Review Article | Open Access
Evolving Therapeutic Paradigms in Bladder Cancer: The Impact of Immunotherapy and Antibody-Drug Conjugates
Shahd Mustafa Ibrahim1, Ahmed Attia Ahmed Abdelmoaty2, Ahmed Helmy Abdelhaseb3
1Department of Clinical Pharmacy, Faculty of Pharmacy, Alamein International University, Alamein 51718, Egypt.
2Department of Pharmacology, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt.
3College of Biotechnology, Misr University for Science and Technology (MUST), 6th of October City, Giza 12566, Egypt.
Correspondence: Shahd Mustafa Ibrahim (Department of Clinical Pharmacy, Faculty of Pharmacy, Alamein International University, Alamein 51718, Egypt; Email: shahdmostafa7272@gmail.com).
Annals of Urologic Oncology 2025, 8(3): 156-166. https://doi.org/10.32948/auo.2025.09.25
Received: 22 Aug 2025 | Accepted: 09 Oct 2025 | Published online: 24 Oct 2025
Key words bladder cancer, immunotherapy, immune checkpoint inhibitors, antibody-drug conjugate, combination therapy
Over the past decade, the therapeutic management of bladder cancer has transformed by the introduction of immune checkpoint inhibitors (ICIs). Leveraging the intrinsic immunogenicity, ICIs restore antitumor immune responses and have become standard treatments across multiple cancer types including bladder cancer [11]. Antibody-drug conjugates (ADCs) have entered clinical practice as well, offering a targeted cytotoxic approach that links tumor-specific antibodies with potent chemotherapeutic payloads to induce cancer cell apoptosis while minimizing systemic toxicity [12]. Together, ICIs and ADCs are redefining the bladder cancer management strategies. Combination regimens such as enfortumab vedotin (an ADC) plus pembrolizumab (an ICI) have demonstrated remarkable efficacy in advanced bladder cancer, achieving response rates of approximately 68% and one-year survival near 79%, exceeding traditional chemotherapy outcomes substantially [13]. Thus, immunotherapy and ADCs represent highly promising therapeutic approaches for patients with bladder cancer.
In this review, we discuss the evolving therapeutic landscape of bladder cancer, emphasizing the integration of both immunotherapy and ADCs into current clinical treatment paradigms. Furthermore, we explore the combination strategies that merge the two modalities, highlighting their potential to define the next generation of personalized therapy for bladder cancer.
ADCs belong to a distinct precision-based strategy for targeted cytotoxic drug delivery. Mechanistically, ADC integrates three components: a monoclonal antibody directed against a tumor-specific antigen, a potent cytotoxic payload, and a linker connecting the two [22]. The antibody component ensures tumor selectivity, while the payload, commonly a microtubule inhibitor such as monomethyl auristatin E (MMAE) or a topoisomerase I inhibitor such as SN-38, provides highly potent cell-killing activity at nanomolar concentrations [23]. Cleavable linkers respond to tumor-specific conditions, such as acidic pH or lysosomal enzymes, while non-cleavable linkers release the payload only after antibody degradation within the cell [24, 25]. Upon antigen binding, the ADC-antigen complex is internalized, and intracellular linker cleavage releases the active drug, which induces apoptotic death primarily through DNA damage or microtubule disruption [26]. Some ADCs also exhibit a “bystander effect,” in which membrane-permeable payloads diffuse inside the neighboring tumor cells, eradicating antigen-negative cells as well within the heterogeneous tumors [27]. The antibody moiety can also trigger immune effector functions such as antibody-dependent cellular cytotoxicity, further amplifying the therapeutic response [28]. Overall, ICIs and ADCs employ complementary mechanisms, one reawakening the immune surveillance, and the other delivering precise cytotoxic attack, marking both classes as essential pillars in the field of modern cancer therapeutics (Figure 1).
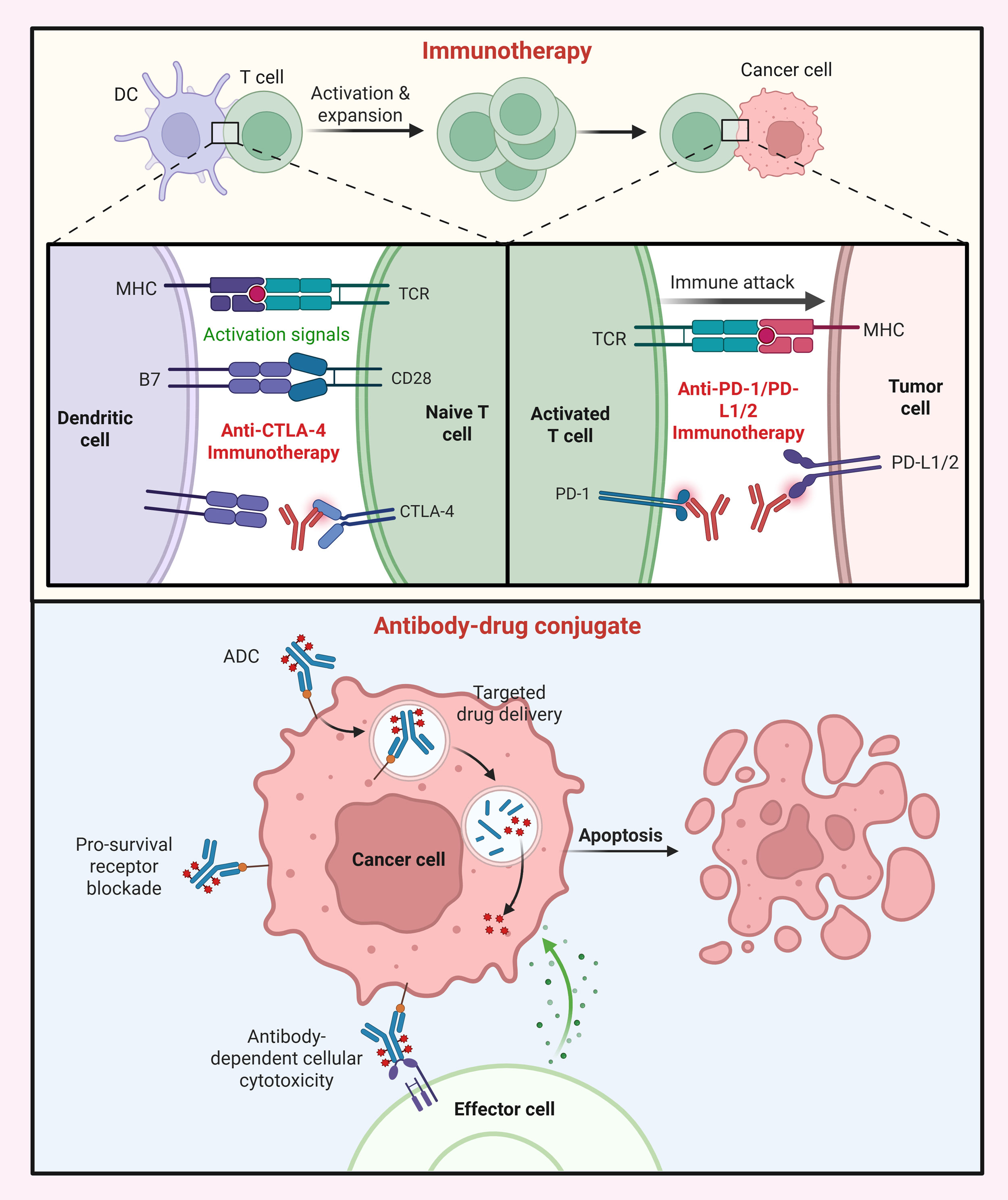 Figure 1. Mechanistic basis of immunotherapy and ADCs. (Top) Immunotherapy (ICIs) restore antitumor T-cell activity by blocking inhibitory pathways exploited by tumors. Anti-CTLA-4 antibodies enhance T-cell priming by preventing CTLA-4-mediated suppression, while anti-PD-1/PD-L1/2 antibodies reinvigorate exhausted T cells in the tumor microenvironment, promoting immune cell-driven tumor cell destruction. (Bottom) ADCs combine a tumor-targeting antibody with a cytotoxic payload linked by a cleavable or non-cleavable linker. Following antigen binding and internalization, the payload is released to induce apoptosis, while additional mechanisms such as antibody-dependent cellular cytotoxicity may further enhance tumor cell killing.
Figure 1. Mechanistic basis of immunotherapy and ADCs. (Top) Immunotherapy (ICIs) restore antitumor T-cell activity by blocking inhibitory pathways exploited by tumors. Anti-CTLA-4 antibodies enhance T-cell priming by preventing CTLA-4-mediated suppression, while anti-PD-1/PD-L1/2 antibodies reinvigorate exhausted T cells in the tumor microenvironment, promoting immune cell-driven tumor cell destruction. (Bottom) ADCs combine a tumor-targeting antibody with a cytotoxic payload linked by a cleavable or non-cleavable linker. Following antigen binding and internalization, the payload is released to induce apoptosis, while additional mechanisms such as antibody-dependent cellular cytotoxicity may further enhance tumor cell killing.
On the adjuvant therapy frontier, CheckMate-274 established nivolumab as key option for high-risk muscle-invasive bladder cancer following cystectomy [35]. Pateints on adjuvant nivolumab exhibited almost double median disease-free survival time compared with those on placebo, and updated results reported a median overall survival of nearly 70 months in the treatment arm versus around 50 months in controls [36]. The benefit was observed across subgroups, regardless of nodal involvement or prior chemotherapy. In the CheckMate-032 trial, the nivolumab-ipilimumab combination has been shown to yield higher response rates than nivolumab monotherapy, particularly with optimized dosing schedules [37]. Building on these, the MODERN trial employs ctDNA-guided escalation with nivolumab plus relatlimab for ctDNA-positive cases, while ctDNA-negative patients undergo immunotherapy or surveillance depending on biomarker status [38]. Retrospective analyses of IMvigor010 trial of adjuvant atezolizumab suggested survival improvement in patients with detectable ctDNA, prompting the IMvigor011 study to prospectively evaluate adjuvant atezolizumab in this biomarker-defined population [39, 40]. This evolving ctDNA-based framework may aid in personalizing adjuvant immunotherapy by distinguishing patients who need additional systemic treatment from those who do not.
Intravesical and vaccine-based immunotherapies are also being explored. The SunRISe-4 trial examined TAR-200, a gemcitabine-releasing intravesical device, combined with the PD-1 inhibitor cetrelimab in cisplatin-ineligible patients. The combination produced a 42% complete pathological response rate compared with 23% with cetrelimab alone [41]. On the same lines, the CORE-002 trial investigated the oncolytic adenovirus CG0070 (armed with GM-CSF) plus nivolumab, achieving a 42% complete response rate and one-year recurrence-free survival exceeding 70% [42]. Another line of investigation involves cytokine modulation: the phase III PIVOT IO 009 trial evaluates nivolumab with bempegaldesleukin (an IL-2 agonist) as neoadjuvant and adjuvant therapy for cisplatin-ineligible patients [43]. For advanced disease, the CheckMate-901 trial confirmed that adding nivolumab to first-line gemcitabine-cisplatin improved both progression-free and overall survival compared with chemotherapy alone, reinforcing the role of chemo-immunotherapy in cisplatin-eligible patients [44]. Meanwhile, the KEYMAKER-U04 platform study is examining pembrolizumab with the LAG-3 inhibitor favezelimab in previously untreated metastatic disease. Personalized vaccine strategies are also emerging. In a pilot study, a neoantigen-based mRNA vaccine (PGV001) given with atezolizumab prevented metastatic recurrence in three of four treated patients with high-risk disease [45]. The V940 (mRNA-4157) vaccine encoding patient-specific neoantigens is being tested with pembrolizumab ± enfortumab vedotin in the perioperative setting [46]. Another personalized mRNA construct, autogene cevumeran, is under evaluation alone or with nivolumab for high-risk adjuvant therapy in resected disease.
Beyond systemic treatment, bladder-preserving approaches incorporating immunotherapy are gaining attention. A multicenter phase II study combining gemcitabine, pembrolizumab, and hypofractionated radiotherapy achieved a two-year bladder-intact disease-free survival of 71% and overall survival of 83% [47]. The IMMUNOPRESERVE trial tested durvalumab plus tremelimumab with radiotherapy in patients unwilling or ineligible for cystectomy, resulting in an 81% complete response rate and 2-year survival above 80% [48]. Similarly, interim analysis from another phase II study combining chemoradiation with nivolumab showed superior two-year relapse-free survival compared to chemoradiation alone [49]. Phase III programs, INTACT (atezolizumab with chemoradiation) and KEYNOTE-992 (pembrolizumab with chemoradiation), have both completed accrual, with results anticipated soon [50, 51]. Collectively, immunotherapy has evolved from a salvage option to a foundation of multimodal bladder cancer management (Table 1). It now spans neoadjuvant, adjuvant, intravesical, and organ-sparing applications. Integration with ctDNA monitoring, radiotherapy, and novel agents continues to expand its utility, bringing the field closer to individualized, biomarker-guided immuno-oncology for bladder cancer.
|
Table 1. List of clinical trials testing immunotherapy in bladder cancer. |
||||
|
Trial No. |
Regimen |
Settings |
Mechanism |
Phase |
|
NCT03359239 |
Atezolizumab + PGV001 |
Adjuvant |
Neoantigen vaccine; Anti-PD-1 |
1 |
|
NCT03740256 |
CAdVEC + HER2 CAR-T |
Refractory |
Oncolytic adenovirus; CAR-T |
1 |
|
NCT04601857 |
Pembrolizumab + Fudibatinib |
First-line |
Pan-FGFR inhibitor; Anti-PD-1 |
2 |
|
NCT04919512 |
Cetrelimab ± TAR-200 (gemcitabine) |
Neoadjuvant |
Anti-PD-1; intravesical gemcitabine device |
2 |
|
NCT06534983 |
Nivolumab + Autogene cevumeran |
Adjuvant |
mRNA vaccine; Anti-PD-1 |
2 |
|
NCT04601857 |
Pembrolizumab + Fudibatinib |
First-line |
Pan-FGFR inhibitor; Anti-PD-1 |
2 |
|
NCT04919512 |
Cetrelimab ± TAR-200 (gemcitabine) |
Neoadjuvant |
Anti-PD-1; intravesical gemcitabine device |
2 |
|
NCT02632409 |
Nivolumab |
Adjuvant |
Anti-PD-1 |
3 |
|
NCT03036098 |
Ipilimumab/Nivolumab; GC ± NIVOLUMAB |
First-line |
Anti-CTLA-4; Anti-PD-1 |
3 |
|
NCT03661320 |
Nivolumab ± Linrodostat |
Perioperative |
Anti-PD-1; IDO1 inhibitor |
3 |
|
NCT03732677 |
Durvalumab |
Perioperative |
Anti-PD-L1 |
3 |
|
NCT03924856 |
Pembrolizumab |
Perioperative |
Anti-PD-1 |
3 |
|
NCT04209114 |
Nivolumab ± Bempeg (NKTR-214) |
Perioperative |
Anti-PD-1; IL-2 prodrug |
3 |
ADC named enfortumab vedotin, which targets Nectin-4, has exhibited another transformative finding in advanced bladder cancer. In the phase III EV-302 trial, the combination of enfortumab vedotin and pembrolizumab nearly doubled both progression-free and overall survival compared with standard chemotherapy, with median overall survival extending beyond 30 months [60, 61]. This regimen has now been established as the new global first-line standard for locally advanced or metastatic bladder cancer, applicable to both cisplatin-eligible and -ineligible populations. Common toxicities included neuropathy, rash, and alopecia, reflecting ADC-specific rather than chemotherapy-related profiles [60, 61]. Several next-generation Nectin-4 ADCs are under development. 9MW2821, which employs a site-specific linker to ensure a consistent drug-to-antibody ratio, showed an objective response rate of 62% and median progression-free survival of nearly 9 months in early-phase studies [62]. Large-scale trials are ongoing to evaluate it both as monotherapy and in combination with immune checkpoint inhibitors [62, 63]. Similarly, SHR-A2102, a topoisomerase-I payload-based ADC targeting Nectin-4, produced an objective response rate of 38% in heavily pretreated patients, with higher activity observed at increased dosing levels [64], offering alternatives for patients who develop resistance or intolerance to enfortumab vedotin.
Datopotamab deruxtecan (Dato-DXd), a Trop-2-directed ADCs, has shown early promise in patients previously treated with chemotherapy and immunotherapy, achieving a 25% overall response rate and a median progression-free survival of about 7 months [65]. The TROPION series of trials is aimed at optimizing the dosage to clarify the impact of Trop-2 expression on response [66]. While sacituzumab govitecan demonstrated encouraging early data in the TROPHY-U-01 trial, its confirmatory phase III study (TROPiCS-04) did not meet its survival endpoint, leading to withdrawal of its indication in bladder cancer [67]. A newer Trop-2 ADC, sacituzumab tirumotecan, links the antibody to a novel topoisomerase-I payload with enhanced stability. In the MK-2870-001 trial, response rates reached 46% in second-line and 26% in later-line therapy, with median progression-free survival extending several months [68]. Trials combining sacituzumab tirumotecan with enfortumab vedotin and pembrolizumab are now underway, potentially enabling dual antigen targeting with immune activation.
Beyond single-agent ADCs, dual-ADC regimens have entered exploration. The DAD study tested sacituzumab govitecan with enfortumab vedotin in metastatic bladder cancer refractory to prior therapies, achieving a 70% response rate with durable responses in several patients [69]. Toxicities were frequent but consistent with known class effects. A subsequent cohort (DAD-IO) is evaluating the addition of pembrolizumab, reflecting a growing interest in multi-target ADC and checkpoint inhibitor combinations. Innovative constructs such as BL-B01D1, a bispecific EGFR-HER3 ADC carrying a topoisomerase-I payload, have also shown early efficacy. In a phase Ib/II trial, the objective response rate approached 44%, with manageable hematologic toxicities [70]. Multiple phase II and III trials are ongoing to determine whether bispecific targeting can overcome antigen heterogeneity and resistance mechanisms in advanced disease. Smaller conjugate designs, small-molecule drug conjugates (SMDCs), are also entering clinical testing. Zelenectide pevedotin (BT8009), a Nectin-4-directed bicyclic peptide conjugate, has been shown to achieve a 45% response rate in patients previously treated with checkpoint inhibitors and platinum therapy [71]. The Duravelo-2 study is now evaluating BT8009 alone and with pembrolizumab as a first-line option in bladder cancer [72]. BT7480, combines Nectin-4 and CD137 (4-1BB) targeting in a single bicyclic construct to stimulate both tumor and immune cells, while BT5528, directed at EphA2, has demonstrated promising activity with confirmed responses in 45% of the bladder cancer patients [73, 74]. Therefore, bicyclic conjugates may offer a next generation of ADC-like precision therapies with improved pharmacokinetics. ADCs within bladder-preserving protocols are also being explored. The RAD-SG study is evaluating concurrent delivery of sacituzumab govitecan with adaptive radiotherapy following maximal transurethral resection, testing whether localized ADC exposure can enhance tumor control while preserving organ function [75].
ADCs have now transitioned from experimental tools to clinically viable therapeutic options in bladder cancer. Refining antigen selection, linker chemistry, and payload design are expected to yield more effective results, and position ADCs as a core pillar of personalized therapy in bladder cancer.
|
Table 2. List of clinical trials testing antibody-drug conjugates in bladder cancer. |
||||
|
Trial No. |
Regimen |
Settings |
Mechanism |
Phase |
|
NCT03401385 |
Datopotamab deruxtecan |
Refractory |
Trop-2 ADC |
1 |
|
NCT05735275 |
SHR-A2102 |
Unspecified |
Nectin-4 ADC |
1 |
|
NCT06238479 |
LY4101174 |
Refractory |
Nectin-4 ADC |
1 |
|
NCT04152499 |
Sacituzumab tirumotecan |
Refractory |
Trop-2 ADC |
1/2 |
|
NCT05460273 |
Datopotamab deruxtecan |
Unspecified |
Trop-2 ADC |
1/2 |
|
NCT03288545 |
Enfortumab vedotin |
Perioperative |
Nectin-4 ADC |
1b/2 |
|
NCT05216965 |
9MW2821 |
Unspecified |
Nectin-4 ADC |
1a/2 |
|
NCT04482309 |
Trastuzumab deruxtecan |
Refractory |
HER2 ADC |
2 |
|
NCT05785039 |
BL-B01D1 |
Refractory |
EGFR-HER3 ADC (bispecific) |
2 |
|
NCT06857175 |
BL-B01D1 |
Refractory |
EGFR-HER3 ADC (bispecific) |
3 |
|
*ADC: Antibody-drug conjugate. |
||||
Other combination treatments entailing ICIs and ADCs are also actively being explored in bladder cancer. For instance, TROPHY-U-01 trial encompassing patients who had progressed after platinum chemotherapy but had not received checkpoint-inhibitor treatment evaluated the sacituzumab govitecan plus pembrolizumab regimen. The combination produced an objective response rate of 41%, and 20% patients achieved complete responses. The median duration of response was 11 months with median overall survival of 13 months [80]. Adverse events were consistent with known drug profiles, primarily including neutropenia, leukopenia, and diarrhea. As enfortumab vedotin plus pembrolizumab now anchors first-line management, sacituzumab govitecan-based combinations may have future roles in post-platinum or post-ADC settings, particularly in biomarker-defined patient subgroups [60]. This affirms that ADC-ICI synergy extends beyond a single drug pair and may serve as a broader therapeutic option across multiple tumor targets in bladder cancer.
Combining ADCs with immunotherapy has rapidly evolved from a conceptual rationale to a clinically tested reality in bladder cancer (Table 3). Ongoing studies involving HER2-, Trop-2-, and Nectin-4-directed ADCs, as well as dual ADC or bispecific constructs, are expected to extend these benefits further in the realm of bladder cancer treatment [60, 80]. Identification of biomarkers that predict synergy, optimization of sequence and dosing, and management of overlapping toxicities is warranted to sustain durable immune-cytotoxic synergy of combining ICIs and ADCs in bladder cancer.
|
Table 3. List of clinical trials testing combinations of immunotherapy and antibody-drug conjugates in bladder cancer. |
||||
|
Trial No. |
Regimen |
Settings |
Mechanism |
Phase |
|
NCT04606472 |
SI-B003 |
Refractory |
PD-1/CTLA-4 (bispecific) |
1 |
|
NCT05297552 |
Disitamab vedotin + Toripalimab |
Neoadjuvant |
HER2 ADC; Anti-PD-1 |
2 |
|
NCT05535218 |
Sacituzumab govitecan + Pembrolizumab |
Neoadjuvant/ Periop |
Trop-2 ADC; Anti-PD-1 |
2 |
|
NCT06405425 |
BL-B01D1 + Anti-PD-1 (unspecified) |
First-line |
EGFR-HER3 ADC (bispecific); Anti-PD-1 |
2 |
|
NCT06823427 |
9MW2821 ± Toripalimab |
First-line |
Nectin-4 ADC; Anti-PD-1 |
2 |
|
NCT03924895 |
Enfortumab vedotin ± Pembrolizumab |
Perioperative |
Nectin-4 ADC; Anti-PD-1 |
3 |
|
NCT04223856 |
Enfortumab vedotin + Pembrolizumab |
First-line |
Nectin-4 ADC; Anti-PD-1 |
3 |
|
NCT04700124 |
Enfortumab vedotin + Pembrolizumab |
Perioperative |
Nectin-4 ADC; Anti-PD-1 |
3 |
|
NCT04960709 |
Enfortumab vedotin + Durvalumab ± Tremelimumab |
Neoadjuvant |
Nectin-4 ADC; Anti-PD-L1; Anti-CTLA-4 |
3 |
|
NCT05302284 |
Disitamab vedotin + Toripalimab |
First-line |
HER2 ADC; Anti-PD-1 |
3 |
|
NCT05911295 |
Disitamab vedotin + Pembrolizumab |
First-line |
HER2 ADC; Anti-PD-1 |
3 |
|
NCT06196736 |
9MW2821 + Toripalimab |
Refractory |
Nectin-4 ADC; Anti-PD-1 |
3 |
|
NCT06592326 |
9MW2821 + Toripalimab |
First-line |
Nectin-4 ADC; Anti-PD-1 |
3 |
|
*ADC: Antibody-drug conjugate. |
||||
Treatment planning equipped with molecular diagnostics and ctDNA analyses is also a big-step forward. ctDNA has emerged as a robust biomarker for detecting minimal residual disease and assessing relapse risk, enabling adjuvant therapies to be guided by molecular evidence rather than clinical suspicion [38, 40]. Genomic profiling is aiding by precise and accurate patient selection for treatment with ADCs and targeted agents, which is eventually bringing precision oncology into everyday clinical decision-making. Multimodal approaches that combine maximal transurethral resection, chemoradiation, and immunotherapy have demonstrated promising results, particularly with respect to bladder preservation. Several phase II trials have reported two-year bladder-intact survival rates exceeding 70% [47, 48]. T Enfortumab vedotin plus pembrolizumab has been established as the recommended first-line regimen for both cisplatin-eligible and -ineligible populations in the metastatic setting [60], as it doubled overall survival compared with chemotherapy and offered a more manageable toxicity profile with reduction in hematologic suppression. For patients who are unable to tolerate ADC-based therapy due to neuropathy, autoimmune conditions, or dermatologic toxicity, platinum-based chemotherapy is unfortunately still a valuable alternative. Resistance to both ICIs and ADCs remains a major obstacle. Immune evasion mechanisms, such as loss of antigen presentation, exclusion of T cells, or activation of alternative checkpoints, can limit immunotherapy efficacy, while ADC resistance often results from target downregulation, defective internalization, or enhanced drug efflux [81, 82]. Dissecting these adaptive processes will be essential for achieving durable responses and long-term disease control.
Key priorities that are essential to elevate the next phase of progress in bladder cancer therapy include: 1) Refinement of biomarker-guided patient selection to personalize therapy and predict benefit; 2) Expansion of ADC target repertoires beyond Nectin-4 and Trop-2 to address tumor heterogeneity; and 3) Designing of rational multi-agent combinations that integrate ICIs, ADCs, and molecularly targeted drugs to delay resistance. These priorities signify a pivotal transition from traditional, stage-based management to a precision-oriented, mechanism-informed treatment paradigm. Through continued integration of immunotherapy, ADCs, and molecular profiling, the field is progressing toward better therapeutic management in bladder cancer.
None.
Ethical policy
Non applicable.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
Ahmed Helmy Abdelhaseb contributed to design of the work, data collection, and drafting the article; Shahd Mustafa Ibrahim draw the figure and checked the tables; Ahmed Attia Ahmed Abdelmoaty revised the final manuscript.
Competing interests
The author declares no competing interests.
Funding
None.
- Bray F, Laversanne M, Sung H: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74(3): 229-263.
- Wéber A, Vignat J, Shah R, Morgan E, Laversanne M, Nagy P, Kenessey I, Znaor A: Global burden of bladder cancer mortality in 2020 and 2040 according to GLOBOCAN estimates. World J Urol 2024, 42(1): 237.
- Pemov A, Wegman-Ostrosky T, Kim J, Koutros S, Douthitt B, Jones K, Zhu B, Baris D, Schwenn M, Johnson A et al: Identification of Genetic Risk Factors for Familial Urinary Bladder Cancer: An Exome Sequencing Study. JCO Precis Oncol 2021, 5: PO.21.00115.
- Zhang Y, Rumgay H, Li M, Yu H, Pan H, Ni J: The global landscape of bladder cancer incidence and mortality in 2020 and projections to 2040. J Glob Health 2023, 13: 04109.
- Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM et al: Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005, 66(6 Suppl 1): 4-34.
- Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A: Epidemiology of Bladder Cancer. Med Sci (Basel) 2020, 8(1): 15.
- Grabe-Heyne K, Henne C, Mariappan P, Geiges G, Pöhlmann J, Pollock RF: Intermediate and high-risk non-muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Front Oncol 2023, 13: 1170124.
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005, 23(21): 4602-4608.
- Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, Dreicer R, Vogelzang N, Sternberg CN, Bajorin DF et al: Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol 2011, 29(17): 2432-2438.
- Sonpavde G, Sternberg CN, Rosenberg JE, Hahn NM, Galsky MD, Vogelzang NJ: Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium. Lancet Oncol 2010, 11(9): 861-870.
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL et al: Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017, 389(10064): 67-76.
- Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, Raitano A, Nadell R, Liu W, Lortie DR et al: Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res 2016, 76(10): 3003-3013.
- Yajima S, Hirose K, Masuda H: Enfortumab Vedotin With or Without Pembrolizumab in Metastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. JAMA Netw Open 2025, 8(3): e250250.
- Crispen PL, Kusmartsev S: Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother 2020, 69(1): 3-14.
- Cao W, Chen J, Fu Y, Jiang H: A next-generation anti-CTLA-4 probody mitigates toxicity and enhances anti-tumor immunity in mice. Nat Commun 2025, 16(1): 9029.
- Ribas A, Wolchok JD: Cancer immunotherapy using checkpoint blockade. Science 2018, 359(6382): 1350-1355.
- Wojtukiewicz MZ, Rek MM, Karpowicz K, Górska M, Polityńska B, Wojtukiewicz AM, Moniuszko M, Radziwon P, Tucker SC, Honn KV: Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev 2021, 40(3): 949-982.
- Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S: PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol 2021, 18(6): 345-362.
- Rui X, Gu TT, Pan HF, Zhang HZ: Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: A meta-analysis. Int Immunopharmacol 2019, 67: 378-385.
- Samstein RM, Lee CH, Shoushtari AN: Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 2019, 51(2): 202-206.
- Kato M, Uchida J: Recent advances in immune checkpoint inhibitors in the treatment of urothelial carcinoma: A review. Int J Urol 2023, 30(12): 1068-1077.
- Fu Z, Li S, Han S, Shi C, Zhang Y: Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther 2022, 7(1): 93.
- Aggarwal D, Yang J, Salam MA, Sengupta S, Al-Amin MY, Mustafa S, Khan MA, Huang X, Pawar JS: Antibody-drug conjugates: the paradigm shifts in the targeted cancer therapy. Front Immunol 2023, 14: 1203073.
- McCombs JR, Owen SC: Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J 2015, 17(2): 339-351.
- Matsuda Y, Chang JR, Mendelsohn BA: Advanced Antibody-Drug Conjugates Design: Innovation in Linker Chemistry and Site-Specific Conjugation Technologies. Chembiochem 2025, https://doi.org/10.1002/cbic.202500305. Epub ahead of print.: e2500305.
- Ponziani S, Di Vittorio G, Pitari G, Cimini AM, Ardini M: Antibody-Drug Conjugates: The New Frontier of Chemotherapy. Int J Mol Sci 2020, 21(15): 5510.
- Wang Y, Cheng X, Li X, Chen W, Zhang X, Liu Y: Bystander effect in antibody-drug conjugates: Navigating the fine line in tumor heterogeneity. Crit Rev Oncol Hematol 2025, 216: 104979.
- Zippelius A, Tolaney SM, Tarantino P, Balthasar JP: Unveiling the molecular and immunological drivers of antibody-drug conjugates in cancer treatment. Nat Rev Cancer 2025, https://doi.org/10.1038/s41568-025-00869-w. Epub ahead of print.
- Powles T, Catto JWF: Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N Engl J Med 2024, 391(19): 1773-1786.
- Powles T, Heijden MSVD, Wang Y, Catto JWF, Meeks JJ, Al-Ahmadie H, Nishiyama H, Mortazavi AM, Vu TQ, Antonuzzo L et al: Circulating tumor DNA (ctDNA) in patients with muscle-invasive bladder cancer (MIBC) who received perioperative durvalumab (D) in NIAGARA. J Clin Oncol 2025, 43(16_suppl): 4503-4503.
- Siefker-Radtke AO, Steinberg GD, Bedke J, Nishiyama H, Fang X, Kataria R, Moreno BH, Hoimes CJ: Phase III study of perioperative pembrolizumab (pembro) plus neoadjuvant chemotherapy (chemo) versus placebo plus neoadjuvant chemo in cisplatin-eligible patients (pts) with muscle-invasive bladder cancer (MIBC): KEYNOTE-866. J Clin Oncol 2020, 38(6_suppl): TPS599-TPS599.
- Sonpavde G, Necchi A, Gupta S, Steinberg GD, Gschwend JE, Van Der Heijden MS, Garzon N, Ibrahim M, Raybold B, Liaw D et al: ENERGIZE: a Phase III study of neoadjuvant chemotherapy alone or with nivolumab with/without linrodostat mesylate for muscle-invasive bladder cancer. Future Oncol 2020, 16(2): 4359-4368.
- Mercinelli C, Moschini M, Cigliola A: First Results of NURE-Combo: A Phase II Study of Neoadjuvant Nivolumab and Nab-Paclitaxel, Followed by Postsurgical Adjuvant Nivolumab, for Muscle-Invasive Bladder Cancer. J Clin Oncol 2024, 42(35): 4196-4205.
- Satkunasivam R, Lim K: A phase II clinical trial of neoadjuvant sasanlimab and stereotactic body radiation therapy as an in situ vaccine for cisplatin-ineligible MIBC: the RAD VACCINE MIBC trial. Future Oncol 2022, 18(25): 2771-2781.
- Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat SF, Park SH et al: Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med 2021, 384(22): 2102-2114.
- Galsky MD, Witjes JA, Gschwend JE, Milowsky MI: Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274. J Clin Oncol 2025, 43(1): 15-21.
- Sharma P, Siefker-Radtke A, de Braud F, Basso U, Calvo E, Bono P, Morse MA, Ascierto PA, Lopez-Martin J, Brossart P et al: Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J Clin Oncol 2019, 37(19): 1608-1616.
- Jackson-Spence F, Toms C, O'Mahony LF, Choy J, Flanders L, Szabados B: IMvigor011: a study of adjuvant atezolizumab in patients with high-risk MIBC who are ctDNA+ post-surgery. Future Oncol 2023, 19(7): 509-515.
- Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz M, Degaonkar V et al: Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2021, 22(4): 525-537.
- Powles T, Assaf ZJ, Degaonkar V, Grivas P, Hussain M, Oudard S, Gschwend JE, Albers P, Castellano D, Nishiyama H et al: Updated Overall Survival by Circulating Tumor DNA Status from the Phase 3 IMvigor010 Trial: Adjuvant Atezolizumab Versus Observation in Muscle-invasive Urothelial Carcinoma. Eur Urol 2024, 85(2): 114-122.
- Necchi A, Guerrero-Ramos F, Crispen PL, Herrera Imbroda B, Garje R, Powles TB, Peyton CC, Pradere B, Ku JH, Shore ND et al: LBA84 TAR-200 plus cetrelimab (CET) or CET alone as neoadjuvant therapy in patients (pts) with muscle-invasive bladder cancer (MIBC) who are ineligible for or refuse neoadjuvant cisplatin-based chemotherapy (NAC): Interim analysis of SunRISe-4 (SR-4). Ann Oncol 2024, 35: S1271-S1272.
- Li R, Villa NY, Yu X: Oncolytic immunotherapy with nivolumab in muscle-invasive bladder cancer: a phase 1b trial. Nat Med 2025, 31(1): 176-188.
- Grivas P, Heijden MSVD, Necchi A, Siefker-Radtke AO, Cutuli H, Qureshi AH, Kreiser S, Hodari M, Ravimohan S, Zakharia Y: PIVOT IO 009: A phase 3, randomized study of neoadjuvant and adjuvant nivolumab (NIVO) plus bempegaldesleukin (BEMPEG; NKTR-214) versus NIVO alone versus standard of care (SOC) in patients (pts) with muscle-invasive bladder cancer (MIBC) who are cisplatin (cis)-ineligible. J Clin Oncol 2022, 40(6_suppl): TPS596-TPS596.
- van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, Bedke J et al: Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med 2023, 389(19): 1778-1789.
- Saxena M, Anker JF, Kodysh J, O'Donnell T, Kaminska AM, Meseck M, Hapanowicz O, Niglio SA, Salazar AM, Shah HR et al: Atezolizumab plus personalized neoantigen vaccination in urothelial cancer: a phase 1 trial. Nat Cancer 2025, 6(6): 988-999.
- Sonpavde GP, Valderrama BP, Chamie K, Gupta S, Santis MD, Banerjee JK, Ojalvo L, Ren Y, Bavle A, Powles T: Phase 1/2 INTerpath-005 study: V940 (mRNA-4157) plus pembrolizumab with or without enfortumab vedotin (EV) for resected high-risk muscle-invasive urothelial carcinoma (MIUC). J Clin Oncol 2025, 43(5_suppl): TPS893-TPS893.
- Economides MP, Milowsky MI, O'Donnell PH, Alva AS, Kollmeier M, Rose TL, Pitroda SP, Rosenberg JE, Hochman T, Goldberg JD et al: Long-term outcomes of pembrolizumab (pembro) in combination with gemcitabine (gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIUC): A multicenter phase 2 trial. J Clin Oncol 2023, 41(16_suppl): 4509-4509.
- Garcia-Del-Muro X, B PV: Bladder Preservation with Durvalumab plus Tremelimumab and Concurrent Radiotherapy in Patients with Localized Muscle-Invasive Bladder Cancer (IMMUNOPRESERVE): A Phase II Spanish Oncology GenitoUrinary Group Trial. Clin Cancer Res 2025, 31(4): 659-666.
- Kougioumtzopoulou A, Koutsoukos K, Zakopoulou R, Tzannis K, Kyriazoglou A, Damatopoulou A, Liontos M, Pispirigkou MK, Ntoumas K, Stravodimos K et al: 1961O Nivolumab plus chemoradiotherapy in patients with non-metastatic muscle-invasive bladder cancer (nmMIBC), not undergoing cystectomy: A phase II, randomized study by the Hellenic GU Cancer Group. Ann Oncol 2024, 35: S1133-S1134.
- Singh P, Tangen C, Efstathiou JA, Lerner SP, Jhavar SG, Hahn NM, Costello BA, Sridhar SS, Du W, Meeks JJ et al: INTACT: Phase III randomized trial of concurrent chemoradiotherapy with or without atezolizumab in localized muscle invasive bladder cancer—SWOG/NRG1806. J Clin Oncol 2020, 38(6_suppl): TPS586-TPS586.
- Gupta S, Fujii Y, Heijden MSVD, Weickhardt AJ, James ND, Shariat SF, Michalski JM, Imai K, Fang X, Kapadia E et al: Phase 3 KEYNOTE-992 study of pembrolizumab plus chemoradiotherapy versus placebo plus chemoradiotherapy in patients with muscle-invasive bladder cancer (MIBC). J Clin Oncol 2024, 42(4_suppl): TPS720-TPS720.
- Fong JY, Phuna Z, Chong DY, Heryanto CM, Low YS, Oh KC, Lee YH, Ng AWR, In LLA, Teo MYM: Advancements in antibody-drug conjugates as cancer therapeutics. J Natl Cancer Cent 2025, 5(4): 362-378.
- Kanaan MR, Schmitz J, Braesen JH, Kuczyk MA, Tezval H: Comparison of molecular profiles (Nectin-4 and TROP-2) in upper tract urothelial carcinoma with a positive history of urinary bladder cancer vs. UTUC only in the era of ADCs. BMC Cancer 2025, 25(1): 1525.
- Nieto-Jiménez C, Sanvicente A, Díaz-Tejeiro C, Moreno V, Lopez de Sá A: Uncovering therapeutic opportunities in the clinical development of antibody-drug conjugates. Clin Transl Med 2023, 13(9): e1329.
- Meric-Bernstam F, Makker V: Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol 2024, 42(1): 47-58.
- Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M: Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv 2022, 29(1): 1335-1344.
- Sheng X, Wang L, He Z, Shi Y, Luo H, Han W, Yao X, Shi B, Liu J: Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J Clin Oncol 2024, 42(12): 1391-1402.
- Zhou L, Yang K, Zhang S, Yan X, Li S, Xu H, Li J, Chi Z, Mao L, Lian B et al: 1979P Disitamab vedotin (DV) plus toripalimab (T) in unresectable locally advanced or metastatic urothelial carcinoma (la/mUC): Long-term outcomes from a phase Ib/II study. Ann Oncol 2024, 35: S1145.
- Galsky MD, Koshkin VS, Campbell MT, Drakaki A, Bowman I, Rose AAN, Brown JR, Aragon-Ching JB, Gadde S, Harandi A et al: 1967MO Preliminary efficacy and safety of disitamab vedotin (DV) with pembrolizumab (P) in treatment (Tx)-naive HER2-expressing, locally advanced or metastatic urothelial carcinoma (la/mUC): RC48G001 cohort C. Ann Oncol 2024, 35: S1138-S1139.
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C: Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med 2024, 390(10): 875-888.
- Powles T, Heijden MSVD, Loriot Y, Bedke J, Valderrama BP, Iyer G, Kikuchi E, Hoffman-Censits J, Vulsteke C, Drakaki A et al: EV-302: Updated analysis from the phase 3 global study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (chemo) in previously untreated locally advanced or metastatic urothelial carcinoma (la/mUC). J Clin Oncol 2025, 43(5_suppl): 664-664.
- Zhang J, Liu R, Gao S, Yang H, Chen J, Yuan F, Liu J, Guo H, Zhang S, Li X et al: 9MW2821, a nectin-4 antibody-drug conjugate (ADC), in patients with advanced solid tumor: Results from a phase 1/2a study. J Clin Oncol 2024, 42(16_suppl): 3013-3013.
- Zhou W, Fang P: Preclinical Evaluation of 9MW2821, a Site-Specific Monomethyl Auristatin E-based Antibody-Drug Conjugate for Treatment of Nectin-4-expressing Cancers. Mol Cancer Ther 2023, 22(8): 913-925.
- Tang B, Sheng X, Guo J, Niu H, Shen Y, Jiang S, Fu B, Guo J, Wahafu W, Yao K et al: Nectin-4 targeted ADC, SHR-A2102, in patients with advanced or metastatic urothelial carcinoma: A phase 1 study. J Clin Oncol 2025, 43(5_suppl): 657-657.
- Meric-Bernstam F, Alhalabi O, Lisberg A, Drakaki A, Garmezy B, Kogawa T, Spira AI, Salkeni MA, Gao X, Tolcher AW et al: Datopotamab deruxtecan (Dato-DXd) in locally advanced/metastatic urothelial cancer: Updated results from the phase 1 TROPIONPanTumor01 study. J Clin Oncol 2025, 43(5_suppl): 663-663.
- Janjigian YY, Oaknin A, Lang JM, Ciombor KK, Ray-Coquard IL, Oza AM, Yonemori K, Xu R-H, Zhao J, Gajavelli S et al: TROPION-PanTumor03: Phase 2, multicenter study of datopotamab deruxtecan (Dato-DXd) as monotherapy and in combination with anticancer agents in patients (pts) with advanced/metastatic solid tumors. J Clin Oncol 2023, 41(16_suppl): TPS3153-TPS3153.
- Powles T, Tagawa S, Vulsteke C, Gross-Goupil M, Park SH, Necchi A, De Santis M, Duran I, Morales-Barrera R, Guo J et al: Sacituzumab govitecan in advanced urothelial carcinoma: TROPiCS-04, a phase III randomized trial. Ann Oncol 2025, 36(5): 561-571.
- Ye D, Jiang S, Yuan F, Zhou F, Jiang K, Zhang X, Li X, Seneviratne LC, Yu G, Zhang M et al: Efficacy and safety of sacituzumab tirumotecan monotherapy in patients with advanced urothelial carcinoma who progressed on or after prior anti-cancer therapies: Report from the phase 1/2 MK-2870-001 study. J Clin Oncol 2025, 43(5_suppl): 796-796.
- McGregor BA, Sonpavde GP, Kwak L, Regan MM, Gao X, Hvidsten H, Mantia CM, Wei XX, Berchuck JE, Berg SA et al: The Double Antibody Drug Conjugate (DAD) phase I trial: sacituzumab govitecan plus enfortumab vedotin for metastatic urothelial carcinoma. Ann Oncol 2024, 35(1): 91-97.
- Ye D, Bian X, Yang T, Jiang S, Cao M, Hua X, Xiao S, Wang H, Zhu H, Zhu Y: 1959O BL-B01D1, an EGFR x HER3 bispecific antibody-drug conjugate (ADC), in patients with locally advanced or metastatic urothelial carcinoma (UC). Ann Oncol 2024, 35: S1133.
- Reig Torras O, Crouzet L, Necchi A, Baldini C, Lostes Bardaji MJ, Doger de Spéville B, Italiano A, Verlingue L, Boni V, Carter L et al: 652P BT8009 monotherapy in enfortumab vedotin (EV)-naïve patients (pts) with metastatic urothelial carcinoma (mUC): Updated results of Duravelo-1. Ann Oncol 2024, 35: S515-S516.
- Loriot Y, Siefker-Radtke AO, Friedlander TW, Necchi A, Wei AZ, Sridhar SS, Garmezy B, Arroyo S, Gartside E, Liu J et al: A phase 2/3 study of Bicycle toxin conjugate BT8009 targeting nectin-4 in patients with locally advanced or metastatic urothelial cancer (la/mUC): Duravelo-2. J Clin Oncol 2024, 42(16_suppl): TPS4619-TPS4619.
- Papadopoulos KP, Dowlati A, Lopez JS, Rodon J, Spira AI, Stein M, Zibelman M, Ortuzar Feliu WI, Dickson A, De A et al: 650P Initial results from a phase I/II study of BT7480, a novel nectin-4/CD137 bicycle tumor-targeted immune cell agonist, in patients (pts) with advanced solid tumors. Ann Oncol 2024, 35: S513-S514.
- Fontana E, Wang JS, McKean M, Aljumaily R, Machiels JP, Doger de Spéville B, Vieito M, Carter L, Prenen H, Falchook GS et al: 647P EphA2-targeting bicycle toxin conjugate (BTC) BT5528 in patients (pts) with advanced solid tumors: A phase I/II study. Ann Oncol 2024, 35: S511-S512.
- Gupta S, Almassi N, Bukavina L, Wee CE, Stephans KL, Diaz-Montero CM, Tendulkar RD, Mian OY, Chan TA-t: RAD-SG: Adaptive radiation therapy with concurrent sacituzumab govitecan (SG) for bladder preservation in patients (pts) with muscle invasive bladder cancer (MIBC). J Clin Oncol 2025, 43(5_suppl): TPS896-TPS896.
- Chang HL, Schwettmann B, McArthur HL, Chan IS: Antibody-drug conjugates in breast cancer: overcoming resistance and boosting immune response. J Clin Invest 2023, 133(18): e172156.
- Shi X, Tang K, Zhang Q, Han Q, Quan L, Li Y, Cui J, Feng N, Gong J, Shang B et al: Antibody-drug conjugate combinations in cancer treatment: clinical efficacy and clinical study perspectives. Front Pharmacol 2025, 16: 1556245.
- Vasan N, Baselga J, Hyman DM: A view on drug resistance in cancer. Nature 2019, 575(7782): 299-309.
- O'Donnell PH, Milowsky MI: Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol 2023, 41(25): 4107-4117.
- Grivas P, Pouessel D, Park CH, Barthelemy P, Bupathi M, Petrylak DP: Sacituzumab Govitecan in Combination With Pembrolizumab for Patients With Metastatic Urothelial Cancer That Progressed After Platinum-Based Chemotherapy: TROPHY-U-01 Cohort 3. J Clin Oncol 2024, 42(12): 1415-1425.
- Mandal K, Barik GK: Overcoming resistance to anti-PD-L1 immunotherapy: mechanisms, combination strategies, and future directions. Mol Cancer 2025, 24(1): 246.
- Li S, Zhao X, Fu K, Zhu S, Pan C, Yang C, Wang F, To KKW, Fu L: Resistance to antibody-drug conjugates: A review. Acta Pharm Sin B 2025, 15(2): 737-756.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript