [7-9]. Advanced AI techniques, including deep learning (DL), machine learning (ML) [10], and natural language processing, and offer considerable promise for enhancing research in the field of RC [11]. These technologies employ extensive and varied datasets, including radiological images, genomic data, histopathological results, and clinical records, to facilitate early detection, prognostic evaluation, treatment planning, and monitoring of therapeutic outcomes [12, 13]. The primary factors that lead to kidney carcinoma are illustrated in Figure 2.
In recent years, several promising studies have been published on using AI for treating RC and other urologic tumors. Several analyses have been performed to summarize and evaluate the role of AI in RC; however, there is a scarcity of comprehensive systematic assessments specifically addressing AI-related studies in RC [14, 15]. Our primary objective in writing this narrative review is to focus solely on AI and its role in RC, providing medical professionals and researchers with valuable information with the ultimate aim of advancing patient outcomes and updating the RC intervention standards.
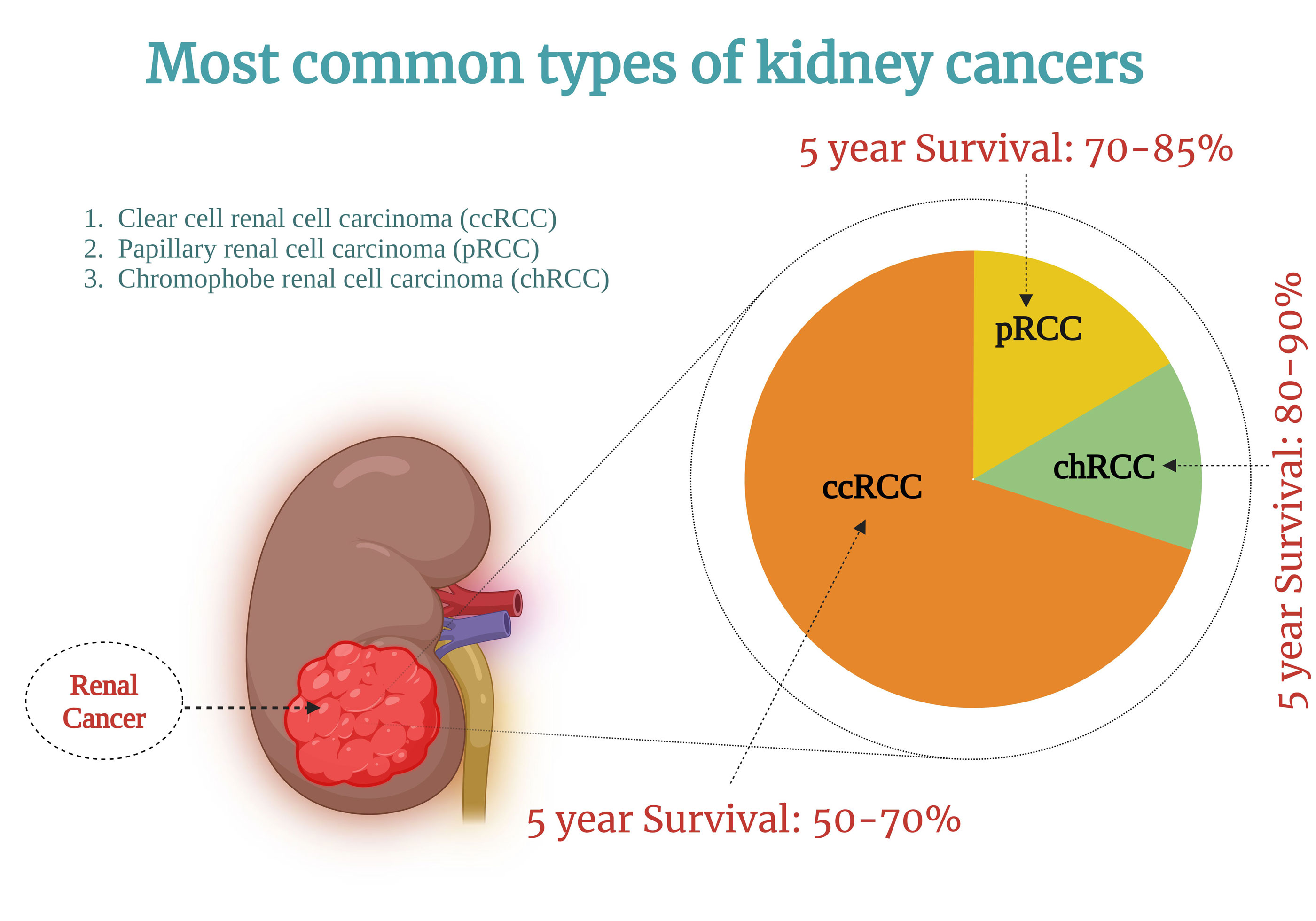 Figure 1. Illustration of various forms of renal cancer along with their associated survival rates.
Figure 1. Illustration of various forms of renal cancer along with their associated survival rates.
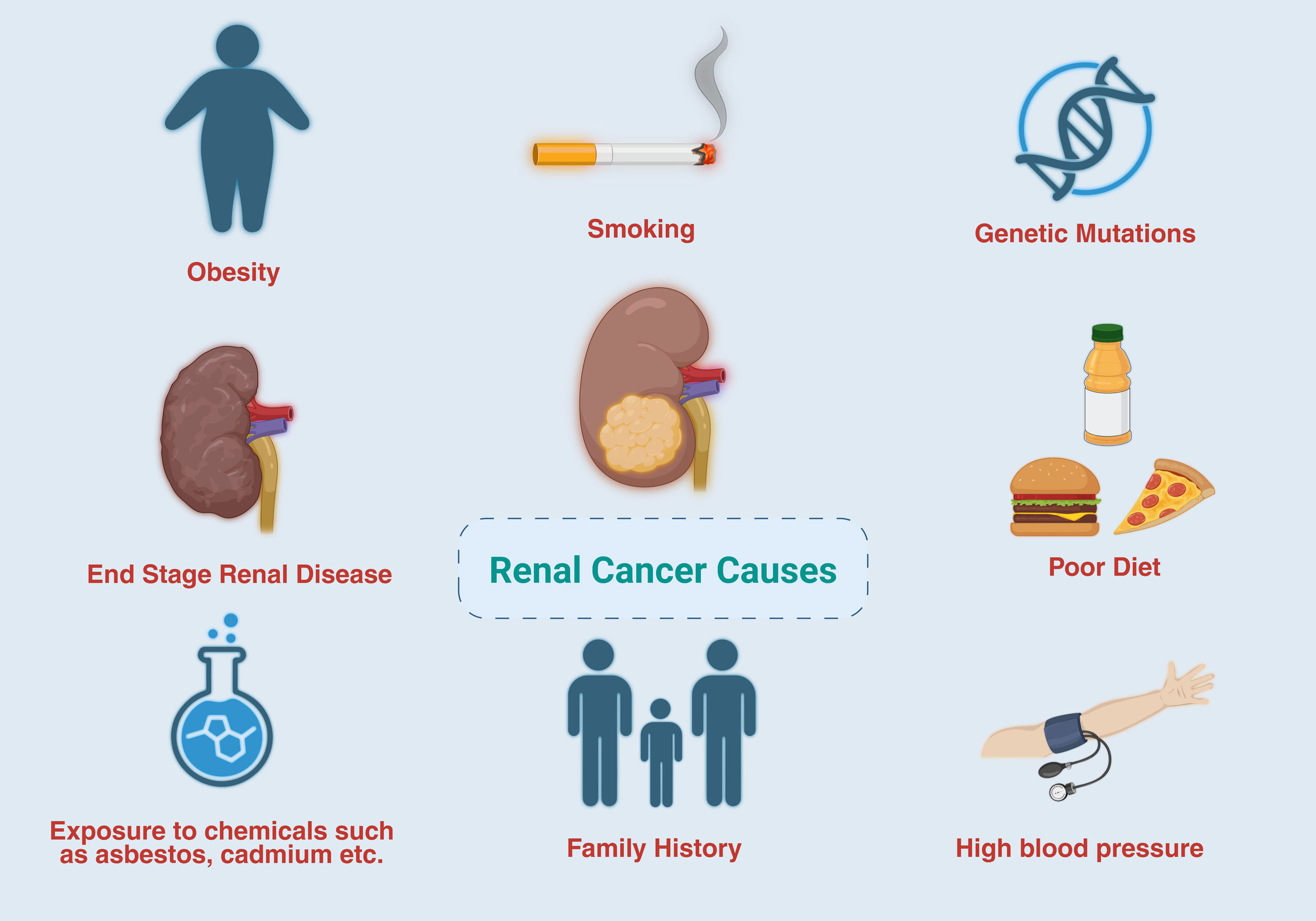 Figure 2. The principal factors contributing to kidney cancer are presented.
Figure 2. The principal factors contributing to kidney cancer are presented.
Initially, AI platforms depended on rule-based thinking performed by computer systems in accordance with a set of steps and protocols developed by human specialists [25]. However, these systems are deficient in the cognitive capabilities necessary for handling “exceptional cases” that are not explicitly specified within the knowledge base [26]. Over the past decade, algorithms that facilitate the automation of image-based processes have evolved significantly. This transition has been marked by the resurgence of neural networks, an ML algorithm based on understanding human brain function.
Data is the primary requirement for all algorithms. This includes not only baseline patient details (e.g., age or comorbidities) but also data obtained during surgical procedures, such as surgical footage, staff engagement, and intraperitoneal pressure [27]. Owing to the accessibility of larger datasets, advances in algorithm development, and improvements in computing capabilities, there has been a surge in interest in this field of study, leading to the development of new “deeper” neural networks [28]. Using training data, algorithms can automatically learn the map of ‘hidden neurons’ connecting the input and output nodes without reasoning rules. DL algorithms exhibit a superior learning capacity to earlier AI models, effectively identifying complex, non-linear relationships within datasets. Therefore, DL has the potential to gradually resemble or even surpass human capabilities for highly complicated tasks and has been used in several healthcare settings [29].
Over the past two decades, whole-slide images (WSIs) have progressed significantly, facilitating DP and high-quality slide storage [34]. WSI is a technology that facilitates the creation and viewing of high-quality digital images of microscopic slides on computer screens [35].
Initially, DP was defined to encompass the digital capture of WSIs using sophisticated slide-scanning methods. Its definition has since broadened to include the application of AI techniques for the detection, segmentation, diagnosis, and analysis of computerized images (Figure 3) [36]. To the best of our knowledge, Mukhopadhyay et al. conducted the first large-scale, multidisciplinary evaluation of the diagnostic accuracy of DP and traditional microscopy. The study consisted of samples from 1,992 patients with distinct tumor classifications, and 16 surgical pathologists collaborated on the study. The outcome of this research suggests that the primary diagnostic effectiveness of WSIs is not inferior to that of traditional microscopy-based methods, with a significant variation rate from the normal range of 4.9% for WSIs and 4.6% for microscopy [37]. The comprehensive data offered by WSIs, in conjunction with other data modalities, enhances ML models in healthcare settings, resulting in improved accuracy and facilitating individualized healthcare [38].
The integration of WSI and AI holds significant promise for oncology and precision medicine [39]. This emerging innovation holds promise for transforming cancer diagnostic processes [40]. It offers advantages such as image and data sharing, improved productivity, and integrated diagnosis. Moreover, it streamlines the processes within pathology workflows, elevates the quality of patient care, encourages collaborative efforts, strengthens physician responsibility, and reduces expenses by enhancing staff efficiency [41]. The combined use of DP and AI technologies can potentially improve cancer care by integrating quantitative tissue analysis with subjective assessments by experienced pathologists. Utilizing digital imaging and AI can provide deeper insights into cancer characteristics, leading to more focused and effective treatment methods. However, the pathway to this vision is not without its difficulties or challenges. Table 1 outlines the limitations of AI use in DP.
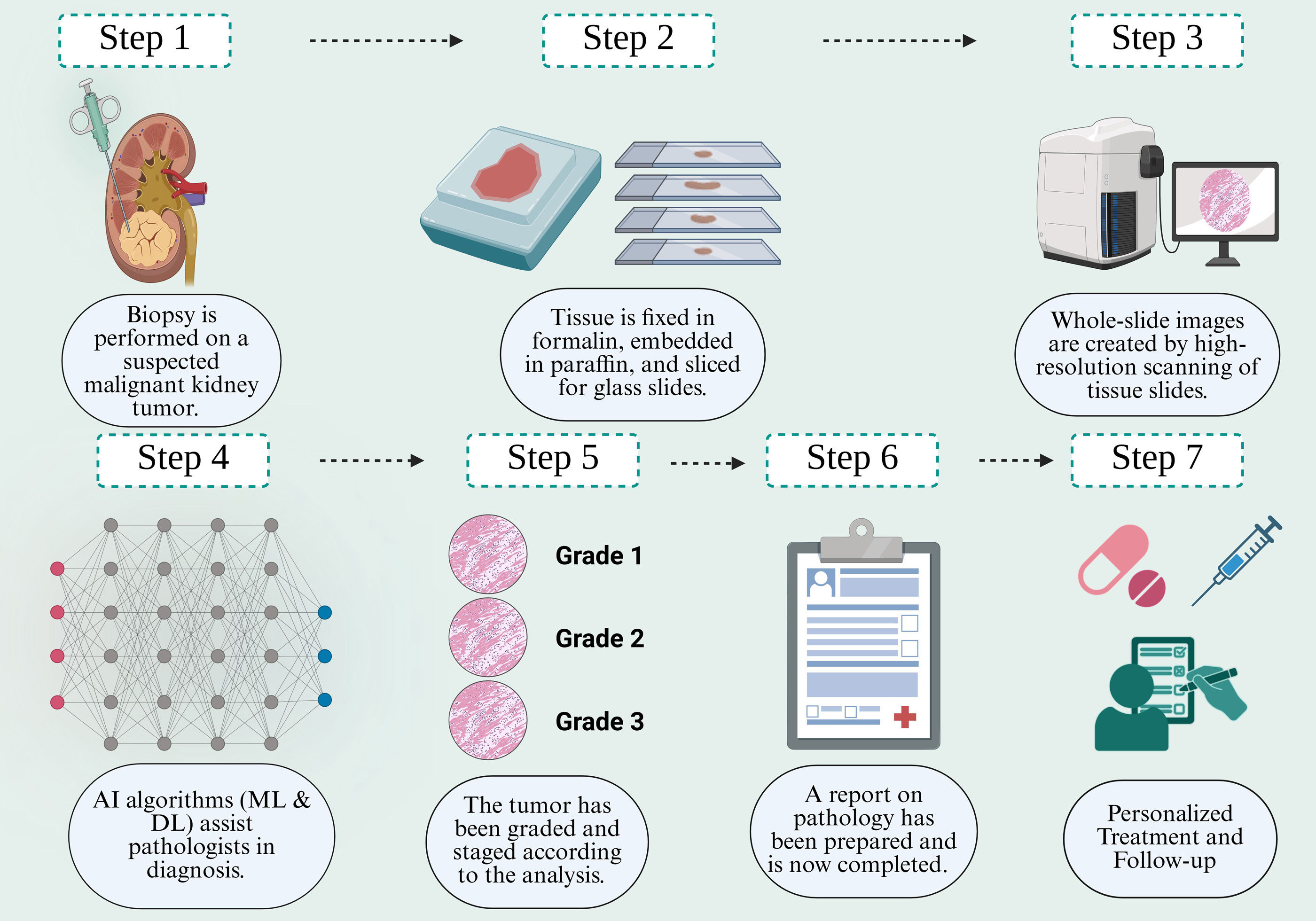 Figure 3. A comprehensive series of steps for DP in treating RC encompasses the procedure from biopsy to the outcome.
Figure 3. A comprehensive series of steps for DP in treating RC encompasses the procedure from biopsy to the outcome.
Utilizing AI for the early detection of renal malignancy
Renal cell biopsy is regarded as the definitive method for diagnosing renal cancer before definitive treatment; however, imaging characteristics observed through CT and MRI also play a crucial role. The integration of AI in the early detection of RC has the potential to improve outcomes by facilitating timely diagnosis. It aims to enhance the non-invasive characterization of kidney tumors. The study looked at how to describe kidney tumours using CT imaging, mainly using radiomic features like texture and intensity from multi-stage CT-based images (preliminary contrast, corticomedullary junction, nephrographic, and, less often, excretory phase) [57, 58]. They used an ML classifier to differentiate between non-cancerous and cancerous pathologies or to group different subtypes [59]. Models aimed at differentiating benign from malignant kidney tumours have primarily concentrated on separating fat-poor angiomyolipoma’s from RC. These models have demonstrated excellent outcomes, with area under the receiver operating characteristic curve (AUC) performance metrics ranging from 0.9029 [60] to 0.96 [61, 62].
Erdim et al. analyzed the differentiation of oncocytomas and fat-poor angiomyolipoma’s from all RC, achieving a favorable AUC of 0.91 [63]. Efforts to differentiate ccRCC from non-ccRCC have consistently achieved high AUC values of 0.91 [64], 0.93 [65], and 0.95 [66], respectively. However, distinguishing chrRCC from non-chrRCCs remains difficult, as evidenced by an AUC of 0.82. Han et al. observed a significant decline in performance when utilizing advanced DL techniques to classify three distinct RC subtypes: ccRCC, pRCC, and chrRCC, achieving an accuracy of 73%. This performance is notably lower than the 85% accuracy obtained in the binary classification task of distinguishing ccRCC from non-ccRCC [67].
The utilization of MRI, with its multiple sequences for the detailed characterization of a renal mass, introduces a considerable challenge in the realm of AI algorithm development [68]. This challenge stems from the need to create and train algorithms that can effectively process the expanded dataset and accommodate the variability in noise and signals across various scans [69]. Xi et al. recently highlighted the enhanced accuracy of a composite model utilizing a DL ResNet framework. This model, which integrates pre-surgical T2-weighted and T1-after contrast MRI series with clinical parameters such as gender, age group, and lesion mass, was evaluated against an imaging-based model and expert analysis for differentiating benign from cancerous renal lesions in a sample of 1,162 patients. Despite the model’s innovative design, its reported overall accuracy of 70% (95% CI, 60%–77%), sensitivity of 92% (95% CI, 82%–97%), and specificity of 41% ( 95% CI, 28%–55%) indicate a pressing need for further refinement to enhance the AI model performance for broader clinical use [70].
Utilizing AI for the grading of renal cancer
Grading is crucial for estimating the prognosis of RC patients. Similar to distinguishing between indolent and aggressive RC subtypes, differentiating low-grade RCs from their more aggressive high-grade forms is essential for developing effective clinical management strategies. Despite being primarily replaced by the WHO/ISUP grading classification system, the Fuhrman grading system remains a significant independent prognostic tool, associated with an increased risk of cancer recurrence and a reduced likelihood of patient survival [71, 72]. The Fuhrman grading system is primarily concerned with nuclear morphology, focusing on the size and shape of the nucleus and the presence of prominent nucleoli. Nonetheless, it is essential to note that there is considerable variability between different observers and within the same observer over time [73].
Chen et al. presented a model termed Retrieval with Clustering-guided Contrastive Learning (RetCCL), which employs weak supervision to classify high-grade Fuhrman predictions [74]. In a related study, Zhen et al. presented the Self-Supervised Learning based Clustering-constrained Attention Multiple Instance Learning (SSL-CLAM) model. This innovative AI model, grounded in self-supervised learning, showed improved efficacy when used in conjunction with pathologists’ diagnostic assessments [75]. Although the Fuhrman grading system is valued for its practical application, its reliability is diminished because of evaluation inconsistencies among observers [76]. In response to these constraints, researchers affiliated with the ISUP and WHO developed a grading framework that classifies tumors into four distinct levels (1 to 4), determined by the degree of nucleoli visibility [77]. Aziz et al. advanced the field by developing a ResNet-50-based attention based model that integrates patch release interpretations with center-based loss, thereby exceeding the performance of current innovative RC-grading models [78]. Koo et al. adopted a combination strategy that leveraged well-established layouts to enhance the accuracy of cancer diagnosis [79]. Chanchal et al. introduced the RCC Grading Network (RCCGNet), which demonstrated superior accuracy compared with traditional models [80].
Recent research has explored the application of AI to assess indirect measures of tumor behavior, particularly the SSIGN score, which evaluates stage, dimensions, severity level, and necrosis to predict ccRCC progression following radical nephrectomy [81]. Choi et al. conducted a study on an AI algorithm designed to preoperatively predict SSIGN scores, differentiating between low and high scores, in patients diagnosed with ccRCC who were to undergo MRI before surgery. The algorithm achieved a notable AUC of 0.94, indicating a high predictive accuracy [82].
Utilizing AI for the staging and prognosis assessment
RC staging plays a pivotal role in shaping therapeutic approaches and anticipating prognostic outcomes [83-85]. As the dimensions and positioning of cancer are pivotal in determining its stage, AI has demonstrated promise in delivering precise and uniform categorization of cancer. Yao et al. utilized the MIL approach to categorize RCC stages 1–4, resulting in a precision rate of 0.8 [86].
Survival risk estimation and analysis is a dynamic and expanding area of study. Beyond traditional statistical approaches like the Cox proportional hazards regression analysis model, the field is witnessing continuous and noteworthy developments [87, 88]. AI utilizes extensive clinical data, genomic data, and tomography datasets to estimate overall, progression-free, and recurrence-free survival accurately. These models support the automated extraction of features from data characterized by high dimensionality, thereby facilitating the discovery of new risk factors and prognostic indicators. Gao et al. focused on predicting lymph node tumor dissemination [89]; however, Liu et al. examined tumor mutation rate classification, reflecting a shift in therapeutic and research directions for RC [90]. Although staging classification shows significant potential, research in this area is comparatively limited when evaluated against studies on subtype classification and grading. The system complexity of creating a thorough analysis necessary for precise staging is partially responsible for this limitation. Moreover, the use of AI in RC research is still gaining momentum, contributing to the limited number of studies in this area.
In conclusion, ongoing research is dedicated to evaluating the roles of CT and MRI in diagnosing RC and forecasting clinical outcomes and therapeutic responses to optimize management strategies. The improvements in the timely detection of RC and the anticipation of treatment outcomes largely depend on recognizing ideal discriminative indicators tailored to specific diagnostic and predictive challenges. This progress is further supported by creating robust, consistent, and versatile AI-based diagnostic and predictive models. By outlining these future initiatives and recommendations, we seek to motivate researchers and investors to close this knowledge gap and realize the objective of developing an integrated system. This system should be reliably utilized for diagnosing renal tumors and predicting clinical outcomes and treatment responses, eventually contributing to advances in health care outcomes.
None.
Ethical policy
Non applicable.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
Darragh Walsh contributed to design of the work, data collection, and drafting the article; Callum Hayes checked the revision manuscript and approved the final submission.
Competing interests
The author declares no competing interests.
Funding
None.
- Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, Gore JL, Sun M, Wood C, Russo P: Epidemiology of Renal Cell Carcinoma. Eur Urol 2019, 75(1): 74-84.
- Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, Rawla P, Barsouk A: Epidemiology of Renal Cell Carcinoma. World J Oncol 2020, 11(3): 79-87.
- Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F, Hora M, Kuczyk MA et al: European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol 2019, 75(5): 799-810.
- Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F: International Variations and Trends in Renal Cell Carcinoma Incidence and Mortality. Eur Urol 2015, 67(3): 519-530.
- Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch H, Grignon DJ et al: The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013, 37(10): 1490-1504.
- Trevisani F, Floris M, Minnei R, Cinque A: Renal oncocytoma: the diagnostic challenge to unmask the double of renal cancer. Int J Mol Sci 2022, 23(5): 2603.
- Al-Qudimat AR, Fares ZE, Elaarag M, Osman M, Al-Zoubi RM, Aboumarzouk OM: Advancing Medical Research Through Artificial Intelligence: Progressive and Transformative Strategies: A Literature Review. Health Sci Rep 2025, 8(2): e70200.
- Mann M, Kumar C, Zeng WF, Strauss MT: Artificial intelligence for proteomics and biomarker discovery. Cell Syst 2021, 12(8): 759-770.
- Kelly BS, Judge C, Bollard SM, Clifford SM, Healy GM, Aziz A, Mathur P, Islam S, Yeom KW, Lawlor A et al: Radiology artificial intelligence: a systematic review and evaluation of methods (RAISE). Eur Radiol 2022, 32(11): 7998-8007.
- Khene Z-E, Bigot P, Doumerc N, Ouzaid I, Boissier R, Nouhaud F-X, Albiges L, Bernhard J-C, Ingels A, Borchiellini D et al: Application of Machine Learning Models to Predict Recurrence After Surgical Resection of Nonmetastatic Renal Cell Carcinoma. Eur Urol Oncol 2023, 6(3): 323-330.
- Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, Aldairem A, Alrashed M, Bin Saleh K, Badreldin HA et al: Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ 2023, 23(1): 689.
- Abbas S, Asif M, Rehman A, Alharbi M, Khan MA, Elmitwally N: Emerging research trends in artificial intelligence for cancer diagnostic systems: A comprehensive review. Heliyon 2024, 10(17): e36743.
- Knudsen JE, Rich JM, Ma R: Artificial Intelligence in Pathomics and Genomics of Renal Cell Carcinoma. Urol Clin North Am 2024, 51(1): 47-62.
- Brodie A, Dai N, Teoh JY, Decaestecker K, Dasgupta P, Vasdev N: Artificial intelligence in urological oncology: An update and future applications. Urol Oncol 2021, 39(7): 379-399.
- Hameed BMZ, AVL SD, Raza SZ, Karimi H, Khanuja HS, Shetty DK, Ibrahim S, Shah MJ, Naik N, Paul R et al: Artificial Intelligence and Its Impact on Urological Diseases and Management: A Comprehensive Review of the Literature. J Clin Med 2021, 10(9): 1864.
- Rajkomar A, Dean J, Kohane I: Machine Learning in Medicine. N Engl J Med 2019, 380(14): 1347-1358.
- Xu Y, Liu X, Cao X, Huang C, Liu E, Qian S, Liu X, Wu Y, Dong F, Qiu CW et al: Artificial intelligence: A powerful paradigm for scientific research. Innovation (Camb) 2021, 2(4): 100179.
- Wang H, Fu T, Du Y, Gao W, Huang K, Liu Z, Chandak P, Liu S, Van Katwyk P, Deac A: Scientific discovery in the age of artificial intelligence. Nature 2023, 620(7972): 47-60.
- Buch VH, Ahmed I, Maruthappu M: Artificial intelligence in medicine: current trends and future possibilities. Br J Gen Pract 2018, 68(668): 143-144.
- Haug CJ, Drazen JM: Artificial intelligence and machine learning in clinical medicine, 2023. N Engl J Med 2023, 388(13): 1201-1208.
- Shehab M, Abualigah L, Shambour Q, Abu-Hashem MA, Shambour MKY, Alsalibi AI, Gandomi AH: Machine learning in medical applications: A review of state-of-the-art methods. Comput Biol Med 2022, 145: 105458.
- Garg A, Mago V: Role of machine learning in medical research: A survey. Comput Sci Rev 2021, 40: 100370.
- Egger J, Gsaxner C, Pepe A, Pomykala KL, Jonske F, Kurz M, Li J, Kleesiek J: Medical deep learning—A systematic meta-review. Comput Methods Programs Biomed 2022, 221: 106874.
- Piccialli F, Somma VD, Giampaolo F, Cuomo S, Fortino G: A survey on deep learning in medicine: Why, how and when? Inf. Fusion 2021, 66: 111-137.
- Xu W, Dainoff MJ, Ge L, Gao Z: Transitioning to human interaction with AI systems: New challenges and opportunities for HCI professionals to enable human-centered AI. Int. J. Hum.–Comput. Interact 2023, 39(3): 494-518.
- van Diest PJ, Flach RN, van Dooijeweert C, Makineli S, Breimer GE, Stathonikos N, Pham P, Nguyen TQ, Veta M: Pros and cons of artificial intelligence implementation in diagnostic pathology. Histopathology 2024, 84(6): 924-934.
- Garrow CR, Kowalewski K-F, Li L, Wagner M, Schmidt MW, Engelhardt S, Hashimoto DA, Kenngott HG, Bodenstedt S, Speidel S: Machine learning for surgical phase recognition: a systematic review. Ann Surg 2021, 273(4): 684-693.
- Boldrini L, Bibault J-E, Masciocchi C, Shen Y, Bittner M-I: Deep learning: a review for the radiation oncologist. Front Oncol 2019, 9: 977.
- Nogales A, Garcia-Tejedor AJ, Monge D, Vara JS, Antón C: A survey of deep learning models in medical therapeutic areas. Artif Intell Med 2021, 112: 102020.
- Aeffner F, Zarella MD, Buchbinder N, Bui MM, Goodman MR, Hartman DJ, Lujan GM, Molani MA, Parwani AV, Lillard K et al: Introduction to Digital Image Analysis in Whole-slide Imaging: A White Paper from the Digital Pathology Association. J Pathol Inform 2019, 10: 9.
- Tizhoosh HR, Pantanowitz L: Artificial Intelligence and Digital Pathology: Challenges and Opportunities. J Pathol Inform 2018, 9: 38.
- Försch S, Klauschen F, Hufnagl P, Roth W: Artificial Intelligence in Pathology. Dtsch Arztebl Int 2021, 118(12): 194-204.
- Jahn SW, Plass M, Moinfar F: Digital Pathology: Advantages, Limitations and Emerging Perspectives. J Clin Med 2020, 9(11): 3697.
- El Nahhas OS, van Treeck M, Wölflein G, Unger M, Ligero M, Lenz T, Wagner SJ, Hewitt KJ, Khader F, Foersch S: From whole-slide image to biomarker prediction: end-to-end weakly supervised deep learning in computational pathology. Nat Protoc 2025, 20(1): 293-316.
- Abels E, Pantanowitz L, Aeffner F, Zarella MD, Van der Laak J, Bui MM, Vemuri VN, Parwani AV, Gibbs J, Agosto‐Arroyo E: Computational pathology definitions, best practices, and recommendations for regulatory guidance: a white paper from the Digital Pathology Association. J Pathol 2019, 249(3): 286-294.
- Faa G, Coghe F, Pretta A, Castagnola M, Van Eyken P, Saba L, Scartozzi M, Fraschini M: Artificial intelligence models for the detection of microsatellite instability from whole-slide imaging of colorectal cancer. Diagnostics 2024, 14(15): 1605.
- Mukhopadhyay S, Feldman MD, Abels E, Ashfaq R, Beltaifa S, Cacciabeve NG, Cathro HP, Cheng L, Cooper K, Dickey GE et al: Whole Slide Imaging Versus Microscopy for Primary Diagnosis in Surgical Pathology: A Multicenter Blinded Randomized Noninferiority Study of 1992 Cases (Pivotal Study). Am J Surg Pathol 2018, 42(1): 39-52.
- Li X, Li C, Rahaman MM, Sun H, Li X, Wu J, Yao Y, Grzegorzek M: A comprehensive review of computer-aided whole-slide image analysis: from datasets to feature extraction, segmentation, classification and detection approaches. Artif. Intell. Rev 2022, 55(6): 4809-4878.
- Lai B, Fu J, Zhang Q, Deng N, Jiang Q, Peng J: Artificial intelligence in cancer pathology: Challenge to meet increasing demands of precision medicine. Int J Oncol 2023, 63(3): 1-30.
- Liu Y, Zhen T, Fu Y, Wang Y, He Y, Han A, Shi H: AI-powered segmentation of invasive carcinoma regions in breast cancer immunohistochemical whole-slide images. Cancers 2023, 16(1): 167.
- Lee M: Recent advancements in deep learning using whole slide imaging for cancer prognosis. Bioengineering 2023, 10(8): 897.
- Haibe-Kains B, Adam GA, Hosny A, Khodakarami F, Waldron L, Wang B, McIntosh C, Goldenberg A, Kundaje A, Greene CS et al: Transparency and reproducibility in artificial intelligence. Nature 2020, 586(7829): E14-e16.
- McKinney SM, Karthikesalingam A, Tse D, Kelly CJ, Liu Y, Corrado GS, Shetty S: Reply to: Transparency and reproducibility in artificial intelligence. Nature 2020, 586(7829): E17-e18.
- Klein C, Zeng Q, Arbaretaz F, Devêvre E, Calderaro J, Lomenie N, Maiuri MC: Artificial intelligence for solid tumour diagnosis in digital pathology. Br J Pharmacol 2021, 178(21): 4291-4315.
- Basak K, Ozyoruk KB, Demir D: Whole slide images in artificial intelligence applications in digital pathology: Challenges and pitfalls. Turk Patoloji Derg 2023, 39(2): 101.
- Dara S, Tumma P: Feature extraction by using deep learning: A survey. Second international conference on electronics, communication and aerospace technology (ICECA) 2018: 1795-1801.
- Ilse M, Tomczak J, Welling M: Attention-based deep multiple instance learning. In: International conference on machine learning: 2018: PMLR; 2018: 2127-2136.
- Lu MY, Williamson DF, Chen TY, Chen RJ, Barbieri M, Mahmood F: Data-efficient and weakly supervised computational pathology on whole-slide images. Nat Biomed Eng 2021, 5(6): 555-570.
- Larrazabal AJ, Nieto N, Peterson V, Milone DH, Ferrante E: Gender imbalance in medical imaging datasets produces biased classifiers for computer-aided diagnosis. Proc Natl Acad Sci U S A 2020, 117(23): 12592-12594.
- McKay F, Williams BJ, Prestwich G, Bansal D, Hallowell N, Treanor D: The ethical challenges of artificial intelligence-driven digital pathology. J Pathol Clin Res 2022, 8(3): 209-216.
- Panch T, Mattie H, Atun R: Artificial intelligence and algorithmic bias: implications for health systems. J Glob Health 2019, 9(2): 010318.
- Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A: Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol 2019, 16(11): 703-715.
- Niazi MKK, Parwani AV, Gurcan MN: Digital pathology and artificial intelligence. Lancet Oncol 2019, 20(5): e253-e261.
- Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F, Hora M et al: European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol 2022, 82(4): 399-410.
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74(3): 229-263.
- Capitanio U, Montorsi F: Renal cancer. Lancet 2016, 387(10021): 894-906.
- Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D: Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014, 5(1): 4006.
- Gillies RJ, Kinahan PE, Hricak H: Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278(2): 563-577.
- Bhandari A, Ibrahim M, Sharma C, Liong R, Gustafson S, Prior M: CT-based radiomics for differentiating renal tumours: a systematic review. Abdom Radiol (NY) 2021, 46(5): 2052-2063.
- Yang R, Wu J, Sun L, Lai S, Xu Y, Liu X, Ma Y, Zhen X: Radiomics of small renal masses on multiphasic CT: accuracy of machine learning-based classification models for the differentiation of renal cell carcinoma and angiomyolipoma without visible fat. Eur Radiol 2020, 30(2): 1254-1263.
- Cui EM, Lin F, Li Q, Li RG, Chen XM, Liu ZS, Long WS: Differentiation of renal angiomyolipoma without visible fat from renal cell carcinoma by machine learning based on whole-tumor computed tomography texture features. Acta Radiol 2019, 60(11): 1543-1552.
- Feng Z, Rong P, Cao P, Zhou Q, Zhu W, Yan Z, Liu Q, Wang W: Machine learning-based quantitative texture analysis of CT images of small renal masses: Differentiation of angiomyolipoma without visible fat from renal cell carcinoma. Eur Radiol 2018, 28(4): 1625-1633.
- Erdim C, Yardimci AH, Bektas CT, Kocak B, Koca SB, Demir H, Kilickesmez O: Prediction of Benign and Malignant Solid Renal Masses: Machine Learning-Based CT Texture Analysis. Acad Radiol 2020, 27(10): 1422-1429.
- Yu H, Scalera J, Khalid M, Touret AS, Bloch N, Li B, Qureshi MM, Soto JA, Anderson SW: Texture analysis as a radiomic marker for differentiating renal tumors. Abdom Radiol (NY) 2017, 42(10): 2470-2478.
- Sun XY, Feng QX, Xu X, Zhang J, Zhu FP, Yang YH, Zhang YD: Radiologic-Radiomic Machine Learning Models for Differentiation of Benign and Malignant Solid Renal Masses: Comparison With Expert-Level Radiologists. AJR Am J Roentgenol 2020, 214(1): W44-w54.
- Li ZC, Zhai G, Zhang J, Wang Z, Liu G, Wu GY, Liang D, Zheng H: Differentiation of clear cell and non-clear cell renal cell carcinomas by all-relevant radiomics features from multiphase CT: a VHL mutation perspective. Eur Radiol 2019, 29(8): 3996-4007.
- Han S, Hwang SI, Lee HJ: The Classification of Renal Cancer in 3-Phase CT Images Using a Deep Learning Method. J Digit Imaging 2019, 32(4): 638-643.
- Wang W, Cao K, Jin S, Zhu X, Ding J, Peng W: Differentiation of renal cell carcinoma subtypes through MRI-based radiomics analysis. Eur Radiol 2020, 30(10): 5738-5747.
- Goyal A, Razik A, Kandasamy D, Seth A, Das P, Ganeshan B, Sharma R: Role of MR texture analysis in histological subtyping and grading of renal cell carcinoma: a preliminary study. Abdom Radiol (NY) 2019, 44(10): 3336-3349.
- Xi IL, Zhao Y, Wang R, Chang M, Purkayastha S, Chang K, Huang RY, Silva AC, Vallières M, Habibollahi P et al: Deep Learning to Distinguish Benign from Malignant Renal Lesions Based on Routine MR Imaging. Clin Cancer Res 2020, 26(8): 1944-1952.
- Dall'Oglio MF, Ribeiro-Filho LA, Antunes AA, Crippa A, Nesrallah L, Gonçalves PD, Leite KR, Srougi M: Microvascular tumor invasion, tumor size and Fuhrman grade: a pathological triad for prognostic evaluation of renal cell carcinoma. J Urol 2007, 178(2): 425-428.
- Lin F, Cui EM, Lei Y, Luo LP: CT-based machine learning model to predict the Fuhrman nuclear grade of clear cell renal cell carcinoma. Abdom Radiol (NY) 2019, 44(7): 2528-2534.
- Bektas S, Bahadir B, Kandemir NO, Barut F, Gul AE, Ozdamar SO: Intraobserver and Interobserver Variability of Fuhrman and Modified Fuhrman Grading Systems for Conventional Renal Cell Carcinoma. Kaohsiung J Med Sci 2009, 25(11): 596-600.
- Chen S, Wang X, Zhang J, Jiang L, Gao F, Xiang J, Yang S, Yang W, Zheng J, Han X: Deep learning-based diagnosis and survival prediction of patients with renal cell carcinoma from primary whole slide images. Pathology 2024, 56(7): 951-960.
- Zheng Q, Yang R, Xu H, Fan J, Jiao P, Ni X, Yuan J, Wang L, Chen Z, Liu X: A Weakly Supervised Deep Learning Model and Human–Machine Fusion for Accurate Grading of Renal Cell Carcinoma from Histopathology Slides. Cancers 2023, 15(12): 3198.
- Dagher J, Delahunt B, Rioux-Leclercq N, Egevad L, Srigley JR, Coughlin G, Dunglinson N, Gianduzzo T, Kua B, Malone G et al: Clear cell renal cell carcinoma: validation of World Health Organization/International Society of Urological Pathology grading. Histopathology 2017, 71(6): 918-925.
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE: The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. European Urology 2016, 70(1): 106-119.
- Aziz MA, Javadian F, Mathew SS, Gopal A, Stegmaier J, Singh S, Jose A: Deep Learning Approach for Renal Cell Carcinoma Detection, Subtyping, And Grading. IEEE International Conference on Image Processing (ICIP) 2024: 3861-3867.
- Koo JC, Ke Q, Hum YC, Goh CH, Lai KW, Yap W-S, Tee YK: Non-annotated renal histopathological image analysis with deep ensemble learning. Quant Imaging Med Surg 2023, 13(9): 5902-5920.
- Chanchal AK, Pandey DSK: Review of Prediction of Delay in Flights using Machine Learning Techniques. Smart Moves Journal Ijoscience 2023, 9(5): 1-5.
- Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H: An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol 2002, 168(6): 2395-2400.
- Choi JW, Hu R, Zhao Y, Purkayastha S, Wu J, McGirr AJ, Stavropoulos SW, Silva AC, Soulen MC, Palmer MB et al: Preoperative prediction of the stage, size, grade, and necrosis score in clear cell renal cell carcinoma using MRI-based radiomics. Abdom Radiol (NY) 2021, 46(6): 2656-2664.
- Najem EJ, Shaikh MJS, Shinagare AB, Krajewski KM: Navigating advanced renal cell carcinoma in the era of artificial intelligence. Cancer Imaging 2025, 25(1): 16.
- Bellin M-F, Valente C, Bekdache O, Maxwell F, Balasa C, Savignac A, Meyrignac O: Update on renal cell carcinoma diagnosis with novel imaging approaches. Cancers 2024, 16(10): 1926.
- Chi S, Ma J, Ding Y, Lu Z, Zhou Z, Wang M, Li G, Chen Y: Integrated multi-omics analysis identifies a machine learning-derived signature for predicting prognosis and therapeutic vulnerability in clear cell renal cell carcinoma. Life Sciences 2025, 363: 123396.
- Yao J, Wei L, Hao P, Liu Z, Wang P: Application of artificial intelligence model in pathological staging and prognosis of clear cell renal cell carcinoma. Discov Oncol 2024, 15(1): 545.
- Adam N, Wieder R: AI Survival Prediction Modeling: The Importance of Considering Treatments and Changes in Health Status over Time. Cancers (Basel) 2024, 16(20): 3527.
- Timsit JF, Alberti C, Chevret S : Le modèle de Cox [Cox proportional hazards regression analysis. Rev Mal Respir 2005, 22(6 Pt 1): 1058-1064.
- Gao F, Jiang L, Guo T, Lin J, Xu W, Yuan L, Han Y, Yang J, Pan Q, Chen E et al: Deep learning-based pathological prediction of lymph node metastasis for patient with renal cell carcinoma from primary whole slide images. J Transl Med 2024, 22(1): 568.
- Liu X, Liu Z, Yan Y, Wang K, Wang A, Ye X, Wang L, Wei W, Li B, Sun C et al: Development of Prognostic Biomarkers by TMB-Guided WSI Analysis: A Two-Step Approach. IEEE J Biomed Health Inform 2023, 27(4): 1780-1789.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript