Research Article | Open Access
Immunoexpression of Estrogen Receptor-β in Urothelial Carcinoma and Its Correlation with Histological Grade
Jyotsna Bhateja1, Anuradha Kusum2, Smita Chandra2, Manoj Biswas3
1Department of Pathology, Shri Guru Ram Rai Institute of Medical & Health Sciences, Dehradun, Uttarakhand, India.
2Department of Pathology, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun, Uttarakhand, India.
3Department of Urology, Medilife Superspeciality Hospital, Ajabpur Khurd, Dehradun, Uttarakhand, India.
Correspondence: Jyotsna Bhateja (Department of Pathology, Shri Guru Ram Rai Institute of Medical & Health Sciences, Ladpur Raipur Road, Dehradun, Uttarakhand 248001, India; Email: khushi.bhateja.91@gmail.com).
Annals of Urologic Oncology 2025, 8(4): 222-226. https://doi.org/10.32948/auo.2025.12.29
Received: 08 Dec 2025 | Accepted: 19 Dec 2025 | Published online: 30 Dec 2025
Objectives To evaluate the immunohistochemical expression of ER-β in urothelial carcinoma of the urinary bladder and to analyze its association with histological grade and muscle invasion.
Materials and Methods This cross-sectional observational study included 100 cases of urothelial carcinoma diagnosed on transurethral resection or cystectomy specimens. Tumors were classified as low-grade or high-grade according to WHO criteria. Immunohistochemical staining for ER-β was performed, and nuclear expression was assessed semi-quantitatively using a three-tier scoring system. Statistical analysis was performed using the chi-square test.
Results ER-β expression was observed in all cases. Moderate to strong ER-β expression (scores 2 and 3) was significantly more frequent in high-grade tumors compared to low-grade tumors (p < 0.001). A statistically significant association was also noted between higher ER-β expression and muscle-invasive disease (p = 0.027).
Conclusion ER-β is consistently expressed in urothelial carcinoma, with higher expression significantly associated with adverse histopathological features such as high tumor grade and muscle invasion. These findings support the potential role of ER-β as a prognostic biomarker and a possible therapeutic target in urothelial carcinoma.
Key words urothelial carcinoma, ER-β, histological grade, muscle invasion, histopathological features
Several environmental and lifestyle-related risk factors contribute to bladder carcinogenesis, of which cigarette smoking remains the most significant, accounting for nearly half of all cases [3]. Occupational exposure to aromatic amines, chronic inflammation, and certain chemotherapeutic agents have also been implicated. Despite advances in diagnostic modalities and therapeutic strategies, bladder cancer continues to pose challenges due to its high recurrence rates, variable biological behavior, and progression to muscle-invasive disease in a significant subset of patients.
Histopathological grade and depth of invasion remain the most important prognostic indicators in urothelial carcinoma. However, tumors with similar stage and grade may demonstrate heterogeneous clinical outcomes, underscoring the need for reliable molecular biomarkers to refine prognostication and guide targeted therapy [4, 5]. In this context, steroid hormone signaling pathways have gained increasing attention in urothelial tumor biology.
Estrogen receptors, particularly estrogen receptor beta (ER-β), are the predominant estrogen receptor subtype expressed in normal urothelium and bladder tumors. Experimental and clinical studies have demonstrated that ER-β plays a role in urothelial carcinogenesis, tumor progression, and invasion [6-8]. Higher ER-β expression and reduced ER-α expression have been associated with high-grade tumors and muscle-invasive bladder cancer, suggesting a possible role in aggressive tumor behavior.
The present study was undertaken to evaluate the immunohistochemical expression of ER-β in urothelial carcinoma of the urinary bladder and to analyze its correlation with histological grade and muscle invasion.
This cross-sectional observational study was conducted over a period of one year in the Department of Pathology at a tertiary care teaching hospital. A total of 100 consecutive patients with histopathologically confirmed urothelial carcinoma of the urinary bladder were included in the study.
Specimens were obtained from patients who underwent transurethral resection of bladder tumor (TURBT) or radical cystectomy for clinically suspected bladder neoplasms. Clinical details including age, sex, occupation, smoking status, and presenting symptoms were retrieved from hospital medical records. Histopathological parameters such as tumor grade and presence or absence of detrusor muscle invasion were recorded following microscopic examination.
Tumors were classified as low-grade urothelial carcinoma (LGUC) or high-grade urothelial carcinoma (HGUC) based on the World Health Organization (WHO) classification of tumors of the urinary tract. Muscle invasion was defined as invasion into the detrusor muscle (pathological stage pT2 or higher).
Ethical approval and informed consent
The study protocol was reviewed and approved by the Institutional Ethics Committee of the participating institution. Patient confidentiality was maintained throughout the study.
Immunohistochemistry assay
Immunohistochemical staining for estrogen receptor beta (ER-β) was performed on formalin-fixed, paraffin-embedded (FFPE) tissue sections. Representative tumor blocks were selected for each case, and 3–4 μm thick sections were cut and mounted on poly-L-lysine–coated slides.
Sections were deparaffinized in xylene and rehydrated through graded alcohols. Antigen retrieval was performed using a heat-induced epitope retrieval method in citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked using hydrogen peroxide.
Immunostaining was carried out using a primary monoclonal antibody against ER-β. Detection was achieved using a standard secondary antibody and chromogen system. Appropriate positive and negative controls were included with each staining run. Nuclear staining of tumor cells was considered specific and positive for ER-β expression.
Assessment of ER-β expression
Evaluation of ER-β immunoexpression was performed independently blinded to the clinical and histopathological data. Only nuclear staining was considered for scoring.
For each case, tumor cells were evaluated in representative high-power fields. ER-β expression was assessed semi-quantitatively based on staining intensity and the percentage of positive tumor cells, and categorized as follows:
Score 1: Weak nuclear staining in 10–50% of tumor cells;
Score 2: Moderate nuclear staining in >50% of tumor cells;
Score 3: Strong nuclear staining in >50% of tumor cells.
Cases showing any degree of nuclear positivity were considered ER-β positive. The immunohistochemical scores were subsequently correlated with histological grade and muscle invasion status.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) software, version 22.0. Categorical variables were expressed as frequencies and percentages.
The association between ER-β expression scores and histological grade as well as muscle invasion was analyzed using the chi-square test. A p-value of less than 0.05 was considered statistically significant.
Histopathological evaluation revealed that 75 cases (75%) were classified as high-grade urothelial carcinoma, while 25 cases (25%) were low-grade urothelial carcinoma. Muscle invasion was identified in 54% of cases overall, with a higher frequency observed in HGUC (60%) compared to LGUC (36%) (Table 1).
Immunohistochemical analysis demonstrated ER-β nuclear positivity in all 100 cases (100%), although the intensity of staining varied. Score 1 expression was observed in 25% of cases, score 2 in 42%, and score 3 in 33%. Higher ER-β expression (scores 2 and 3 combined) was noted in 75% of tumors (Table 1). The range of ER-β immunohistochemical expression, from weak to strong nuclear staining in low-grade and high-grade urothelial carcinoma, is illustrated in Figure 1.
A statistically significant association was observed between ER-β expression and histological grade. High-grade tumors showed a greater proportion of moderate to strong ER-β expression compared to low-grade tumors (χ² = 16.45, df = 2, p < 0.001) (Table 2). Similarly, ER-β expression demonstrated a significant correlation with muscle invasion, with higher scores being more frequently observed in muscle-invasive tumors (χ² = 7.24, df = 2, p = 0.027) (Table 2).
|
Table 1. Clinical characteristics of participants, selected histomorphological features and ER-β immunoexpression scores. |
|||||
|
Clinical characteristics (n = 100) |
Selected histomorphological features (n=100) |
ER-β immunoexpression scores (n=100) |
|||
|
Clinical variable |
Findings |
Feature |
n (%) |
Score |
n (%) |
|
Number of patients |
100 |
High-grade (HGUC) |
75 (75) |
1 |
25 (25) |
|
Mean age (years) |
60.7 |
Low-grade (LGUC) |
25 (25) |
2 |
42 (42) |
|
Age range (years) |
30-90 |
Muscle invasion |
54 (54) |
3 |
33 (33) |
|
Sex (Male : Female) |
11.5: 1 |
Muscle invasion( HGUC) |
45/75 (60) |
2 + 3 |
75 (75) |
|
Occupation - Farming |
35% |
Muscle invasion( LGUC) |
9/25 (36) |
All cases showed some degree of ER-β positivity (no score 0) |
|
|
Smoking history |
63% |
- |
- |
||
|
Presenting symptom - Hematuria |
96% |
||||
|
HGUC, high-grade urothelial carcinoma; LGUC, low-grade urothelial carcinoma. ER-β, estrogen receptor-β. Scoring: Score 1 = weak nuclear staining in 10–50% of tumor cells; Score 2 = moderate nuclear staining in >50% of tumor cells; Score 3 = strong nuclear staining in >50% of tumor cells. |
|||||
|
Table 2. ER-β score by histological grade and muscle invasion. |
||||||
|
Items |
Grade/Invasion |
Score 1 |
Score 2 |
Score 3 |
Total |
Comparison |
|
ER-β score by histological grade |
LGUC |
9 |
16 |
0 |
25 |
χ² = 16.45, df = 2, p < 0.001 |
|
HGUC |
16 |
26 |
33 |
75 |
||
|
Total |
25 |
42 |
33 |
100 |
- |
|
|
ER-β score by muscle invasion |
Absent |
18 |
22 |
6 |
46 |
χ² = 7.24, df = 2, p = 0.027 |
|
Present |
7 |
20 |
27 |
54 |
||
|
Total |
25 |
42 |
33 |
100 |
- |
|
|
ER-β, estrogen receptor-β; LGUC, low-grade urothelial carcinoma; HGUC, high-grade urothelial carcinoma. Muscle invasion defined as invasion into detrusor muscle (pT2 or higher). p: chi-square test. |
||||||
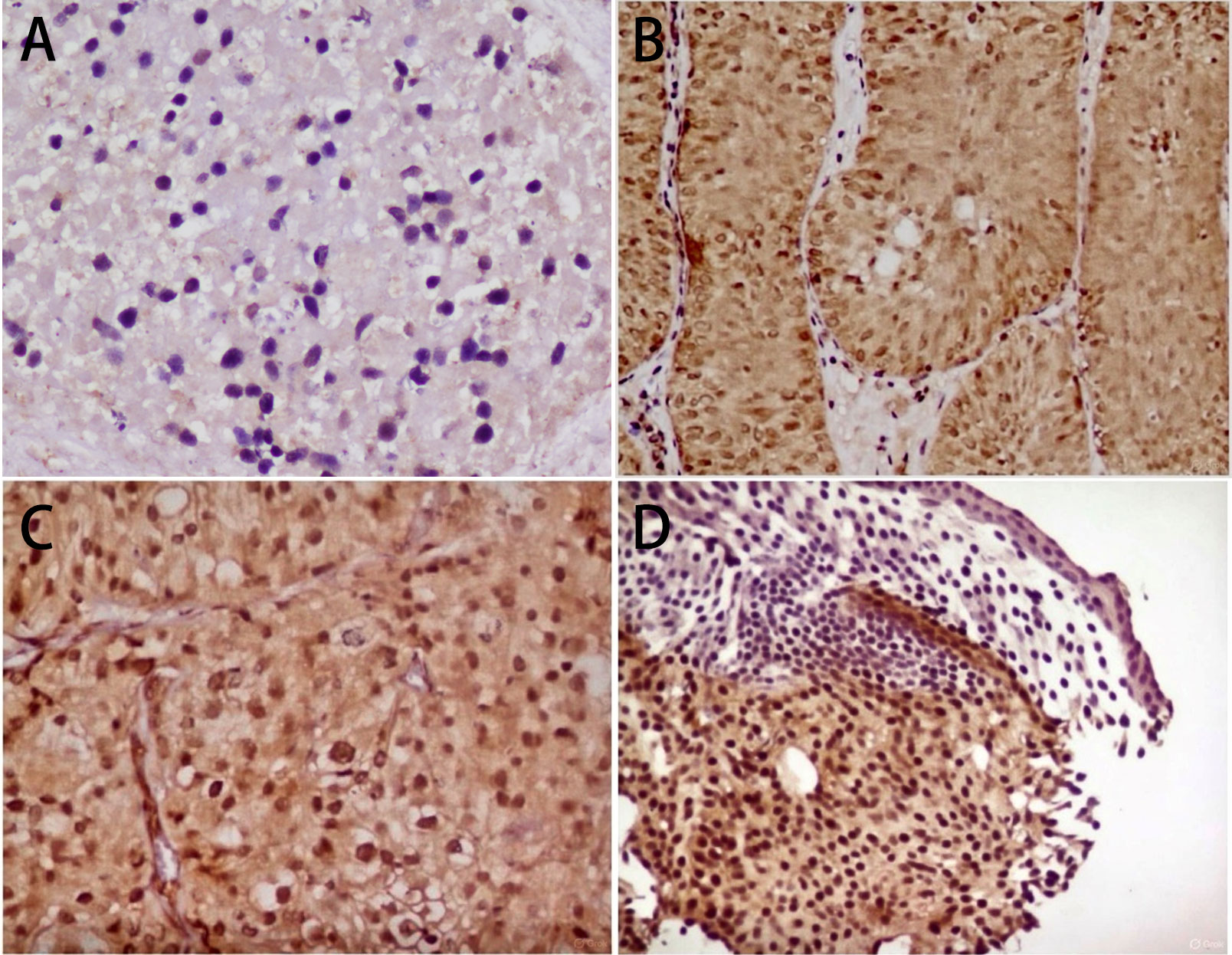 Figure 1. Representative immunohistochemical photomicrographs showing ER-β expression in urothelial carcinoma of varying histological grades. (A) Score 1 + of ER-β immunostain low grade urothelial carcinoma (40x); (B) Score 2 + of ER-β immunostain in low grade in urothelial carcinoma (10x); (C) Score 2 + of ERβ immunostain in high grade urothelial carcinoma (20x); (D) Score 3 + of ERβ immunostain in high grade urothelial carcinoma (10x).
Figure 1. Representative immunohistochemical photomicrographs showing ER-β expression in urothelial carcinoma of varying histological grades. (A) Score 1 + of ER-β immunostain low grade urothelial carcinoma (40x); (B) Score 2 + of ER-β immunostain in low grade in urothelial carcinoma (10x); (C) Score 2 + of ERβ immunostain in high grade urothelial carcinoma (20x); (D) Score 3 + of ERβ immunostain in high grade urothelial carcinoma (10x).
In the present study, smoking emerged as a major risk factor, consistent with extensive epidemiological evidence supporting its central role in urothelial carcinogenesis. Tobacco-related carcinogens induce DNA damage, oxidative stress, and epigenetic alterations that contribute to tumor initiation and progression [3]. The predominance of hematuria as the presenting symptom further reinforces its importance as an early clinical indicator warranting prompt evaluation.
A key finding of this study was the universal nuclear expression of estrogen receptor beta (ER-β) in urothelial carcinoma, with increasing staining intensity observed in high-grade and muscle-invasive tumors. Strong ER-β expression was seen exclusively in high-grade urothelial carcinoma, suggesting a close association between ER-β overexpression and aggressive tumor biology. These findings are concordant with previous studies demonstrating higher ER-β expression in advanced-stage and high-grade bladder tumors [6-8].
Multiple studies have reported that elevated ER-β expression correlates with adverse prognostic features, including increased recurrence, disease progression, and reduced disease-specific survival [7-9]. Cross-talk between estrogen and androgen receptor pathways has also been implicated in urothelial carcinogenesis and may partly explain sex-related differences in disease incidence and outcomes [10].
Variant histologies, including urothelial carcinoma with squamous differentiation and pure squamous cell carcinoma, are associated with more aggressive clinical behavior and inferior outcomes compared with conventional urothelial carcinoma [11]. Although the present study focused on conventional urothelial carcinoma, these observations highlight the biological heterogeneity of bladder cancer.
Upper tract urothelial carcinoma shares several molecular and biological characteristics with bladder urothelial carcinoma. Prior studies have demonstrated that estrogen receptor expression in upper tract tumors correlates with advanced pathological stage and poor prognosis, suggesting that estrogen receptor–mediated signaling may represent a common oncogenic pathway across urothelial malignancies irrespective of anatomical location [12].
Estrogen receptor–mediated signaling has been increasingly recognized as a key regulator of urothelial carcinogenesis, tumor progression, and invasion [13-15]. Experimental data suggest that ER-β signaling may promote tumor cell proliferation, inhibit apoptosis, and facilitate epithelial–mesenchymal transition, thereby enhancing invasive behavior [9, 14, 15].
The significant association between ER-β expression and muscle invasion observed in the present study further supports a role for ER-β in tumor progression. Muscle-invasive bladder cancer represents a biologically aggressive subset with high metastatic potential and poor prognosis. Sex-related differences in bladder cancer incidence and outcomes may also be influenced by hormonal signaling pathways [16].
Emerging evidence suggests that hormonal receptor expression patterns may differ in variant histologies and mixed tumors, potentially influencing tumor biology and therapeutic response [17, 18]. Further studies addressing ER-β expression across variant subtypes are warranted.
From a therapeutic perspective, increasing interest has focused on hormonal signaling pathways as potential targets in urothelial carcinoma. Preclinical studies have shown that modulation of ER-β activity may suppress tumor growth and enhance responsiveness to conventional and systemic therapies [19]. While ER-β–targeted treatments are not yet incorporated into standard clinical practice, ongoing research may open new avenues for personalized therapy, particularly in advanced or treatment-resistant disease [20].
The limitations of this study include the absence of survival and recurrence data due to limited follow-up and the lack of comparative evaluation of estrogen receptor alpha (ER-α). Future prospective studies incorporating long-term outcomes, molecular subtyping, and combined assessment of estrogen receptor subtypes may further clarify the prognostic and therapeutic significance of ER-β in urothelial carcinoma.
None.
Ethical policy
The study was done in accordance with the Declaration of Helsinki. Informed patient consent was taken.
Availability of data and materials
All data was included in publication.
Author contributions
AK contributed to concept and design of the manuscript; JB & MB conducted the data; JB & AK focus on images and editing; SC & JB are in charge of proofreading.
Competing interests
The authors declare that they have no competing interests.
Funding
None.
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide. CA Cancer J Clin 2024, 74(3): 229-263.
- Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM: WHO Classification of Tumours of the Urinary System and Male Genital Organs. Eur Urol 2016, 70(1): 106-119.
- Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, Kiemeney L, Lotan Y, Pang K, Silverman DT et al: Epidemiology of bladder cancer. Eur Urol 2018, 74(6): 784-795.
- Ho ME, Quek SI, True LD, Seiler R, Fleischmann A, Bagryanova L, Kim SR, Chia D, Goodglick L, Shimizu Y, et al. AGR2 expression in bladder cancer. Oncotarget 2016, 7(2): 15747-15756.
- Omran OM: CD10 and E-cadherin expression in bladder carcinoma. J Environ Pathol Toxicol Oncol 2012, 31(3): 203-212.
- Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, Sonpavde G, Ayala GE, Younes M, Lerner SP: Estrogen receptor expression in bladder cancer. Cancer 2006, 106(12): 2610-2616.
- Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, Chang C, Messing EM, Netto GJ, Yeh S: Hormone receptor expression and prognosis in bladder cancer. BJU Int 2012, 109(11): 1716-1726.
- Tuygun C, Kankaya D, Imamoglu A, Sertcelik A, Zengin K, Oktay M, Sertcelik N: Sex-specific hormone receptors in urothelial carcinoma. Urol Oncol 2011, 29(1): 43-51.
- Izumi K, Taguri M, Miyamoto H: ER-β–targeted therapy in bladder cancer. J Urol 2014, 191(4): 1185-1192.
- Boorjian S, Ugras S, Mongan NP: Androgen receptor signaling in bladder cancer. Expert Rev Anticancer Ther 2007, 7(12): 1817-1825.
- Jagtap SV, Jagtap SS, Kaur P, Vartak S: Squamous cell carcinoma of the urinary bladder. Ann Urol Oncol 2021, 4(1): 10-17.
- Kashiwagi E, Fujita K, Yamaguchi S, Fushimi H, Ide H, Inoue S, Mizushima T, Reis LO, Sharma R, Netto GJ, et al: Steroid hormone receptors in upper tract urothelial carcinoma. Cancer Biol Ther 2016, 17(11): 1189-1197.
- Hsu I, Vitkus S, Da J, Yeh S: Estrogen receptors in bladder cancer development. Nat Rev Urol 2013, 10(6): 317-326.
- Teng J, Wang ZY, Proctor M: Differential roles of estrogen receptor subtypes. Cancer Res 2008, 68(20): 789-798.
- Ide H, Miyamoto H: Steroid hormone signaling in urothelial tumorigenesis. Cancer Sci 2015, 106(8): 979-986.
- Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA: Gender and bladder cancer. Eur Urol 2016, 69(2): 300-310.
- Mitra AP, Bartsch CC, Bartsch G Jr, Miranda G, Skinner EC, Daneshmand S: Squamous differentiation in urothelial carcinoma. Urol Oncol 2014, 32(1): 31.e1-31.e7.
- Scosyrev E, Ely BW, Messing EM: Mixed histological features in urothelial carcinoma. Urology 2009, 73(2): 316-320.
- Nelson AW, Tilley WD, Neal DE, Carroll JS: Estrogen receptor beta in cancer biology. Endocr Relat Cancer 2014, 21(4): T219-T234.
- Humphries MP, Sundaram SK: Hormone receptor signaling in urothelial carcinoma. Clin Genitourin Cancer 2019, 17(5): e900-e907.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript