Review Article | Open Access
Prostate-Specific Membrane Antigen (PSMA) PET-CT: Revolutionizing Staging, Restaging, and Treatment Response Assessment
Theo Smith1, Mia Harper11Department of Biotechnology, University of Canterbury, Canterbury, 8140, New Zealand.
Correspondence: Mia Harper (Department of Biotechnology, University of Canterbury, 20 Kirkwood Avenue Christchurch, Canterbury, 8140, New Zealand; Email: biotech_2001@outlook.com).
Annals of Urologic Oncology 2025, 8(4): 200-210. https://doi.org/10.32948/auo.2025.12.15
Received: 08 Oct 2025 | Accepted: 20 Dec 2025 | Published online: 28 Dec 2025
Prostate-specific membrane antigen (PSMA) positron emission tomography–computed tomography (PET-CT), has become the benchmark imaging tool in the management of prostate cancer, due to its unparalleled precision in staging, restaging and assessing of treatment response. Traditional imaging modalities, such as computed tomography (CT), magnetic resonance imaging (MRI) and bone scintigraphy, suffer from subpar sensitivity, particularly in detection of small volume nodal disease and early metastatic spread. Conversely, PSMA PET-CT takes advantage of the upregulation of PSMA on prostate cancer cells to offer highly specific and sensitive molecular imaging. This review provides the contemporary overview of available literature from high-impact clinical trials, systematic reviews and meta-analyses that have described the utility of PSMA PET-CT across all stages of the prostate cancer journey. We emphasize its superior results concerning staging in the high- and intermediate-risk patients, where a better value for nodal or distant metastasis translates into individualized treatment. The review also highlights the growing importance of PSMA PET-CT imaging in biochemical recurrence, which allows early detection of recurrent disease at low prostate-specific antigen levels and individualized treatment decision making including salvage and metastasis-directed therapies. Novel applications of PSMA PET-CT in response assessment are discussed, including functional imaging biomarkers, quantitative metrics and evolving response criteria. The milestones of development and introduction of new PET tracers to support PEI for PCa are also presented in the light of radiotracers, theranostics, AI and hybrid imaging. In general, PSMA PET-CT is a leap forward in prostate cancer imaging from diagnosis to treatment based on precision medicine. Continued standardization approaches and prospective evaluation will clarify its best application in a clinical context and future oncology care pathways.
Key words prostate-specific membrane antigen, PSMA PET-CT, prostate cancer imaging, biochemical recurrence, treatment response assessment
Other imaging techniques such as CT scan, MRI and technetium-99m bone have been commonly employed for PCa staging and restaging. However, these methods rely on morphological or surrogate functional alterations and are not sensitive for small-volume disease including lymph nodal metastases and early bone invasion [6, 7]. Lymph node staging by CT and MRI is largely based on size, which does not detect micrometastatic disease in otherwise normal-sized lymph nodes. Bone scintigraphy is also nonspecific and may not identify early bony metastases. These constraints often lead to undertreatment or delayed recognition of disease progression, both of which influence treatment planning and outcome [8].
Molecular imaging has revolutionized prostate cancer diagnosis. Of these developments, prostate-specific membrane antigen (PSMA)–targeted radiotracers for positron emission tomography (PET) has been a game changer [9]. PSMA is a type II transmembrane glycoprotein that is greatly upregulated on PCa cells, particularly in high-grade, metastatic, or castration-resistant disease but with low expression levels in most normal tissues [10]. This peculiar expression pattern promoted PSMA as a perfect target for both diagnostic imaging and therapy, heralding the advent of theranostics in prostate cancer treatment [11].
PSMA PET-CT is a new generation of nuclear and molecular imaging that significantly enhances sensitivity using CT to provide high spatial resolution for the detection of PSMA activity including anatomic changes in prostate cancers [12]. Radiotracers including ^68Ga-PSMA-11 and ^18F-labeled PSMA compounds (e.g., ^18F-DCFPyL and 34^18F-PSMA-1007) have shown outstanding reliability for detection of primary tumors, nodal disease, and distant metastasis down to very low prostate-specific antigen (PSA) levels beyond the sensitivity range of standard imaging [13, 14]. This has led to the acceptance of PSMA PET-CT as a new standard in prostate cancer staging and restaging.
For initial staging, PSMA PET-CT has demonstrated high usefulness in patients with intermediate/high-risk PCa in whom evaluation of lymph node/distant metastasis is necessary for defining appropriate local/systemic treatment [15]. Several prospective trials and meta-analyses have demonstrated that PSMA PET-CT is superior to conventional imaging with respect to sensitivity and specificity for nodal and metastatic disease [16]. Significantly, this enhanced diagnostic accuracy has been demonstrated to have a direct impact on clinical management with changes in surgical planning, radiation therapy treatment volumes and systemic treatment plans [17].
In the context of metastatic prostate cancer (mPCa), the value of PSMA PET-CT is even more evident, especially in the setting of biochemical recurrence (BCR), that is, an increase in prostate-specific antigen (PSA) after definitive local treatment such as radical prostatectomy or radiotherapy [18]. Identification of the site of PSA relapse at low PSA levels is essential for early salvage treatments with the possibility to cure [19]. PSMA PET-CT has shown high detection rates with PSA below 0.5 ng/mL for early locoregional recurrences or oligometastatic disease [20]. This capability has revolutionized the strategy of restaging, which can now be aimed at a personalized, targeted therapeutic approach.
In addition to staging and restaging, PSMA PET-CT is receiving increasing attention as a method of treatment response evaluation. Conventional anatomical change based response evaluation criteria frequently do not correlate well with biological response, for example in bone metastases or after new systemic treatments [21, 22]. PSMA PET-CT delivers functional data on tumor survival and PSMA expression, which is more sensitive for monitoring therapeutic response [23]. Novel methodologies for response assessment and also quantitative PET metrics are being developed in order to standardize the interpretation of the results and increase reproducibility both in clinical trials and routine workup [24]. The growing clinical utility of PSMA PET-CT is closely associated with improvements in the development of radiotracers and image analysis [12]. ^18F-labeled PSMA tracers have become available within the last years, and due to their longer half-life and excellent imaging profile they enabled broader clinical implementation as well as superior image quality. Moreover, the combination of artificial intelligence, radiomics and machine learning are providing novel strategies to extract quantitative biomarkers from PSMA PET images with potential benefits for better risk-stratification and prognostication [15, 25].
Nevertheless, several issues persist despite its fast translation into the clinic. Discrepancies in imaging protocols, choice of tracer, interpretation criteria and availability of PET-CT facilities may influence uniform findings and broad utilisation [26]. Furthermore, the position of PSMA PET-CT in certain clinical settings or situations like low-risk disease, routine follow-up and long-term follow-up is still in flux. Current research and international consensus work are focusing on these aspects, to try to define standardized recommendat ions of best use [27].
In this article, we present a detailed overview of evidence underpinning the application of PSMA PET-CT in prostate cancer with an emphasis on studies related to its use in primary staging, restaging post-biochemical relapse and evaluation treatment response. We review critical trials, news emergent approaches, and the horizon ahead to illuminate how PSMA PET-CT is transforming anatomic imaging of prostate cancer and pushing the envelope on precision oncology.
Precise initial stage is one of the mainstays for prostate cancer therapy, since it is critical to risk stratification, management determination and prognosis [28]. Standard imaging techniques of CT, MRI and bone scan have historically been used for staging; however, their accuracy is not ideal, especially in the identification of small volume nodal disease and early metastatic disease spread. These limitations have led to the development of, and clinical uptake in, molecular imaging modalities including PSMA PET-CT which has shown unparalleled diagnostic sensitivity [29, 30].
PSMA PET-CT takes advantage of the high level of expression of prostate-specific membrane antigen in prostate cancer cells, resulting in excellent specific radiotracer binding and imaging of disease foci [31]. Several prospective studies, systematic reviews and meta-analyses have consistently demonstrated the superiority of PSMA PET-CT over conventional imaging in the detection of pelvic lymph node metastases as well as extrapelvic nodal disease, bone metastases and visceral disease (Table 1) [32]. Crucially, PSMA PET-CT can detect mor phologically occult (normal sized) metastases undetectable by CT or MRI addressing the size-based inadequacies of standard nodal assessment [33].
The available meta-analytical data showed that PSMA PET-CT has high specificity in (often >90%) for lymph node involvement and moderate to high sensitivity according to tumour load and disease risk category [34]. Although sensitivity is likely compromised for micrometastatic nodal disease smaller than the spatial resolution of PET imaging, PSMA PET-CT has a markedly higher performance compared to anatomic imaging with CT and MRI alone [35]. For bone involvement, PSMA PET-CT has demonstrated higher accuracy when compared with bone scintigraphy for early or low-volume lesions and decreased false-positive readings of degenerative or inflammatory changes [36].
The diagnostic accuracy of PSMA PET-CT imaging has been demonstrated with various radiotracers, such as ^68Ga-PSMA-11 and ^18F-labeled tracers including^18F-DCFPyL and^18F-PSMA-1007 [13]. Although all are highly sensitive, ^18F-labelled tracers provide a longer half-life in addition to an improved spatial resolution and lower urinary excretion (where applicable) that may be helpful for better visualization of pelvic-based lesions. Comparative analyses indicate an overall uniform accuracy of tracers, while tracer choice may impact detection patterns in certain areas as revealed by the factor scores [14].
Notably, the performance of PSMA PET-CT is even more striking among men with intermediate and high risk PCa in whom the prevalence of metastatic disease is greater [37]. In these subgroups, the PSMA PET-CT assists in a more accurate delineation of disease extension prospecting nap for accurate Tumor-LNode-Metastasis (TNM) staging [38]. Prostatic cancer Prospective trials, such as the key randomized studies, have shown that PSMA PET-CT conspicuously raises staging accuracy versus conventional imaging with higher diagnostic confidence and fewer equivocal findings [27].
There are some limitations of the high accuracy. PSMA can be heterogeneously expressed and rare prostate cancer subtypes may have low expression of PSMA, thus leading to false-negative findings [39]. Furthermore, nontumoral tissues and benign processes like ganglia, fractures or inflammatory lesions can show physiological or not otherwise specified PSMA uptake, requiring expert reading [40]. Nonetheless, when used in the right clinical setting, PSMA PET-CT is the most accurate imaging technique for primary staging of prostate cancer to date [12].
Clinical implications
Beyond accuracy, the clinical relevance of PSMA PET-CT manifests in impact on patient management. Greater staging precision also increases the level of information available to guide treatment, and permits customization of therapy in response to actual disease burden. Various series have shown that PSMA PET-CT impacts upon clinical management to a large extent if used as initial staging, especially in intermediate-high-risk prostate cancer [27, 41].
By far the most important clinical consequence of a positive PSMA PET-CT, is its effect on planning local therapy [42]. Precise determination of pelvic lymph node involvement can impact on a decision to carry out or not an extended pelvic lymphadenectomy as part of radical prostatectomy and to adapt the radiotherapy fields according to involved nodal regions [43]. On the other hand, avoidance of overtreatment by ruling out metastatic disease may lead to less unnecessary aggressive surgical or radiation therapy and subsequent morbidity [44].
PSMA PET-CT is also crucial for detecting occult distant metastases, which can alter management from cureative local therapy to systemic strategies [20]. The recognition of oligometastatic disease (ie, few metastases) has led to change in therapeutic approach and the introduction of treatment strategies such as metastasis-directed therapy by means of stereotactic body radiotherapy or focal surgical resection [45]. In selected patients, PSMA PET-CT may lead to a tailored and possibly aggressive treatment approach through precise identification of sites of metastatic disease [27].
Prospective trial findings suggest that PSMA PET-CT–directed staging leads to management changes in a significant proportion of men, frequently > 20–30% when contrasted against conventional imaging [46]. These modifications may involve changes in the surgical technique, radiation planning, commencement of systemic therapy, or participation in clinical trials. Critically, PSMA PET-CT steers clear of uncertainty and further imaging which is widespread in the staging process - it narrows down a diagnosis and kicks treatment options into gear [27].
From a multidisciplinary point of view, PSMA PET-CT can contribute to optimal communication and equipoise among urologists, radiation oncologists, medical oncologists and nuclear medicine physicians [47]. The comprehensive genomic and fusion-guided anatomical information enabled by PSMA PET-CT underpins consensus-driven decision-making, especially in difficult situations where traditional imaging is inconclusive [48].
There is also a growing body of evidence to support the prognostic utility of PSMA PET-CT staging [49]. Burden and Distribution of PSMA-Avid Disease Have a Strong Impact on Oncologic Outcomes, Such as PFS and BCR [50]. Quantitative PET parameters and whole-body tumor burden measures from PSMA PET-CT are currently evaluated as potential candidates for risk stratification, which suggests that the clinical assessment using PSMA-PET CT may go beyond the mere detection of lesions [51].
However, the implementation of PSMA PET-CT in the routine clinical care needs to be balanced against its costs and availability in addition to need for specialized skills [52]. Although a cost-effectiveness analysis universally favours PSMA PET-CT once inappropriately selected patients because of associated management change, access to PSMA remains heterogeneous between health provider organisations. Moreover, standardized reporting and interpretation system are needed to guarantee a uniform performance of a technique [53].
|
Table 1. Diagnostic performance of PSMA PET-CT in initial staging. |
|||
|
Parameter |
PSMA PET-CT |
Conventional imaging (CT/MRI/Bone Scan) |
Key points |
|
Pelvic lymph node detection |
High sensitivity (moderate to high depending on tumor burden) |
Limited by size criteria; misses micrometastases |
Detects morphologically normal metastatic nodes |
|
Specificity for lymph nodes |
>90% |
Lower |
Reduces false positives |
|
Bone metastases detection |
Superior, even for low-volume lesions |
Lower, may miss early metastases |
Avoids false positives from degenerative/inflammatory changes |
|
Visceral metastases |
Accurate localization |
Often missed |
Important for staging and treatment planning |
|
Radio tracers |
^68Ga-PSMA-11, ^18F-DCFPyL, ^18F-PSMA-1007 |
N/A |
^18F-tracers have longer half-life, better resolution |
|
Patient subgroup impact |
Intermediate- to high-risk prostate cancer |
Less effective |
Improved TNM staging and management decisions |
|
Limitations |
Rare PSMA-negative tumors, benign uptake |
Size-dependent detection |
Requires expert interpretation |
|
Note: PSMA: Prostate-specific membrane antigen, PET-CT: Positron emission tomography-computer tomography, MRI: Magnetic resonance imaging, TNM: Tumor node metastasis classification, N/A: Not applicable. |
|||
Introduction Biochemical recurrence (BCR) is a major clinical problem in the management of prostate cancer and occurs in many patients after definitive local therapy, such as radical prostatectomy and radiotherapy [54]. BCR is usually characterized by an increase in the prostate-specific antigen (PSA) level, whereas no clinical or radiographic signs of disease have been detected [18]. Recurrence disease clearly needs to be identified early, and precisely as possible because there may still be a potential curative effect or postponement of treatment among salvage treatments [54]. However, traditional imaging is less sensitive in this population especially at low PSA levels and may result in deferred or empiric treatments [55].
PSMA PET-CT is the most sensitive imaging modality for detection of recurrence of prostate cancer and has revolutionised the diagnostic paradigm in BCR. Due to the fact that PC expresses the PSMA in excessive amounts, a special opportunity is open: with the help of PSMA PET-CT one can see the disease in a PSA far below those values at which CT, MRI or bone scintigraphy are sensitive enough [12, 56]. A number of reports have shown high detection rates even among patients with a PSA 80% for PSA >2.0 ng/mL [57]. This early sensitivity results in localization of recurrence, frequently predate the appearance of symptoms or later extensive disease and permits more accurate and earlier treatment intervention [58].
Anatomical patterns of recurrence PSMA PET-CT has a greater potential for detecting local recurrence in the prostatic bed, pelvic lymph node metastases, extraprostatic nodal disease and bone or visceral metastasies [59]. Importantly, some of these lesions are small and could be overlooked according to size criteria for imaging. The success of PSMA PET-CT at identifying oligometastatic disease has revolutionized clinical thinking regarding recurrence and the popular contention that a proportion of patients may benefit from targeted, metastasis-directed therapy rather than early systemic therapy [45].
The efficacy of PSMA PET-CT in BCR has been demonstrated using a variety of radiotracers, including ^68Ga-PSMA-11 and ^18F-based agents [60]. ^18F-labelled tracers have advantages regarding image resolution and even better biodistribution, with potential enhanced detection of lesions in selected regions [61]. Although variations in detection rates were observed, diagnostic performance is well maintained for all tracers tested and highlights the strength of PSMA-targeted imag (ing) (e.g. imaging) at time of recurrence [62].
PSMA PET-CT has its inherent limitations notwithstanding its impressive features. False-negative can be seen in low expression of PSMA or neuroendocrine differentiation, while false-positive site are reactive lymph nodes, fractures or inflammatory alterations independent of a treatment response [40, 63]. However, when correlated with clinical and biochemical parameters, PSMA PET-CT is unequaled in terms of diagnostic confidence in the BCR context (Figure 1) [64].
Impact of PSMA PET-CT on clinical management in biochemical recurrence
More so than its diagnostic advantage, the greatest advantage of PSMA PET-CT in biochemical recurrence is that it guides patient’s clinical management directly. By accurately identifying the site of disease recurrence, PSMA PET-CT permits personalized salvage treatment regimen based on patterns and burden of recurrence rather than utilizing a blanket approach based only on PSA kinetics [65]. As summarized in Table 2, this imaging directly affects clinical decision-making across several key scenarios.
Management issues One of the major implications regarding management would be to salvage radiotherapy. The correct diagnosis of local recurrence and pelvic nodal involvement enables radiation oncologists to adjust the target volumes, to intensify doses to PSMA-avid lesions as well as to minimize unnecessary irradiation of non-involved areas [66]. It has been demonstrated that PSMA PET-CT findings have resulted in changes of salvage radiotherapy plans in a significant number of patients with a resultant improvement in biochemical control on follow-up [67].
PSMA PET-CT has also paved the way for metastasis-directed therapy (MDT) of oligometastatic recurrence [68]. When few metastatic lesions are found by PSMA PET-CT, stereotactic body radiotherapy or surgical metastasectomy can be applied. Initial clinical data indicates that PSMA PET-CT directed MDT has the potential to sustain use of androgen deprivation therapy, maintain QoL and affect cancer related outcomes in the appropriate patient population [47].
If PSMA PET-CT demonstrates widespread or distant metastatic disease, treatment options are typically focused on systemic therapy, such as androgen deprivation therapy, androgen receptor pathway inhibitors, or chemotherapy [69]. In this regard, PSMA PET-CT can spare inappropriate local salvage therapies and facilitate prompt commencement of suitable systemic treatment. Critically, this stratified strategy reduces over-treatment while ensuring that treatment intensity is congruent with the disease burden [70].
Numerous studies have demonstrated that PSMA PET-CT affects management in 30–60% of patients with BCR. These include modifications in radiation fields, surgical planning, institution or deferral of systemic therapy, and candidacy for clinical trials [64]. This level of impact to management illustrates the clinical significance of PSMA PET-CT, beyond the diagnostic performance.
In terms of prognostication, imaging with PSMA PET-CT has been correlated clinically with survival outcomes such as progression-free and time to develop castrate-resistant disease. The volume and pattern of PSMA-avid lesions and the quantitative PET parameters are under evaluation as potential biomarkers, which can be used to inform risk-adapted treatment approaches [71].
A risk of integration with PSMA PET-CT is its disruptive impact, and there are ongoing efforts to deconvolute evolving BCR management algorithms [65]. Heterogeneity in imaging protocols, reporting, and treatment thresholds may act as confounders of clinical decision making. There are current international efforts to create consensus guidelines and reporting standards for uniform interpretation and application between institutions [72].
|
Table 2. Impact of PSMA PET-CT on management in biochemical recurrence (BCR). |
|||
|
Clinical scenario |
Role of PSMA PET-CT |
Effect on management |
Evidence / Notes |
|
Salvage radiotherapy |
Identifies local recurrence & pelvic nodes |
Adjusts target volumes, escalates doses, avoids unnecessary irradiation |
Improves biochemical control |
|
Oligometastatic recurrence |
Detects limited metastatic lesions |
Guides metastasis-directed therapy (MDT) like stereotactic radiotherapy or surgery |
Delays systemic therapy, preserves QoL |
|
Extensive/distant metastases |
Identifies systemic spread |
Initiates appropriate systemic therapy (ADT, AR inhibitors, chemotherapy) |
Avoids futile local salvage treatments |
|
Overall management change |
N/A |
30–60% of patients with BCR |
Includes surgery, radiation, systemic therapy, trial eligibility |
|
Prognostic utility |
PSMA-avid lesion burden & distribution |
Risk stratification for progression-free survival and CRPC |
Quantitative PET parameters under study |
|
Limitations |
Low PSMA expression, neuroendocrine differentiation, false positives |
Requires careful interpretation with clinical context |
Standardization ongoing |
|
Note: PSMA: Prostate-specific membrane antigen, PET-CT: Positron emission tomography-computer tomography, BCR: Biochemical recurrence, MDT: Multi-disciplinary treatment, ADT: Androgen-deprivation therapy, CRPC: Castration-resistant prostate cancer, QoL: Quality of life, N/A: Not applicable. |
|||
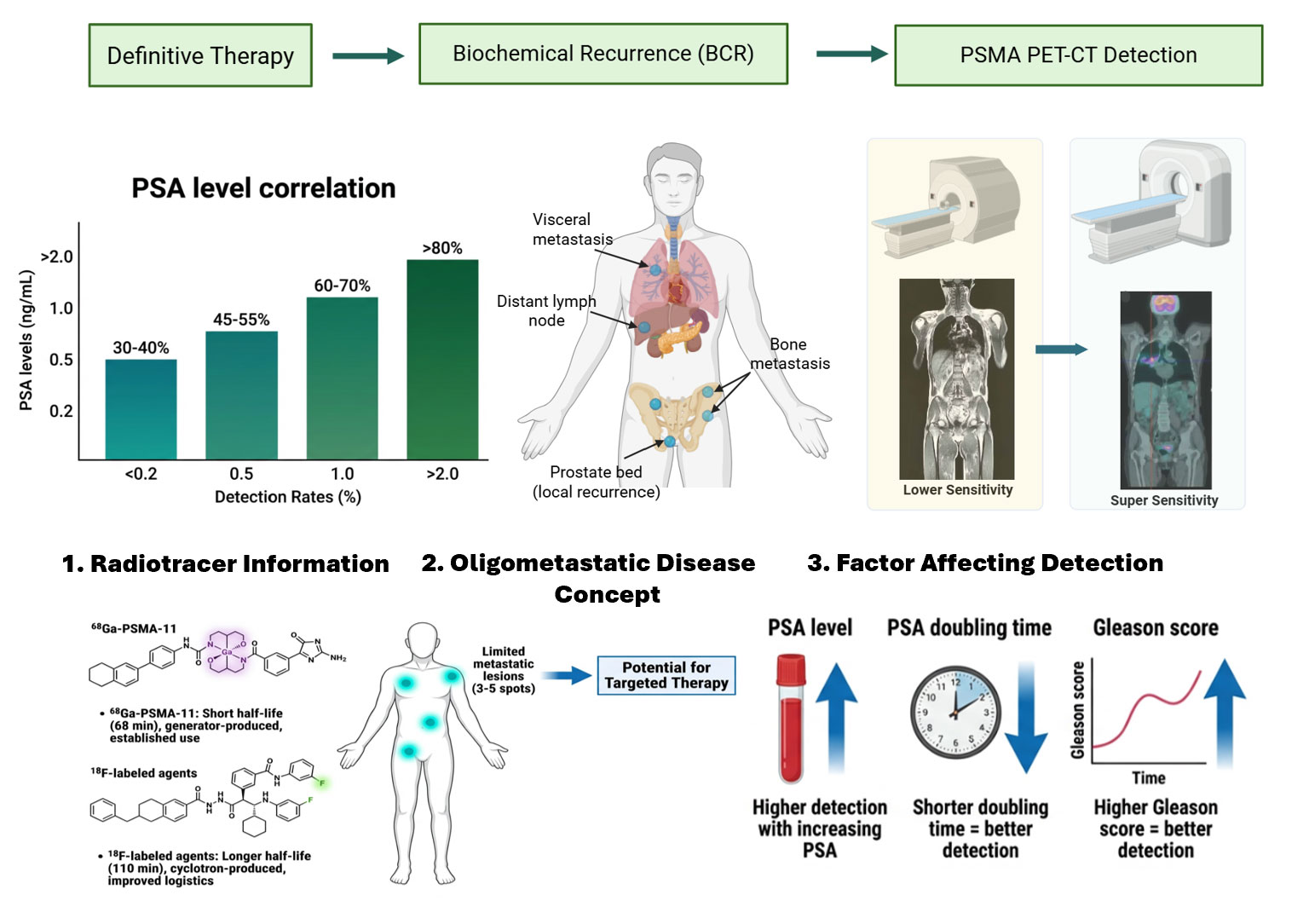 Figure 1. PSMA PET-CT in guiding prostate cancer recurrence management. Flow diagram for the use of PSMA PET-CT in the assessment of biochemical recurrence following definitive treatment for prostate cancer. The model demonstrates how PSA drives imaging choice, with PSMA PET-CT having greater sensitivity than traditional imaging for local and metastatic recurrence. The detection rate increases with increasing PSA-value, shorter PSA doubling time, and higher Gleason score. Identification of oligometastatic disease (3-5 lesions) allows for personalized treatment modalities.
Figure 1. PSMA PET-CT in guiding prostate cancer recurrence management. Flow diagram for the use of PSMA PET-CT in the assessment of biochemical recurrence following definitive treatment for prostate cancer. The model demonstrates how PSA drives imaging choice, with PSMA PET-CT having greater sensitivity than traditional imaging for local and metastatic recurrence. The detection rate increases with increasing PSA-value, shorter PSA doubling time, and higher Gleason score. Identification of oligometastatic disease (3-5 lesions) allows for personalized treatment modalities.
Monitoring treatment response is essential to prostate cancer care, which helps clinical decision-making and provides a predictor of prognosis [73]. Historically, serum prostate-specific antigen (PSA) levels, anatomic imaging and clinical symptoms have been used as measures of response. Yet these measures have significant shortcomings [74]. PSA kinetics may not accurately represent tumor load in all situations, particularly during novel systemic agents and conventional imaging has difficulty distinguishing viable tumour from treatment-induced changes, especially in bone metastases [75]. In this setting PSMA PET-CT has become appealing, being a functional imaging modality potentially able to offer relevant biological information about therapy response.
PSMA PET-CT provides the doctrine of the visualization of vital tumor, which is defined by PSMA expression but not size or morphology based assessment. This attribute is of particular relevance in mCRPC, where lesions may appear stabilization in size despite biological progression or response [76]. The early clinical trials have shown that, in many cases, the variation of PSMA uptake on PET-CT is known to precede anatomical changes which can be used as an indicator of early therapeutic response (or failure) using PSMA PET-CT [77].
The use of PSMA PET-CT has been investigated with regard to different treatment options, including androgen deprivation therapy (ADT), ARPI, chemotherapy, radiotherapy and PSMA anticancer radioligand [78]. In patients on systemic hormonal therapy, PSMA uptake may decrease in responding lesions, whereas stable or increased uptake can signify resistant disease. Transient increases in PSMA expression, the so-called “flare phenomenon,” which regularly occur early after ADT initiation have also been described and should be considered when interpreting serial imaging results [77, 79].
In the radiotherapy context, PSMA PET-CT has been shown to have value in differentiating residual or recurrent disease from post-treatment fibrosis or inflammation. This isn't always possible to be seen with standard imaging and makes a big difference in how we plan next steps [80]. In addition, in patients receiving chemotherapy, changes on PSMA PET parameters have been associated with clinical response indicating a potential role in early treatment monitoring [81].
PSMA PET-CT has also become increasingly important in the era of PSMA-targeted radioligand therapy, where imaging is used diagnostically and predictively [82]. Base-line PSMA PET-CT ensures the expression of target before therapy; follow-up imaging can reflect the efficacy and heterogeneity of disease. Response patterns on PSMA PET-CT has been related with survival, emphasizing its importance as a prognostic marker [83].
However, despite these encouraging results, the number of studies of PSMA PET-CT for treatment response assessment is relatively limited, and it has not yet become a widespread clinical application [84]. One issue is the absence of an accepted response criteria. Classical approaches such as RECIST are not readily applicable to molecular imaging, and in particular for bone-dominant disease. Therefore, the interpretation of PSMA PET-CT response is currently varied by qualitative assessment or institution specific quantitative cut-off values [85].
Emerging criteria, quantitative metrics, and future directions
Acknowledging the lack of standardized response evaluation, several attempts for PSMA PET-specific response criteria are emerging[21]. These frameworks seek to address variations in PSMA uptake, lesion number and whole-body tumour load to standardize more reliable treatment response assessment [21]. In this context, progression rules and emerging criteria (eg RECIP [Response Evaluation Criteria in PSMA Imaging for Prostate Cancer]) are important steps toward standardization [86].
Quantitative PSMA PET-CT parameters, such as SUV, PSMA tumor volume and total lesion PSMA expression are increasingly being studied as potential surrogates for response. Such parameters may instigate development beyond binary categorization (response–vs–progression) and might support a more differential evaluation of treatment effects [70]. The latter (ie, changes in PSMA uptake above the threshold of normal tissue) has been demonstrated to be associated with improved progression-free and overall survival, indicating its prognostic significance [87].
Imaging analysis techniques (radiomics and artificial intelligence) continue to broaden the scope of how PSMA PET-CT can be used in response assessment. Radiomic-features derived from PSMA PET images could have the potential to represent intratumoral heterogeneity and treatment-related alterations below the level of detection by visual assessment [88, 89]. Machine-learning algorithms that incorporate PSMA PET imaging with clinical and genomic data are being constructed to predict response to treatment and personalize therapy choice [90].
An additional possible utility of PSMA PET-CT is adaptive treatment planning. By localizing sites of residual PSMA-avid disease during or after treatment, oncologists could adapt intensity of therapy details such as dose escalation to refractory locations or more expeditious changes in systemic therapy at an earlier stage[65]. This personalized adaptive mechanism is consistent with the principles of precision cancer therapy and may offer benefit in terms of response without undue toxicity [91].
However, many hurdles must still be overcome before PSMA PET-CT is integrated in regular treatment response evaluations [21]. It remains critical to standardize acquisition protocols, moment for follow-up scans and reporting criteria to obtain consistency among institutions and clinical trials [92]. Furthermore, the biological variance of PSMA expression, especially under treatment selection pressure, needs to be taken into account to prevent misinterpretation of imaging results [93].
The prospective trials underway are poised to critically validate the PSMA PET-based response criteria and define their role in predicting with PSA response versus survival end points [77]. As the data definition evolves, PSMA PET-CT will likely be included in response assessment algorithms for advanced and heavily pre-treated patients with prostate cancer (Figure 2) [94] .
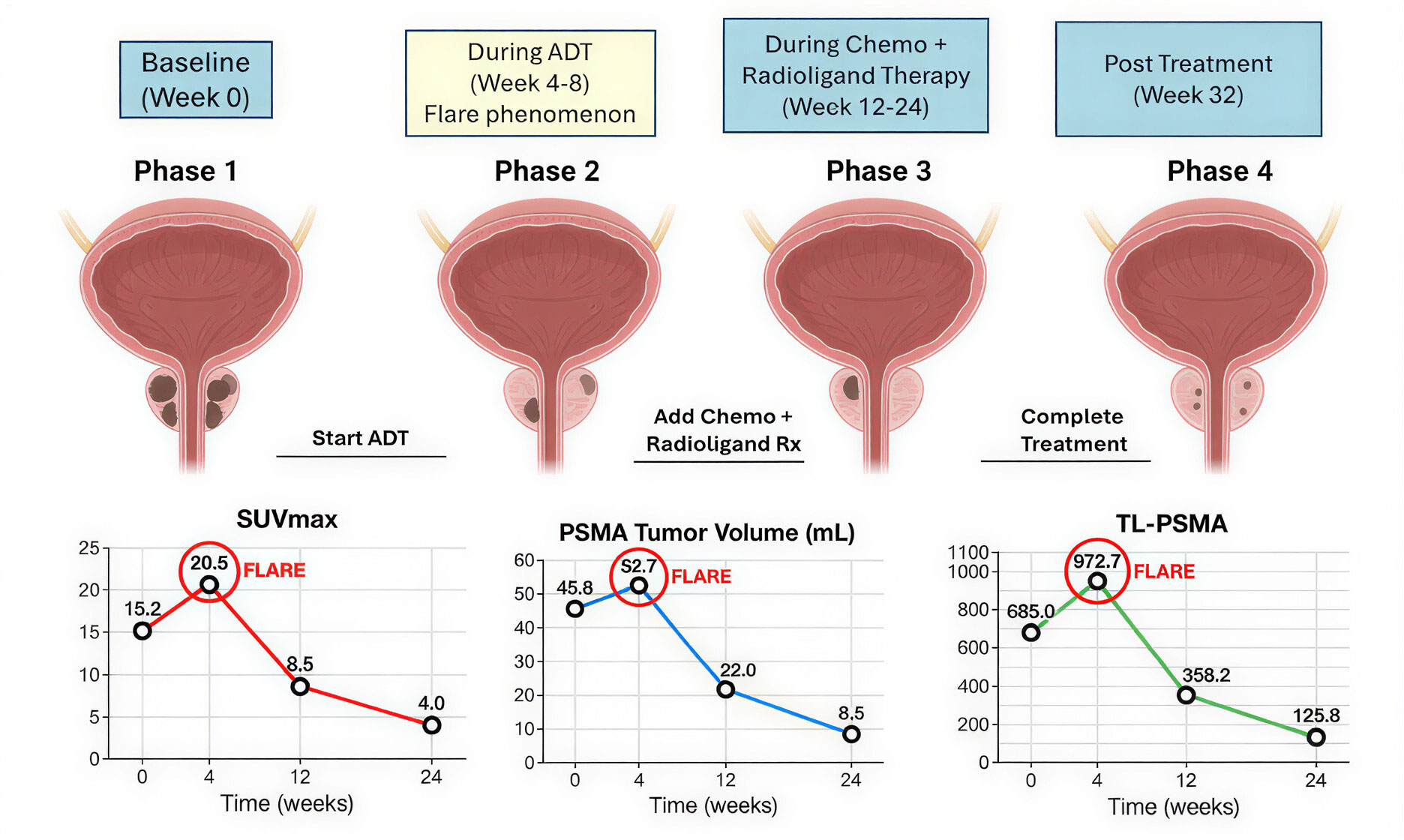 Figure 2. PET-CT in treatment response assessment. Sequential PSMA PET-CT scans and quantitative parameters at baseline, during, and after various treatments in a patient with PCa treated with ADT, chemotherapy, and/or radioligand therapy. The heat maps show PSMA uptake (red-yellow, high; blue, low). SUVmax, PSMA tumor volume and TL-PSMA reflect the change of tumour burden. A temporary “flare phenomenon” is detected after initiation of ADT, and then lesion response. Persistent uptake indicates resistant lesions.
Figure 2. PET-CT in treatment response assessment. Sequential PSMA PET-CT scans and quantitative parameters at baseline, during, and after various treatments in a patient with PCa treated with ADT, chemotherapy, and/or radioligand therapy. The heat maps show PSMA uptake (red-yellow, high; blue, low). SUVmax, PSMA tumor volume and TL-PSMA reflect the change of tumour burden. A temporary “flare phenomenon” is detected after initiation of ADT, and then lesion response. Persistent uptake indicates resistant lesions.
The development of radiolabelled tracers has in addition contributed to the implementation of PSMA PET-CT into theranostic concepts and thereby established an interconnection between diagnostic imaging and targeted radionuclide treatment [11]. PSMA PET-CT is central for selecting patients who are eligible to undergo PSMA-targeted radioligand therapy as it verifies adequate target expression and imaging of disease site [82]. Radio-ligand therapies with beta-emitting isotopes (e.g., ^177Lu-PSMA) have shown strong systemic treatment benefits in metastatic castration-resistant prostate cancer, and PSMA PET-CT plays a key role in patient selection, therapy monitoring, and dosimetry [99]. The advent of alpha-emitting monoclonal PSMA targeted agents have further broadened therapeutic options, especially in CRPC; however, dosing, and safety is still evolving as a field [100].
PSMA PET-CT has also been improved with the development of technological innovations in PET imaging systems. Facility of digital PET detectors, better time-of-flight technology and new reconstruction algorithms has led to superior spatial resolution, improved lesion detection and lower acquisition duration [101]. These enhancements have improved the ability to detect micrometastases, and could pave the way for a reduction in radiotracer dose without sacrificing image quality. Thus metal artefact reduced (MAR) PSMA PET-CT has also become more effective and better tolerated, remaining accurate systematically [17, 102].
Simultaneously, quantitative imaging and artificial intelligence have broadened the analytical horizon of PSMA PET-CT. Semi-quantitative parameters measured on PSMA PET/CT, such as SUVmax, PSMA-derived tumor volume and whole-body tumor burden, are being more accepted as potential quantitative biomarkers for disease characterization and therapy response evaluation [103]. Radiomics and machine-learning techniques complexify the identification of imaging features that reflect tumor heterogeneity, biological activity beyond visual assessment. Integration of data derived from PSMA PET and more traditional clinical, biochemical and genomics-based information appears particularly promising for prognostication and personalized treatment [104].
Combining PSMA PET with other imaging modalities and clinical workflows constitutes a second key area of innovation [105]. Molecular-sensitive with excellent soft-tissue characterization, hybrid approaches such as PET-MRI hold promise for local staging and pelvic disease assessment. In addition, PSMA PET-CT findings are incorporated more frequently into radiation therapy planning, surgical navigation and adaptive treatment strategies highlighting the increasing role of this modality in precision oncology [48].
Nevertheless, obstacles persist for the broader applicability of the new PSMA PET-CT techniques. Variable radiotracer availability, imaging protocols and interpretation criteria can influence reproducibility and clinical symmetry [27]. Currently work is ongoing to develop and validate standard acquisition protocols, reporting schemes and quantitative measures to ensure consistent application at various sites [106]. Furthermore, biological variability of the PSMA expression, particularly under therapeutic pressure needs to be taken into account upon interpreting imaging results and making treatment decisions [93].
In the primary diagnostic setting, PSMA PET-CT has emerged as the most accurate tool for detection of nodal and metastatic disease, especially for patients with intermediate to high-risk prostate cancer. It has allowed the identification of clinically relevant metastases, which would have otherwise been missed, contributed to better treatment planning, and reduced the uncertainty characterizing the diagnostic phase. Likewise, in BCR, PSMA PET-CT allows a timely visualization of recurrent disease at low PSA levels for individualized salvage and metastasis directed therapies and to avoid an unnecessary or highly ineffective treatment.
In addition to the role in disease detection, PSMA PET-CT is increasingly being considered for evaluation of treatment response. With standardized response criteria still in development, preliminary evidence suggests that PSMA PET-derived measures can serve as functional biomarkers that capture tumor biology and treatment response better than traditional results. Quantitative analysis, radiomics, and artificial intelligence further contribute to the increasingly strong role of PSMA PET-CT imaging for precision oncology and adaptive therapeutic decisions.
Technological development such as with new radiotracers, theranostic approaches, improved imaging systems further extend the clinical utility of PSMA PET-CT. These developments are not only driving diagnostic accuracy and accessibility, but also fostering greater linkage between imaging and image-guided therapeutic strategies in a manner that underpins the personalized model of prostate cancer care.
Such enthusiasm has not come without challenges, however, namely lack of imaging biostandardization (imaging protocol harmonization), interpretation criteria and access to PSMA PET-CT. Continued prospective trials, consensus programs and interaction among multidisciplinary teams will be necessary to refine the clinical indications and establish evidence based guidelines for its best utilization.
In conclusion, PSMA PET-CT is a new milestone in the imaging of prostate cancer which links diagnostics, treatment and prognostication. With progressive development of clinical data and technological capacity, PSMA PET-CT is set to assume a central role in tailoring personalized care and improving outcomes for patients with prostate cancer.
Non applicable.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
TS contributed to design of the work, data collection, and drafting the article. MH revised the draft manuscript and approved the final submission.
Competing interests
The author declares no competing interests.
None.
- Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, Stattin P, Van Poppel H, La Vecchia C: Epidemiology and prevention of prostate cancer. Eur Urol Oncol 2021, 4(6): 877-892.
- Rawla P: Epidemiology of prostate cancer. World J Oncol 2019, 10(2): 63.
- Tonry C, Finn S, Armstrong J, Pennington SR: Clinical proteomics for prostate cancer: understanding prostate cancer pathology and protein biomarkers for improved disease management. Clin Proteomics 2020, 17(1): 41.
- Agrawal S, Vagha S: A comprehensive review of artificial intelligence in prostate cancer care: state-of-the-art diagnostic tools and future outlook. Cureus 2024, 16(8): e66225.
- Brönimann S, Pradere B, Karakiewicz P, Abufaraj M, Briganti A, Shariat SF: An overview of current and emerging diagnostic, staging and prognostic markers for prostate cancer. Expert Rev Mol Diagn 2020, 20(8): 841-850.
- Zhu M, Liang Z, Feng T, Mai Z, Jin S, Wu L, Zhou H, Chen Y, Yan W: Up-to-date imaging and diagnostic techniques for prostate cancer: A literature review. Diagnostics 2023, 13(13): 2283.
- Leung D, Krishnamoorthy S, Schwartz L, Divgi C: Imaging approaches with advanced prostate cancer: techniques and timing. Can J Urol 2014, 21(2): 42-47.
- Hövels A, Heesakkers R, Adang E, Jager G, Strum S, Hoogeveen Y, Severens J, Barentsz J: The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008, 63(4): 387-395.
- Rowe SP, Gorin MA, Pomper MG: Imaging of prostate-specific membrane antigen with small-molecule PET radiotracers: from the bench to advanced clinical applications. Annual review of medicine 2019, 70(1): 461-477.
- Adnan A, Basu S: Dual-Tracer PET-Computed Tomography Imaging for Precision Radio-Molecular Theranostics of Prostate Cancer: A Futuristic Perspective. PET clinics 2022, 17(4):641-652.
- Sallam M, Nguyen N-T, Sainsbury F, Kimizuka N, Muyldermans S, Benešová-Schäfer M: PSMA-targeted radiotheranostics in modern nuclear medicine: then, now, and what of the future? Theranostics 2024, 14(8): 3043.
- Farolfi A, Calderoni L, Mattana F, Mei R, Telo S, Fanti S, Castellucci P: Current and emerging clinical applications of PSMA PET diagnostic imaging for prostate cancer. J Nucl Med 2021, 62(5): 596-604.
- Yang Y-Y, Liu Z-M, Peng R-C: Diagnostic performance of 18F-labeled PSMA PET/CT in patients with biochemical recurrence of prostate cancer: a systematic review and meta-analysis. Acta Radiol 2023, 64(10): 2791-2801.
- Awenat S, Piccardo A, Carvoeiras P, Signore G, Giovanella L, Prior JO, Treglia G: Diagnostic role of 18F-PSMA-1007 PET/CT in prostate cancer staging: a systematic review. Diagnostics 2021, 11(3): 552.
- Werner RA, Derlin T, Lapa C, Sheikbahaei S, Higuchi T, Giesel FL, Behr S, Drzezga A, Kimura H, Buck AK: 18F-labeled, PSMA-targeted radiotracers: leveraging the advantages of radiofluorination for prostate cancer molecular imaging. Theranostics 2020, 10(1): 1-16.
- Satapathy S, Singh H, Kumar R, Mittal BR: Diagnostic accuracy of 68Ga-PSMA PET/CT for initial detection in patients with suspected prostate cancer: a systematic review and meta-analysis. Am J Roentgenol 2021, 216(3): 599-607.
- Mari A, Cadenar A, Giudici S, Cianchi G, Albisinni S, Autorino R, Di Maida F, Gandaglia G, Mir MC, Valerio M et al: A systematic review and meta-analysis to evaluate the diagnostic accuracy of PSMA PET/CT in the initial staging of prostate cancer. Prostate Cancer Prostatic Dis 2025, 28(1): 56-69.
- Shore ND, Moul JW, Pienta KJ, Czernin J, King MT, Freedland SJ: Biochemical recurrence in patients with prostate cancer after primary definitive therapy: treatment based on risk stratification. Prostate Cancer Prostatic Dis 2024, 27(2): 192-201.
- Nguyen PL, D’Amico AV, Lee AK, Warren Suh W: Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate‐specific antigen failure: a systematic review of the literature. Cancer 2007, 110(7): 1417-1428.
- Alberto M, Yim A, Papa N, Siva S, Ischia J, Touijer K, Eastham JA, Bolton D, Perera M: Role of PSMA PET-guided metastases-directed therapy in oligometastatic recurrent prostate cancer. Front Oncol 2022, 12: 929444.
- Gafita A, Schroeder JA, Ceci F, Oldan JD, Khandani AH, Lecouvet FE, Solnes LB, Rowe SP: Treatment Response Evaluation in Prostate Cancer Using PSMA PET/CT. J Nucl Med 2025, 66(7): 995-1004.
- Barbosa FG, Queiroz MA, Ferraro DA, Nunes RF, Dreyer PR, Zaniboni EC, Costa LB, Bastos DA, Gomes Marin JF, Buchpiguel CA: Prostate-specific membrane antigen PET: therapy response assessment in metastatic prostate cancer. Radiographics 2020, 40(5): 1412-1430.
- Adnan A, Basu S: PSMA receptor-based PET-CT: the basics and current status in clinical and research applications. Diagnostics 2023, 13(1): 158.
- Doot RK, McDonald ES, Mankoff DA: Role of PET quantitation in the monitoring of cancer response to treatment: review of approaches and human clinical trials. Clin Transl Imaging 2014, 2(4): 295-303.
- Capasso G, Stefanucci A, Tolomeo A: A systematic review on the current status of PSMA-targeted imaging and radioligand therapy. Eur J Med Chem 2024, 263: 115966.
- McDougald WA: Standardisation of preclinical PET/CT protocols across multiple research centres. University of Edinburgh 2020, PhD Thesis.
- von Stauffenberg F, Poyet C, Beintner-Skawran S, Maurer A, Schmid FA: Current clinical applications of PSMA-PET for prostate cancer diagnosis, staging, and treatment. Cancers 2024, 16(24): 4263.
- Reina Y, Villaquirán C, García-Perdomo HA: Advances in high-risk localized prostate cancer: Staging and management. Current Problems in Cancer 2023, 47(4): 100993.
- Borley N, Feneley MR: Prostate cancer: diagnosis and staging. Asian J Androl 2008, 11(1): 74.
- Taneja SS: Imaging in the diagnosis and management of prostate cancer. Rev Urol 2004, 6(3): 101-113.
- Rowe S, Gorin M, Allaf M, Pienta K, Tran P, Pomper M, Ross A, Cho S: PET imaging of prostate-specific membrane antigen in prostate cancer: current state of the art and future challenges. Prostate Cancer Prostatic Dis 2016, 19(3): 223-230.
- Yang B, Dong H, Zhang S, Ming S, Yang R, Peng Y, Gao X: PSMA PET vs. mpMRI for lymph node metastasis of prostate cancer: A systematic review and head-to-head comparative meta-analysis. Acad Radiol 2025, 32(5): 2797-2814.
- Dondi F, Albano D, Bertagna F, Treglia G: Bone scintigraphy versus PSMA-targeted PET/CT or PET/MRI in prostate cancer: lessons learned from recent systematic reviews and meta-analyses. Cancers (Basel) 2022, 14(18): 4470.
- Matushita CS, Silva AMMd, Schuck PN, Bardisserotto M, Piant DB, Pereira JL, Cerci JJ, Coura Filho GB, Esteves FP, Amorim BJ et al: 68Ga-Prostate-specific membrane antigen (psma) positron emission tomography (pet) in prostate cancer: a systematic review and meta-analysis. Int Braz J Urol 2021, 47(4): 705-729.
- Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, Behr SC, Zhang L, Barbato F, Ceci F et al: Diagnostic accuracy of 68Ga-PSMA-11 PET for pelvic nodal metastasis detection prior to radical prostatectomy and pelvic lymph node dissection: a multicenter prospective phase 3 imaging trial. JAMA Oncol 2021, 7(11): 1635-1642.
- Kirby RS, Poon D, Patel M: Fast Facts: Prostate Cancer: If, when and how to intervene. Fast Facts: Prostate Cancer (11th edition), 2020.
- Cytawa W, Seitz AK, Kircher S, Fukushima K, Tran-Gia J, Schirbel A, Bandurski T, Lass P, Krebs M, Połom W et al: 68Ga-PSMA I&T PET/CT for primary staging of prostate cancer. Eur J Nucl Med Mol Imaging 2020, 47(1): 168-177.
- Mazzone E: Integrating Psma Expression Patterns, Morphological And Clinical Features To Optimize Detection Of Nodal Metastases In Patients Treated With Psma Radio-Guided Robot-Assisted Radical Prostatectomy And Extended Pelvic Lymph Node Dissection. Doctoral Thesis 2024.
- Tsourlakis MC, Klein F, Kluth M, Quaas A, Graefen M, Haese A, Simon R, Sauter G, Schlomm T, Minner S: PSMA expression is highly homogenous in primary prostate cancer. Appl Immunohistochem Mol Morphol 2015, 23(6): 449-455.
- Sheikhbahaei S, Afshar-Oromieh A, Eiber M, Solnes LB, Javadi MS, Ross AE, Pienta KJ, Allaf ME, Haberkorn U, Pomper MG et al: Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging 2017, 44(12): 2117-2136.
- Hoffman A, Amiel GE: The impact of PSMA PET/CT on modern prostate cancer management and decision making—the urological perspective. Cancers 2023, 15(13): 3402.
- Donswijk ML, van Leeuwen PJ, Vegt E, Cheung Z, Heijmink SW, van der Poel HG, Stokkel MP: Clinical impact of PSMA PET/CT in primary prostate cancer compared to conventional nodal and distant staging: a retrospective single center study. BMC cancer 2020, 20(1): 723.
- Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, Hawaux E, Limani K, Otte FX, Peltier A et al: Clinical impact of 68Ga‐prostate‐specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate‐specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int 2017, 120(2): 197-203.
- Wang K, Tepper JE: Radiation therapy‐associated toxicity: Etiology, management, and prevention. CA: a cancer journal for clinicians 2021, 71(5): 437-454.
- Galgano SJ, McDonald AM, West JT, Rais-Bahrami S: Defining oligometastatic disease in the new era of PSMA-PET imaging for primary staging of prostate cancer. Cancers 2022, 14(14): 3302.
- Hofman MS, Murphy DG, Williams SG, Nzenza T, Herschtal A, Lourenco RDA, Bailey DL, Budd R, Hicks RJ, Francis RJ et al: A prospective randomized multicentre study of the impact of gallium‐68 prostate‐specific membrane antigen (PSMA) PET/CT imaging for staging high‐risk prostate cancer prior to curative‐intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int 2018, 122(5): 783-793.
- Suh M, Cheon GJ: Multidisciplinary team approach in prostate-specific membrane antigen theranostics for prostate cancer: A narrative review. J Urol Oncol 2024, 22(1): 11-20.
- Dragonetti V, Colandrea M, Travaini L, Farulla LSA, Ceci F, Mattana F: PSMA-PET Guided Radiotherapy in Prostate Cancer: A Critical Review of Current Applications and Future Directions. Semin Radiat Oncol: 2025, 35(3): 317-324.
- Bagguley D, Ong S, Buteau JP, Koschel S, Dhiantravan N, Hofman MS, Emmett L, Murphy DG, Lawrentschuk N: Role of PSMA PET/CT imaging in the diagnosis, staging and restaging of prostate cancer. Future Oncol 2021, 17(17): 2225-2241.
- Sidhu A, Singh P: Transformation of PSMA-avid to FDG-avid disease in metastatic castration-resistant prostate cancer in the setting of biochemical response with LU177-PSMA-617. Current Problems in Cancer: Case Reports 2025, 17: 100347.
- Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester H-J, Ross TL, Bengel FM, Derlin T: Initial experience with volumetric 68Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med 2017, 58(12): 1962-1968.
- Rovera G, Oprea-Lager DE, Ceci F: Health technology assessment for PSMA-PET: striving towards a cost-effective management of prostate cancer. Clin Transl Imaging 2021, 9(5): 409-412.
- Song R, Jeet V, Sharma R, Hoyle M, Parkinson B: Cost-effectiveness analysis of prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) for the primary staging of prostate cancer in Australia. Pharmacoeconomics 2022, 40(8): 807-821.
- Artibani W, Porcaro AB, De Marco V, Cerruto MA, Siracusano S: Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol Int 2018, 100(3): 251-262.
- Lin X, Kapoor A, Gu Y, Chow MJ, Xu H, Major P, Tang D: Assessment of biochemical recurrence of prostate cancer. Int J Oncol 2019, 55(6): 1194-1212.
- Lisney AR, Leitsmann C, Strauß A, Meller B, Bucerius JA, Sahlmann C-O: The role of PSMA PET/CT in the primary diagnosis and follow-up of prostate cancer—a practical clinical review. Cancers 2022, 14(15): 3638.
- Gilbert SM, Cavallo CB, Kahane H, Lowe FC: Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: review of 36,316 biopsies. Urology 2005, 65(3): 549-553.
- Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, Agoritsas T, Dahm P: Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ 2018, 362: k3519.
- Barbosa FG, Queiroz MA, Nunes RF, Viana PC, Marin JFG, Cerri GG, Buchpiguel CA: Revisiting prostate cancer recurrence with PSMA PET: atlas of typical and atypical patterns of spread. Radiographics 2019, 39(1): 186-212.
- Huang S, Ong S, McKenzie D, Mirabelli A, Chen DC, Chengodu T, Murphy DG, Hofman MS, Lawrentschuk N, Perera M: Comparison of 18F-based PSMA radiotracers with [68Ga] Ga-PSMA-11 in PET/CT imaging of prostate cancer—a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2024, 27(4): 654-664.
- Mertens K, Slaets D, Lambert B, Acou M, De Vos F, Goethals I: PET with 18F-labelled choline-based tracers for tumour imaging: a review of the literature. Eur J Nucl Med Mol Imaging 2010, 37(11): 2188-2193.
- Alberts IL, Seide SE, Mingels C, Bohn KP, Shi K, Zacho HD, Rominger A, Afshar-Oromieh A: Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: a systematic review and network meta-analysis. Eur J Nucl Med Mol Imaging 2021, 48(9): 2978-2989.
- Liu J, Cundy TP, Woon DT, Lawrentschuk N: A systematic review on artificial intelligence evaluating metastatic prostatic cancer and lymph nodes on psma pet scans. Cancers 2024, 16(3): 486.
- Ekmekcioglu Ö, Busstra M, Klass ND, Verzijlbergen F: Bridging the imaging gap: PSMA PET/CT has a high impact on treatment planning in prostate cancer patients with biochemical recurrence—a narrative review of the literature. J Nucl Med 2019, 60(10): 1394-1398.
- Wang L, Wang L, Wang Xa, Wu D: The Evolving Role of PSMA-PET/CT in Prostate Cancer Management: an Umbrella Review of Diagnostic Restaging, Therapeutic Redirection, and Survival Impact. Curr Oncol Rep 27(6): 774-787.
- Kase AM, Tan W, Copland III JA, Cai H, Parent EE, Madan RA: The continuum of metastatic prostate cancer: interpreting PSMA PET findings in recurrent prostate cancer. Cancers 2022, 14(6): 1361.
- Bluemel C, Linke F, Herrmann K, Simunovic I, Eiber M, Kestler C, Buck AK, Schirbel A, Bley TA, Wester H-J et al: Impact of 68Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI research 2016, 6(1): 78.
- Sabbagh A, Mohamad O, Lichter KE, Hope TA: Management of patients with recurrent and metachronous oligometastatic prostate cancer in the era of PSMA PET. Cancers 2022, 14(24): 6194.
- Afshar-Oromieh A, Debus N, Uhrig M, Hope TA, Evans MJ, Holland-Letz T, Giesel FL, Kopka K, Hadaschik B, Kratochwil C et al: Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging 2018, 45(12): 2045-2054.
- Kendrick J: Towards a quantitative management of metastatic prostate cancer using PSMA PET images. Doctor of Philosophy Thesis 2023.
- Lanfranchi F, Belgioia L, Vita D, Passoni J, Mastrogiovanni S, Catanoso A, Sofia L, Pau V, Raffa S, Chiola S et al: Baseline PSMA tumor volume as a prognostic marker in radical radiotherapy for prostate cancer: a propensity score-weighted retrospective analysis. Ann Nucl Med 2025, Epub ahead of print.:1-10.
- deSouza NM, Achten E, Alberich-Bayarri A, Bamberg F, Boellaard R, Clément O, Fournier L, Gallagher F, Golay X, Heussel CP et al: Validated imaging biomarkers as decision-making tools in clinical trials and routine practice: current status and recommendations from the EIBALL* subcommittee of the European Society of Radiology (ESR). Insights into imaging 2019, 10(1): 87.
- Hamdy FC, Donovan JL: Patient-reported outcomes following treatment for localized prostate cancer: helping decision making for patients and their physicians. JAMA 2017, 317(11): 1121-1123.
- Armbruster DA: Prostate-specific antigen: biochemistry, analytical methods, and clinical application. Clin Chem 1993, 39(2): 181-195.
- Evangelista L, Bertoldo F, Boccardo F, Conti G, Menchi I, Mungai F, Ricardi U, Bombardieri E: Diagnostic imaging to detect and evaluate response to therapy in bone metastases from prostate cancer: current modalities and new horizons. Eur J Nucl Med Mol Imaging 2016, 43(8): 1546-1562.
- Davies A, Conteduca V, Zoubeidi A, Beltran H: Biological evolution of castration-resistant prostate cancer. Eur Urol Focus 2019, 5(2): 147-154.
- Fanti S, Hadaschik B, Herrmann K: Proposal for systemic-therapy response-assessment criteria at the time of PSMA PET/CT imaging: the PSMA PET progression criteria. J Nucl Med 2020, 61(5): 678-682.
- Vaz S, Hadaschik B, Gabriel M, Herrmann K, Eiber M, Costa D: Influence of androgen deprivation therapy on PSMA expression and PSMA-ligand PET imaging of prostate cancer patients. Eur J Nucl Med Mol Imaging 2020, 47(1): 9-15.
- Gaertner FC, Halabi K, Ahmadzadehfar H, Kürpig S, Eppard E, Kotsikopoulos C, Liakos N, Bundschuh RA, Strunk H, Essler M: Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget 2017, 8(33): 55094.
- Karagiannis V, Wichmann V, Saarinen J, Eigeliene N, Andersen H, Jekunen A: Radiotherapy treatment modification for prostate cancer patients based on PSMA-PET/CT. Radiat Oncol 2022, 17(1): 19.
- Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, Horn T, Gschwend JE, Schwaiger M, Eiber M et al: Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging 2018, 45(4): 602-612.
- Jones W, Griffiths K, Barata PC, Paller CJ: PSMA theranostics: review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers 2020, 12(6): 1367.
- Shagera QA, Karfis I, Kristanto P, Spyridon S, Diamand R, Santapau A, Peltier A, Roumeguère T, Flamen P, Artigas C: PSMA PET/CT for response assessment and overall survival prediction in patients with metastatic castration-resistant prostate cancer treated with androgen receptor pathway inhibitors. J Nucl Med 2023, 64(12): 1869-1875.
- Fanti S, Goffin K, Hadaschik BA, Herrmann K, Maurer T, MacLennan S, Oprea-Lager DE, Oyen WJ, Rouvière O, Mottet N et al: Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur J Nucl Med Mol Imaging 2021, 48(2): 469-476.
- Cook GJ, Goh V: Molecular imaging of bone metastases and their response to therapy. J Nucl Med 2020, 61(6): 799-806.
- Seifert R, Gafita A, Telli T, Voter A, Herrmann K, Pomper M, Hadaschik B, Rowe SP, Fendler W: Standardized PSMA-PET imaging of advanced prostate cancer. Semin Nucl Med 2024, 54(1): 60-68.
- Roberts MJ, Maurer T, Perera M, Eiber M, Hope TA, Ost P, Siva S, Hofman MS, Murphy DG, Emmett L et al: Using PSMA imaging for prognostication in localized and advanced prostate cancer. Nat Rev Urol 2023, 20(1): 23-47.
- Yazdani E, Geramifar P, Karamzade-Ziarati N, Sadeghi M, Amini P, Rahmim A: Radiomics and artificial intelligence in radiotheranostics: a review of applications for radioligands targeting somatostatin receptors and prostate-specific membrane antigens. Diagnostics 2024, 14(2): 181.
- Zamboglou C, Bettermann AS, Gratzke C, Mix M, Ruf J, Kiefer S, Jilg CA, Benndorf M, Spohn S, Fassbender TF: Uncovering the invisible—prevalence, characteristics, and radiomics feature–based detection of visually undetectable intraprostatic tumor lesions in 68GaPSMA-11 PET images of patients with primary prostate cancer. Eur J Nucl Med Mol Imaging 2021, 48(6): 1987-1997.
- Moazemi S, Erle A, Khurshid Z, Lütje S, Muders M, Essler M, Schultz T, Bundschuh RA: Decision-support for treatment with 177Lu-PSMA: machine learning predicts response with high accuracy based on PSMA-PET/CT and clinical parameters. Ann Transl Med 2021, 9(9): 818.
- Wistuba II, Gelovani JG, Jacoby JJ, Davis SE, Herbst RS: Methodological and practical challenges for personalized cancer therapies. Nat Rev Clin Oncol 2011, 8(3): 135-141.
- Francone M, Budde RP, Bremerich J, Dacher JN, Loewe C, Wolf F, Natale L, Pontone G, Redheuil A, Vliegenthart R: CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting—a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol 2020, 30(5): 2627-2650.
- Bakht MK, Beltran H: Biological determinants of PSMA expression, regulation and heterogeneity in prostate cancer. Nat Rev Urol 2025, 22(1): 26-45.
- Sandhu K, Chen D, Hennes D, Murphy DG, Lawrentschuk N, Perera M: PSMA Theranostics in Prostate Cancer and Beyond: Current and Future Perspectives. Cancers 2025, 17(22): 3717.
- El Fakiri M, Geis NM, Ayada N, Eder M, Eder A-C: PSMA-targeting radiopharmaceuticals for prostate cancer therapy: recent developments and future perspectives. Cancers 2021, 13(16): 3967.
- Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, Haberkorn U: The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med 2016, 57(Supplement 3): 79S-89S.
- Evangelista L, Maurer T, van der Poel H, Alongi F, Kunikowska J, Laudicella R, Fanti S, Hofman MS: [68Ga] Ga-PSMA versus [18F] PSMA positron emission tomography/computed tomography in the staging of primary and recurrent prostate cancer. A systematic review of the literature. Eur Urol Oncol2022, 5(3): 273-282.
- Lengana T: 18F-PSMA-1007 in recurrent prostate cancer, a new frontier in prostate cancer PET imaging. University of Pretoria 2020, PhD Thesis.
- Luining WI, Cysouw MC, Meijer D, Hendrikse NH, Boellaard R, Vis AN, Oprea-Lager DE: Targeting PSMA revolutionizes the role of nuclear medicine in diagnosis and treatment of prostate cancer. Cancers 2022, 14(5): 1169.
- Mattana F, Muraglia L, Barone A, Colandrea M, Saker Diffalah Y, Provera S, Cascio AS, Omodeo Salè E, Ceci F: Prostate-specific membrane antigen-targeted therapy in prostate cancer: history, combination therapies, trials, and future perspective. Cancers 2024, 16(9): 1643.
- Aide N, Lasnon C, Desmonts C, Armstrong IS, Walker MD, McGowan DR: Advances in PET/CT technology: an update. Semin Nucl Med 2022, 52(3): 286-301.
- van der Vos CS, Arens AI, Hamill JJ, Hofmann C, Panin VY, Meeuwis AP, Visser EP, de Geus-Oei L-F: Metal artifact reduction of CT scans to improve PET/CT. J Nucl Med 2017, 58(11): 1867-1872.
- Fragkiadaki V, Ntanasis-Stathopoulos I, Liontos M, Zagouri F, Dimopoulos M-A, Gavriatopoulou M: Semiquantitative Analysis in PET/CT Imaging of Prostate Cancer. J Clin Med 2025, 14(11): 3828.
- Pool M, de Boer HR, Lub-de Hooge MN, van Vugt MA, de Vries EG: Harnessing integrative omics to facilitate molecular imaging of the human epidermal growth factor receptor family for precision medicine. Theranostics 2017, 7(7): 2111.
- Scobioala S, Kittel C, Wolters H, Huss S, Elsayad K, Seifert R, Stegger L, Weckesser M, Haverkamp U, Eich HT: Diagnostic efficiency of hybrid imaging using PSMA ligands, PET/CT, PET/MRI and MRI in identifying malignant prostate lesions. Ann Nucl Med 2021, 35(5): 628-638.
- Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, Eiber M, Haberkorn U, Hofman MS, Hope TA: E-PSMA: the EANM standardized reporting guidelines v1. 0 for PSMA-PET. Eur J Nucl Med Mol Imaging 2021, 48(5): 1626-1638.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript