Case Report | Open Access
The Urological Subtleties of an Uncommon Encounter with Primary Mucinous Adenocarcinoma of the Urinary Bladder with Review of Literature
Anuradha Pandit1, Shaivy Malik1, Nibir Chakma1, Charanjeet Ahluwalia11Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, 110029, India.
Correspondence: Charanjeet Ahluwalia (Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, 110029, India; Email: charanjeetahluwalia70@gmail.com).
Annals of Urologic Oncology 2025, 8(2): 106-115. https://doi.org/10.32948/auo.2025.08.01
Received: 02 Jul 2025 | Accepted: 06 Aug 2025 | Published online: 07 Aug 2025
Key words primary mucinous adenocarcinoma, bladder neoplasms, urinary bladder adenocarcinoma, colloid carcinoma
Herein, we illustrate the key clinical, radiological, gross, histopathological, and immunohistochemical findings of an intriguing case of a 52-year-old male with PMA of the urinary bladder, which provides crucial insights into its diagnosis and adds to the limited pool of cases described internationally.
Systemic physical examination, including the digital rectal examination (DRE) was unremarkable. Ultrasound imaging showed an intra-luminal hypoechogenic mass approximately 40 x 30 mm in size, positioned near the right ureteric orifice within the urinary bladder. This mass caused moderate right-sided hydronephrosis and hydroureter. Mild hepatomegaly was also detected.
CT urography revealed a soft tissue polypoid lesion that enhanced and originated from the right postero-lateral wall of the urinary bladder, extending to the vesico-ureteric junction. This led to moderate right-sided hydroureteronephrosis (Figure 1). No signs of extravesical spread were observed. The findings indicated a neoplastic bladder mass. Furthermore, bilateral renal cortical cysts were identified and categorised as Bosniak type 1 cysts. The left kidney showed normal contrast excretion, whereas the right kidney displayed impaired contrast excretion, with no contrast seen in the right ureter.
The patient underwent a transurethral resection of bladder tumor (TURBT), followed by a right percutaneous nephrostomy (PCN). Intraoperative findings showed a solid and papillary neoplasm measuring 5 x 5 cm on the right posterior-lateral wall of the bladder, encroaching on the right ureteric orifice. The excised TURBT biopsy was sent for histopathological examination, which revealed nests of neoplastic cells floating in abundant extravasated mucin, with singly dispersed and groups of signet ring cells seen in the mucin pools (Figure 2). Mucin was deeply invading the muscularis propria. On immunohistochemistry (IHC), the tumor cells exhibited strong cytoplasmic positivity for CK7, CK 20, p53, membranous and cytoplasmic positivity for beta-catenin (Figure 3), and thrombomodulin, with negative immunoexpression for CDX 2, GATA 3, SATB2, and PSA. Based on the pathological findings, an impression of muscle-invasive, mucinous adenocarcinoma was rendered, and a clinico-radiological correlation was advised to negate the possibility of metastases of adenocarcinoma from a primary elsewhere.
A thorough clinical examination, including colonoscopic evaluation, was done to rule out evidence of any primary tumor in other visceral sites, which turned out to be unremarkable. An 18F-FDG/PET-CT (18F-Fluorodeoxyglucose/Positron Emission Tomography-Computed Tomography) was also performed, which did not reveal any metabolically avid lesion elsewhere, apart from the primary bladder lesion.
Subsequently, a radical cystectomy was done, and the resected specimen was submitted entirely for histopathological diagnosis. On gross examination, a papillary and fungating grey-white growth, with convoluted configuration, measuring 5.2 x 4.8 x 3.7 cm was identified, arising from the right posterolateral bladder wall. The cut surface of the tumor was covered with thick, viscous, slimy gelatinous material, and it appeared to grossly involve the muscularis propria of the bladder wall.
Multiple haematoxylin and eosin-stained sections examined revealed the presence of an infiltrative neoplasm characterised by stratified columnar cells arranged in a glandular architecture and floating within pools of abundant extracellular mucin. These cells were large with abundant mucin-filled cytoplasm. The nuclei displayed hyperchromasia, moderate pleomorphism, loss of polarity, irregular nuclear membranes, and inconspicuous nucleoli. Also seen were admixed singly dispersed signet ring cells with eccentrically pushed hyperchromatic nuclei and mucin-containing goblets within their cytoplasm. Atypical mitoses were identified with a mitotic count of 3-4/high power field. The tumour involved the superficial muscularis propria with deep muscle free of tumor. The resection margins of the prostatic urethra, along with the cut margins of bilateral ureters, showed no tumor involvement. Additionally, the perivesical soft tissue was devoid of neoplastic infiltration, and the prostate and seminal vesicles, which were entirely processed, were also free of tumor. The tumor cells displayed an identical immunohistochemical expression as seen on the TURBT biopsy. All 30 regional lymph nodes submitted exhibited reactive lymphoid hyperplasia, and were free of tumor.
Based on the corroborative clinical, radiological, histopathological and ancillary testing findings, a final impression of G2, moderately-differentiated PMA, urinary bladder, pathologic staging-PT2aN0M0 was rendered.
The patient has been hemodynamically stable in the post-operative period and has been kept on close follow-up in the urology department.
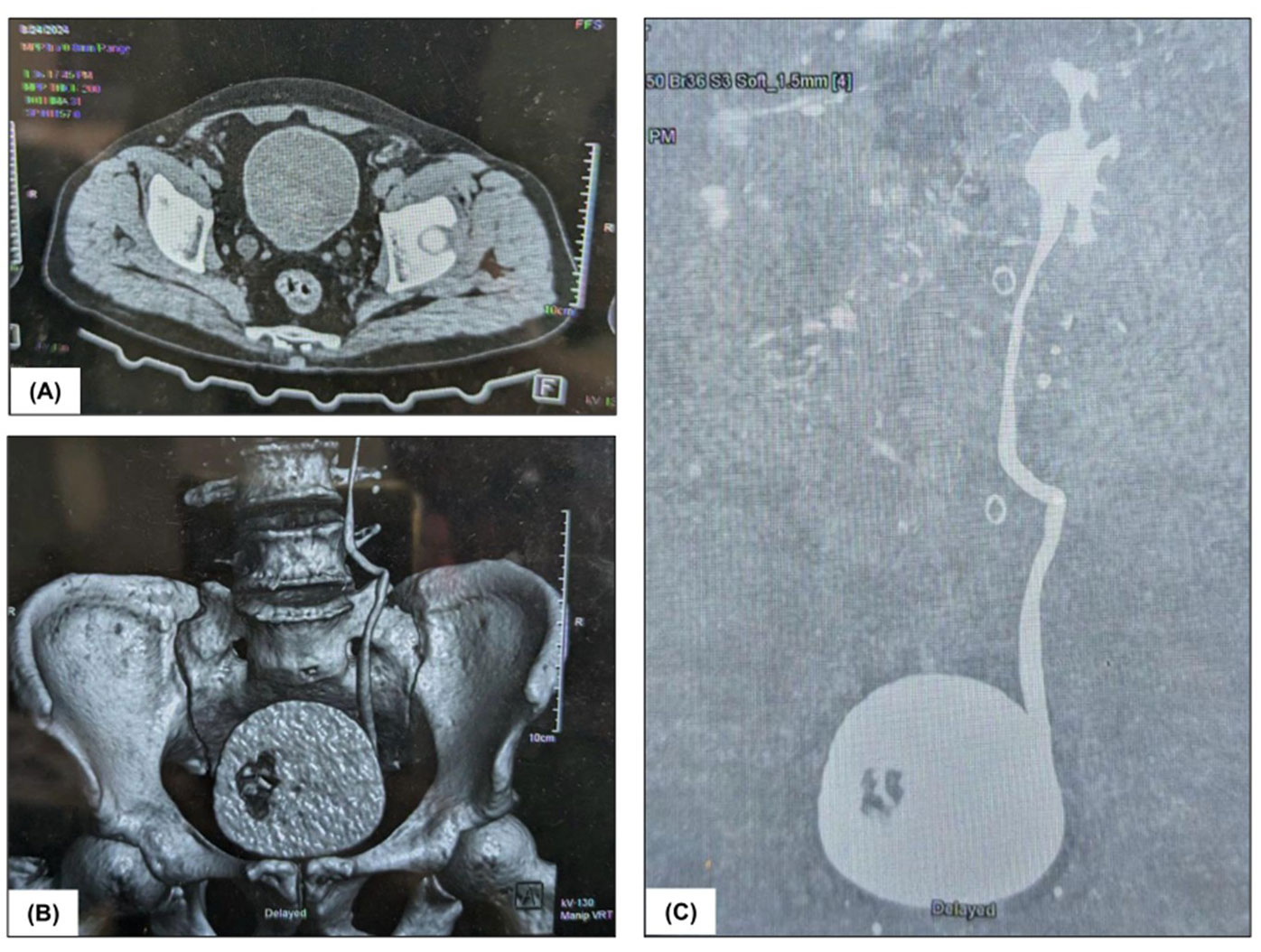 Figure 1. Radiological findings: (A), (B) and (C) CT urography depicting soft tissue polypoidal lesion arising from the right posterolateral wall of the urinary bladder.
Figure 1. Radiological findings: (A), (B) and (C) CT urography depicting soft tissue polypoidal lesion arising from the right posterolateral wall of the urinary bladder.
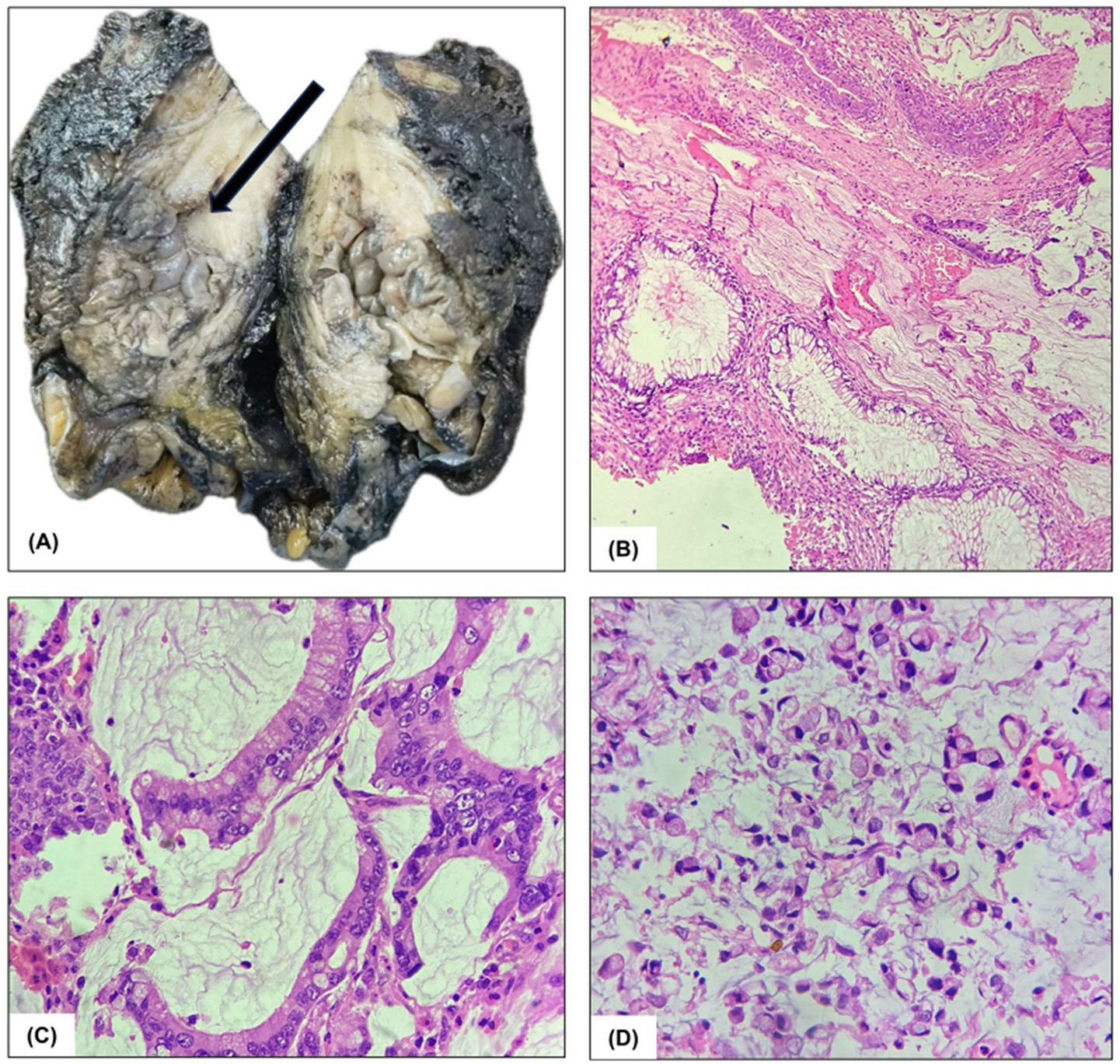 Figure 2. Pathological findings: (A) Gross specimen of the urinary bladder showing a papillary growth in the posterolateral wall. (B) and (C) Hematoxylin and Eosin-stained sections at 10X and 40X magnification, demonstrating abundant pools of extracellular mucin with medium-sized, well-formed glands lined by stratified columnar epithelium. (D) Singly dispersed signet ring cells with eccentrically pushed hyperchromatic nuclei and mucin-containing goblets within their cytoplasm (x400).
Figure 2. Pathological findings: (A) Gross specimen of the urinary bladder showing a papillary growth in the posterolateral wall. (B) and (C) Hematoxylin and Eosin-stained sections at 10X and 40X magnification, demonstrating abundant pools of extracellular mucin with medium-sized, well-formed glands lined by stratified columnar epithelium. (D) Singly dispersed signet ring cells with eccentrically pushed hyperchromatic nuclei and mucin-containing goblets within their cytoplasm (x400).
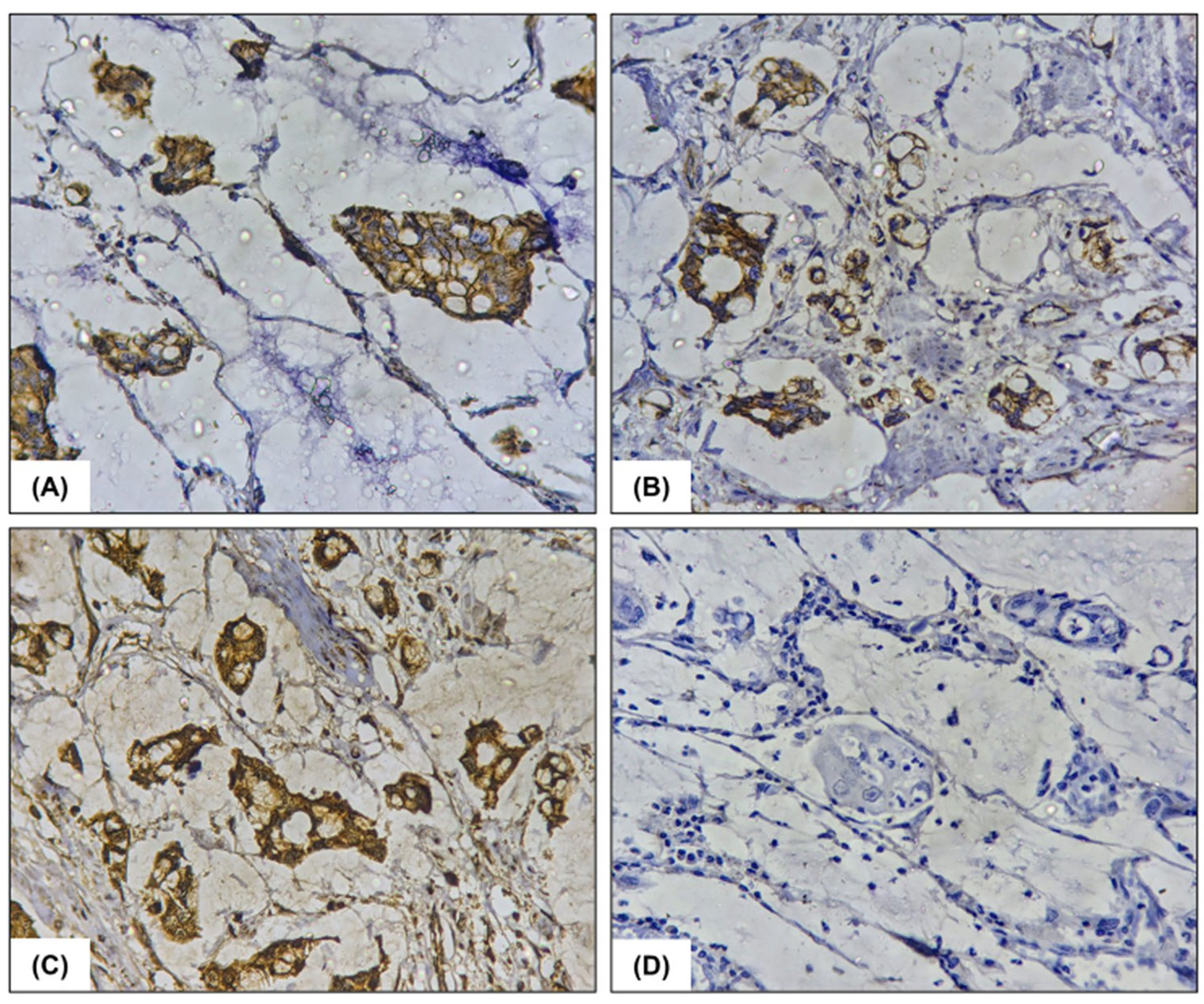 Figure 3. Immunohistochemistry findings: immunohistochemical analysis reveals (A) Cytoplasmic positivity for CK7(x400), (B) Cytoplasmic positivity for CK20(x400), (C) Membranous positivity for beta catenin(x400), and (D) Negative immunoexpression for CDX2(x400).
Figure 3. Immunohistochemistry findings: immunohistochemical analysis reveals (A) Cytoplasmic positivity for CK7(x400), (B) Cytoplasmic positivity for CK20(x400), (C) Membranous positivity for beta catenin(x400), and (D) Negative immunoexpression for CDX2(x400).
Bladder malignancies predominantly consist of urothelial carcinoma, which makes up about 90% of cases. PMA is an uncommon and aggressive form, representing a meagre 0.5% to 2% of all bladder tumors [3].
Bladder adenocarcinoma likely develops from sequential changes in the surface urothelium initiated by chronic inflammation, from cystitis glandularis with intestinal metaplasia to dysplasia, and then finally to adenocarcinoma. A prevailing hypothesis proposes that mucinous adenocarcinoma of the bladder may evolve through a gradual progression from mucinous metaplasia to mucinous adenoma, and eventually to carcinoma. However, this proposed sequence has not yet been confirmed by conclusive research [4]. Supporting this concept, a study conducted by Morton et al. demonstrated that intestinal metaplasia of the bladder exhibits significant telomere shortening and, at times, chromosomal abnormalities, corroborating its role as a potential precursor to bladder adenocarcinoma [5]. It has been associated with various risk factors, including bladder exstrophy, previous augmentation procedures, persistent irritation, urinary obstruction, and a non-functioning bladder. In regions with endemic schistosomiasis, infection caused by Schistosoma haematobium is considered the most significant contributor. Additionally, prolonged exposure to arsenic has been linked to an increased risk of developing this malignancy. It shows distinct molecular alterations, with frequent TP53, KRAS, PIK3CA, CTNNB1, APC, and TERT mutations [6].
Adenocarcinomas arising within the urinary tract encompass a spectrum of well-defined histological subtypes, including enteric (colonic-type), mucinous (colloid), signet-ring cell, not otherwise specified (NOS), and various mixed forms [6]. Our case demonstrated nests of neoplastic cells floating in abundant extravasated mucin, with singly dispersed or groups of signet ring cells within the mucin pools, consistent with the mucinous (colloid) variant. Adenocarcinoma of the urinary bladder may be either primary or secondary, with secondary cases originating from sites such as the colorectum, prostate, endometrium, or cervix. These lesions must be carefully distinguished from a range of histological mimics, including villous adenoma; urothelial carcinoma exhibiting prominent glandular differentiation; metastatic or locally invasive colorectal carcinoma; cystitis glandularis with intestinal metaplasia; nephrogenic adenoma; and the microcystic variant of urothelial carcinoma [7]. Immunohistochemistry aids in distinguishing primary from metastatic adenocarcinoma. Nuclear β-catenin and CK 20 expression and a negative CK 7 immunoexpression favor a colorectal origin, while immunopositivity for CK 7, CK 20, thrombomodulin, and membranous β-catenin staining support a primary bladder origin. Prostatic adenocarcinomas frequently express PSA, PSAP, and NKX3.1. Markers such as CA-125, vimentin, and PAX-8 indicate an endometrial origin [8]. In this case, the possibility of metastatic disease, particularly from common sites such as the colon or prostate, was excluded by a meticulous clinical, radiological, and immunohistochemical evaluation. Clinical assessment, including abdominal and digital rectal examination, revealed no abnormalities suggestive of gastrointestinal or prostatic pathology. Prostate-specific antigen (PSA) levels were within normal limits, and imaging showed no features of prostatomegaly or focal prostatic lesions, thereby excluding a prostatic source. Moreover, colonoscopic examination was unremarkable which further negated the possibility of a primary lesion in the colon.
A panel of immunohistochemical markers, including CDX2, β-catenin, CK7, CK20, SATB2, and GATA3 was employed to differentiate a primary bladder adenocarcinoma from metastatic colorectal adenocarcinoma. While CK20 was positive, CK7 co-expression and the absence of nuclear β-catenin favored a primary bladder origin. CDX2 and SATB2, which are more frequently positive in colorectal adenocarcinoma, were negative as well. Furthermore GATA3, which suggests urothelial differentiation and is generally negative in bladder adenocarcinomas, was negative in the present study as well. A whole-body PET-CT scan demonstrated a solitary, metabolically active lesion localized to the bladder, with no evidence of abnormal uptake elsewhere, effectively ruling out distant primary or secondary malignancy. Collectively, the clinicopathological and immunophenotypic profile substantiated the diagnosis of primary mucinous adenocarcinoma of the bladder, with no evidence suggestive of a metastatic lesion.
The diagnosis of primary mucinous adenocarcinoma of the bladder is primarily established through cystoscopic evaluation, urinary cytology, and histopathological examination following transurethral resection. PMA of the bladder represents a rare malignancy, characterized by aggressive clinical behavior and limited responsiveness to both chemotherapy and radiotherapy [4]. The prognostic outcome for primary bladder adenocarcinoma tends to be unfavourable, largely due to its frequent detection at an advanced stage. Reported disease-specific survival rates are approximately 62% at 2 years, 47% at 5 years, and decline further to around 40% at 10 years post-diagnosis [6]. As in the case of urothelial carcinoma, stage is the most important prognostic factor. Additionally, the pattern and frequency of metastases are also similar to those of high-grade, conventional urothelial carcinoma. The most common treatment for this stage is radical cystectomy with pelvic lymph node dissection, but primary radiation therapy may be considered for those who cannot undergo surgery. Table 1 elucidates the entire clinicopathological spectrum of previously reported cases of PMA of urinary bladder.
|
Table 1. The entire clinicopathological spectrum of previously reported cases of PMA of urinary bladder. |
||||||||||||
|
S No. |
Author |
Year |
Age |
Sex |
Clinical presentation |
Radiological findings |
Diagnostic findings |
Extent of tumor |
Staging of tumor |
IHC/ Molecular findings |
Treatment |
Follow up |
|
1. |
Kiyama Y et al. [9] |
2024 |
48 |
M |
Asymptomatic gross hematuria |
Pelvic Contrast MRI: mass lesion; low signal intensity on T2-Weighted images in bladder with infiltration into transition zone and marginal zone of the base of right lobe of prostate and into both seminal vesicles. Right internal iliac and external iliac lymph node swelling was also observed. |
H&E staining: atypical glandular epithelial cells with mucus production |
Connective tissue lining of the bladder wall. |
Stage IIIb. cT4aN2M0 |
Positive for CK20, CDX2, SATB2, B-Catenin.
Negative for CK7 and NKX3.1 |
Total cystectomy, pelvic lymph node dissection, and ileal conduit creation. Post-op adjuvant chemotherapy, three courses of XELOX therapy (Oxaliplatin 21-day cycle, capecitabine 14 days). |
No recurrence after 11 months of surgery. |
|
2. |
Ghewade P et al. [10] |
2024 |
44 |
F |
Burning micturition for six to eight months, decreased frequency of urination, and urine incontinence. Loss of appetite |
CT scan: A heterogeneously enhancing lobulated soft tissue mass lesion spanning approximately 8.1 x 7.4 x 5.7 cm along the fundic region of the bladder, accompanied by minimal surrounding fat stranding |
Histopathology: neoplastic glands lined by pleomorphic, mucin-producing, pseudostratified columnar epithelium; moderately differentiated adenocarcinoma of the enteric type with invasion up to the muscularis propria.
|
Muscularis propria layer of bladder wall. |
Stage II.
T3aN0M0 |
Positivity for CK7 & CK20 |
Total cystectomy and total abdominal hysterectomy with bilateral salpingo-oophorectomy (anterior exenteration) with ileal conduit creation. Post-op antibiotics and analgesics. |
To follow up 7 days post-surgery. Further details not provided. |
|
3. |
Maja SG et al. [11] |
2022 |
75 |
F |
Hematuria with severe anemia and hypovolemic shock |
Abd. CT scan: a tumor sized 2x2 cm right to the bladder dome. |
Infiltrative malignant epithelial neoplasm composed of atypical glandular structures with nuclear and cytological atypia, and mucin production. |
Fatty tissue lining the bladder |
Stage IIIa.
T3aN2M0 |
IHC showed CK20 and CDX2 expression, positivity of CK7 and beta- catenin, and CA-125 focal expression.
Negative for estrogen receptor, progesterone receptor, and Pax8. |
Partial cystectomy and bilateral lymphadenectomy.
Poor response to chemotherapy and radiotherapy. |
Regular cystoscopy follow-ups every three months for the first year. CT-scan once a year for five years. After 2 years of follow- ups, no signs of recurrence or distant metastasis were found.
|
|
4. |
Wang D et al. [12] |
2022 |
62 |
F |
Difficulties in urination for 1 year. |
USG: hypoechoic mass- 6.5 cm × 3.8 cm × 4.4 cm at the bladder outlet and urethra. Plain CT & Enhanced: thickened wall of bladder, soft tissue density was seen at the bladder outlet and urethra with slight enhancement and an unclear partial boundary. |
Cells arranged in solid clumps or small cords; formation of glandular lumen; mucin production; neuroendocrine differentiation, some signet ring cells and focal calcification also seen.
|
Metastasized to bones. |
Stage IVB |
Positive for CK, CK20, Ki-67, Synaptophysin
CD56 - Partial positive
Chromogranin A - slightly positive
GATA3 - negative CDX2 - negative |
Radical Cystectomy. 2 cycles of FOLFOX (leucovorin calcium + 5-fluorouracil + oxaliplatin)-6 regimen were performed. Zoledronic Acid was used for treating bone metastases. |
The patient survived the treatment. Further follow-up details are not available. |
|
5. |
Gupta S et al. [13] |
2022 |
65 |
M |
Huge supra-pubic lump, which ulcerated into a fungating mass, ripping off, protruding, and eroding through all suprapubic structures along with hematuria, increased frequency and burning micturition. |
Non-contrast CT-scan: large exophytic and endophytic components of polypoidal lobulated soft tissue mass lesion originating from the wall of urinary bladder and also mild cortical irregularity and erosions in superior pubic ramus and pubic symphysis. Multiple enlarged lymph nodes in right inguinal region with loss of fatty hilum, largest measuring 2.7 x 1.6 cm. Lung metastasis; largest nodule-17 x 18 mm in poster basal segment of right lower lobe. |
Well-differentiated adenocarcinoma of the bladder (papillary mucin type).
|
Metastasized to the lungs. |
Stage IVB |
Not available |
Palliative treatment. |
Not available. |
|
6. |
Bijalwan P et al. [14] |
2017 |
70 |
F |
Mucusuria with recurrent UTI. Hematuria absent. Examination revealed the bladder was distended up to the umbilicus. |
CT Abdomen: irregular enhancing focal thickening over the right wall and dome of the bladder with air pockets within the over-distended bladder. |
Histology of biopsy showed adenocarcinoma with extracellular pools of mucin infiltrating into the muscle. |
Tumor invading into the muscle of bladder. |
Stage II.
T2bN0M0 |
CK20 positivity |
Radical cystectomy and pelvic lymph node dissection were performed. |
Not available. |
|
7. |
Pan X et al. [15] |
2016 |
59 |
F |
Epigastric pain for 5 months. |
CT Abdomen: a mass located in the anterior bladder wall, sized 5.0×3.4 cm with thickened surrounding bladder wall and abnormal findings in the pelvic cavity. |
Histopathology: The tumor contained copious amounts of mucus, with irregular cell cords surrounding the mucus pools. |
Invasion into the greater omentum and peritoneum. |
Stage IVa
T4bN0M0 |
Not Available. |
Tumor found to be inoperable due to significant local invasion. |
The patient succumbed to the disease 3 months later. |
|
8. |
Pokuri VK et al. [16] |
2016 |
54 |
M |
Urinary urgency and hematuria for 6 weeks, which progressed to urinary retention. |
CT Abdomen & Pelvis: right hydroureteronephrosis, bladder wall thickening, and an enlarged prostate.
Cystoscopy: prostatomegaly with partial obstruction of prostatic urethra.
MRI: asymmetric thickening of the right posterior wall of urinary bladder, increased enhancement along the mucosa contiguous with enlarged right seminal vesicle (abnormal low T2 signal), suggesting a suspicious mass lesion extending toward the distal right ureterovesical junction causing obstruction. No colorectal masses or pelvic/abdominal lymphadenopathy were identified. |
Histopathology: Prostate gland shows normal and atrophic glands with admixed infiltrating adenocarcinoma with perineural invasion. Features suggestive of primary bladder adenocarcinoma with intestinal differentiation extending into prostate. |
Metastasis to lungs. |
Stage IVb.
T4aN0M1b |
Positive for CK20 and CDX2.
Negative for Prostate-specific antigen, thrombomodulin, and AMACR. |
The patient underwent TURP followed by right percutaneous nephrostomy and antegrade ureteral stenting.
Chemotherapy regimen FOLFOX (5-fuorouracil, folinic acid, oxaliplatin) plus bevacizumab (Bev) for 12 cycles given. CT showed decrease in the bladder wall thickening and stable disease in the lungs. Subsequent imaging 3 months later showed enlargement of pulmonary metastases. So, oxaliplatin changed to irinotecan (FOLFIRI-Bev). The patient received about 28 cycles of FOLFIRI-Bev with intermittent chemotherapy breaks and had radiographic progression in the bladder. Currently stable on FOLFIRI- cetuximab, and urinary symptoms have improved. |
Patient received 3 lines of colorectal cancer regimens, with stable disease for 2.2 years. |
|
9. |
Bauman TM et al. [17] |
2015 |
75 |
M |
A history of spinal cord injury presented with hematuria and SP (suprapubic) discharge after SP catheterization for 51 years. |
CT urography: irregular, infiltrative, and heterogeneous mass arising from the anterior bladder at the level of the suprapubic catheter and extending along the SP tube tract. |
Biopsy: invasive moderately differentiated adenocarcinoma of the anterior bladder and stoma with extensive associated mucin production and a background of acute and chronic inflammation. |
Invasion through superficial lamina propria. |
High-grade Stage II. T2N0M0. |
IHC was not used to help in the diagnosis of this patient. |
Cystoprostatectomy, abdominal wall resection, ileal conduit creation, and abdominal wall reconstruction. |
CT abdomen & pelvis and chest with either CT or x-ray was performed every 6 months for 2 years and every 12 months to monitor for local tumor recurrence or metastatic disease. No evidence of tumor recurrence over the previous 5 years. |
|
10. |
Santos BM et al. [4] |
2015 |
40 |
F |
Recurrent Urinary Tract Infections for over a year. |
USG: 21 mm vegetating bladder lesion, close to the urethra. CT: 19 mm solid bladder lesion, with contrast enhancement |
Histopathology: intestinal type mucinous carcinoma, originated from tubular polyp with low-grade dysplasia.
No gastrointestinal source found. It was concluded that bladder lesion was primary. |
Tumor growing into connective tissue under the lining layer of the bladder.
Invasion into muscle layer not seen. |
Stage I
T1N0M0 |
Positive for Beta Catenin, CDX2, CK20, CK07, Cytokeratins cocktail, and p53.
Negative for CA 19-9, CA 125, alpha-fetoprotein, and CEA. |
Transurethral resection of the lesion |
It was recommended that the patient undergoes a cystoscopy every 3 months, and CT in one year. |
Not applicable.
Ethical policy
Written informed consent was taken from the patient to participate in this study and for the publication of any potentially identifiable images or data included in this article. Ethical review and approval are not required, and a waiver for the publication of case reports is required as per institutional requirements.
Availability of data and materials
No new data were generated during this study.
Author contributions
Anuradha Pandit, Shaivy Malik and Nibir Chakma had a key role in the conceptualization, drafting, and revision of the manuscript and the collection, analysis, and interpretation of the data. Charanjeet Ahluwalia had an important role in diagnosing the case, conceptualization, providing resources, and critically revising the manuscript for important intellectual content.
Competing interests
The authors declare no conflict or competing interests.
Funding
This study was not supported by any sponsor or funder.
- Vasudevan G, Bishnu A, Singh BMK, Nayak DM, Jain P: Bladder adenocarcinoma: a persisting diagnostic dilemma. J Clin Diagn Res 2022, 11(3): ER01-ER04.
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74(3): 229-263.
- Tuazon RJ, Romero RLT, Alpas M: A rare case of mucinous adenocarcinoma of the bladder: a case report. Philipp J Urol 2022, 31(1): 117.
- Santos BM, de Souza JD, Lima RS, de Lima EM: Mucinous bladder adenocarcinoma: case report and literature review. Case Rep Urol 2015, 2015: 783109.
- Morton MJ, Zhang S, Lopez-Beltran A, MacLennan GT, Eble JN, Montironi R, Sung MT, Tan PH, Zheng S, Zhou H: Telomere shortening and chromosomal abnormalities in intestinal metaplasia of the urinary bladder. Clin Cancer Res 2007, 13(20): 6232-6236.
- Gill AJ, Hartmann A, Menon S, Raspollini MR, Rubin MA, Srigley JR, Hoon Tan P, Tickoo SK, Tsuzuki T, Turajlic S: The 2022 World Health Organization classification of tumors of the urinary system and male genital organs-part b: prostate and urinary tract tumors. Eur Urol 2022, 82(5): 469-482.
- Jagtap SV, Beniwal A, Jagtap SS, Huddedar AD: Transitional cell carcinoma with glandular differentiation – a rare variant of urinary bladder carcinoma. Int J Health Sci Res 2015, 5(1): 374-376.
- Yeh TJ, Tang SH, Liu YC, Hsiao HH: Primary adenocarcinoma of the urinary bladder: report of two cases with a literature review. J Cancer Res Pract 2020, 7(3): 130.
- Kiyama Y, Sekii Y, Inoguchi S, Matsumura S, Kitakaze H, Hongo S, Okumi M, Takada S, Kitaoka H: A case of primary adenocarcinoma mucinous subtype of the bladder. Hinyokika Kiyo 2024, 70(4): 89-92.
- Ghewade P, Shukla S, Vagha S, Kalode SS, Gadkari P: Primary adenocarcinoma of the urinary bladder: a case report on a rare malignancy. Cureus 2024, 16(8): e66269.
- Maja SG, Slavica KK, Suad A, Rubens J: Bladder mucinous adenocarcinoma as a diagnostic challenge: a case report. Pan Afr Med J 2022, 42: 221.
- Wang D, Zhang K, Guan L, Wen N: Imaging features of primary mucinous adenocarcinoma of bladder outlet and urethra: a case report and literature review. Transl Cancer Res 2022, 11(7): 2416-2424.
- Gupta S, Dharamshi JD: Adenocarcinoma of urinary bladder with distant metastasis: huge fungating tumor eroding and ripping-off through suprapubic region: a rare presentation. Cureus 2022, 14(5): e24698.
- Bijalwan P, Bindhu MR, Pooleri GK: Primary mucin secreting adenocarcinoma bladder: a case series. Indian J Surg Oncol 2017, 8(4): 634-636.
- Pan X, Jin L, He T, Hu J, Quan J, Zhou L, Ni L, Yang S, Mao X, Lai Y: Mucinous adenocarcinoma of the bladder: a case report and review of the literature. Mol Clin Oncol 2016, 5(4): 447-448.
- Pokuri VK, Sule N, Perfetto C, Duff M, Kopp C, Guru K, Shah D, George S: A novel treatment approach prolonging survival in an uncommon metastatic primary bladder adenocarcinoma. J Community Support Oncol 2016, 14(2): 72-75.
- Bauman TM, Potretzke TA, Potretzke AM, Siegel CL, Brandes SB: Mucinous adenocarcinoma of the bladder associated with long term suprapubic tube: a case report. BMC Urol 2015, 15: 119.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript