Case Report | Open Access
Adenoid Cystic (Basal Cell) Carcinoma of the Prostate: A Rare Entity with Diagnostic Challenges and Therapeutic Uncertainty
Shabana Andleeb Ansari1, Mohd. Mubashir Ali Khan2, Azmat Kamal Ansari3, Beant Kaur1, Nagma Parveen1
1Department of Pathology, UPUMS Saifai, Etawah, Uttar Pradesh, India.
2Department of Urology, UPUMS Saifai, Etawah, Uttar Pradesh, India.
3Department of Biochemistry, UPUMS Saifai, Etawah, Uttar Pradesh, India.
Correspondence: Beant Kaur (Department of Pathology, UPUMS Saifai, Etawah, Uttar Pradesh, India; Email: drbeantpatho@gmail.com).
Annals of Urologic Oncology 2026, 9: 1. https://doi.org/10.32948/auo.2026.01.10
Received: 12 Dec 2025 | Accepted: 08 Jan 2026 | Published online: 18 Jan 2026
Case Report A 69-year-old man presented with hematuria. Digital rectal examination and ultrasonography revealed an enlarged, hard, nodular prostate, while serum prostate-specific antigen (PSA) levels were within normal limits. Transurethral resection of the prostate (TURP) chips revealed a malignant neoplasm composed of basaloid and cribriform patterns. Immunohistochemistry demonstrated diffuse tumor cell positivity for high-molecular-weight cytokeratin (HMWCK) and BCL2, with negativity for PSA, AMACR, GATA3, CK7, synaptophysin, CD56, and chromogranin. A high Ki-67 labeling index (60–65%) supported the diagnosis of adenoid cystic (basal cell) carcinoma.
Conclusion Prostatic ACC/BCC is a rare malignancy that can clinically and histologically mimic other prostatic tumors. Normal PSA levels, in conjunction with a characteristic immunophenotype, are critical for accurate diagnosis. Early recognition is essential given the tumor’s variable biological behavior and potential for aggressive progression.
Key words adenoid cystic carcinoma, basal cell carcinoma, prostate, immunohistochemistry, PSA
Here, we report a case of prostatic adenoid cystic (basal cell) carcinoma in a 69-year-old man presenting with hematuria, treated with radical prostatectomy and remaining clinically stable at six months of follow-up.
Grossly, approximately 8 g of prostatic tissue was received, aggregating to 5.5 × 3.5 × 1.5 cm. Microscopic examination revealed a malignant neoplasm composed of basaloid cells arranged in solid and cribriform patterns, with peripheral palisading. The tumor cells exhibited moderate nuclear pleomorphism and frequent mitotic figures. Foci of necrosis and perineural invasion were identified (Figure 1A, 1B). Figure 1A illustrates solid basaloid tumor nests with central comedo-type necrosis, while Figure 1B highlights marked cytological atypia with frequent mitotic figures at higher magnification.
On immunohistochemistry, tumor cells showed diffuse and strong positivity for HMWCK (Figure 2A), confirming basal cell differentiation, and BCL2 (Figure 2B), supporting a diagnosis of basal cell carcinoma of the prostate. Staining was negative for PSA, AMACR, GATA3, CK7, synaptophysin, CD56, and chromogranin, thereby excluding prostatic adenocarcinoma, urothelial carcinoma, and neuroendocrine neoplasms. The Ki-67 proliferative index was markedly elevated (60-65%) (Figure 2C), indicating a high proliferative activity and supporting a diagnosis of adenoid cystic (basal cell) carcinoma of the prostate. The patient subsequently underwent radical prostatectomy and remained clinically stable without evidence of distant metastasis at six months of follow-up.
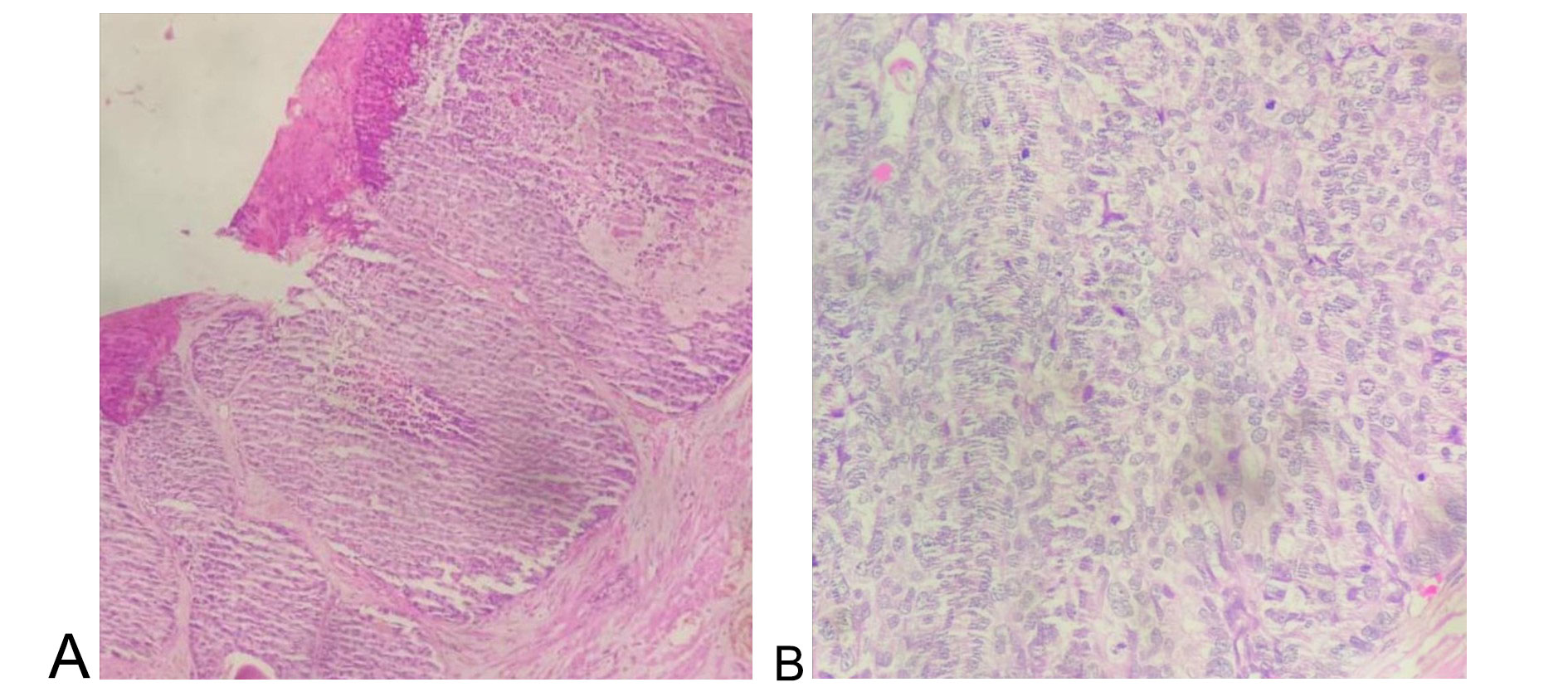 Figure 1. Histomorphology of adenoid cystic (basal cell) carcinoma of the prostate. (A) Tumour cells are arranged in basaloid pattern with central area of necrosis (100X), (B) Tumour cells are showing moderate pleomorphism with frequent mitotic figures (400X).
Figure 1. Histomorphology of adenoid cystic (basal cell) carcinoma of the prostate. (A) Tumour cells are arranged in basaloid pattern with central area of necrosis (100X), (B) Tumour cells are showing moderate pleomorphism with frequent mitotic figures (400X).
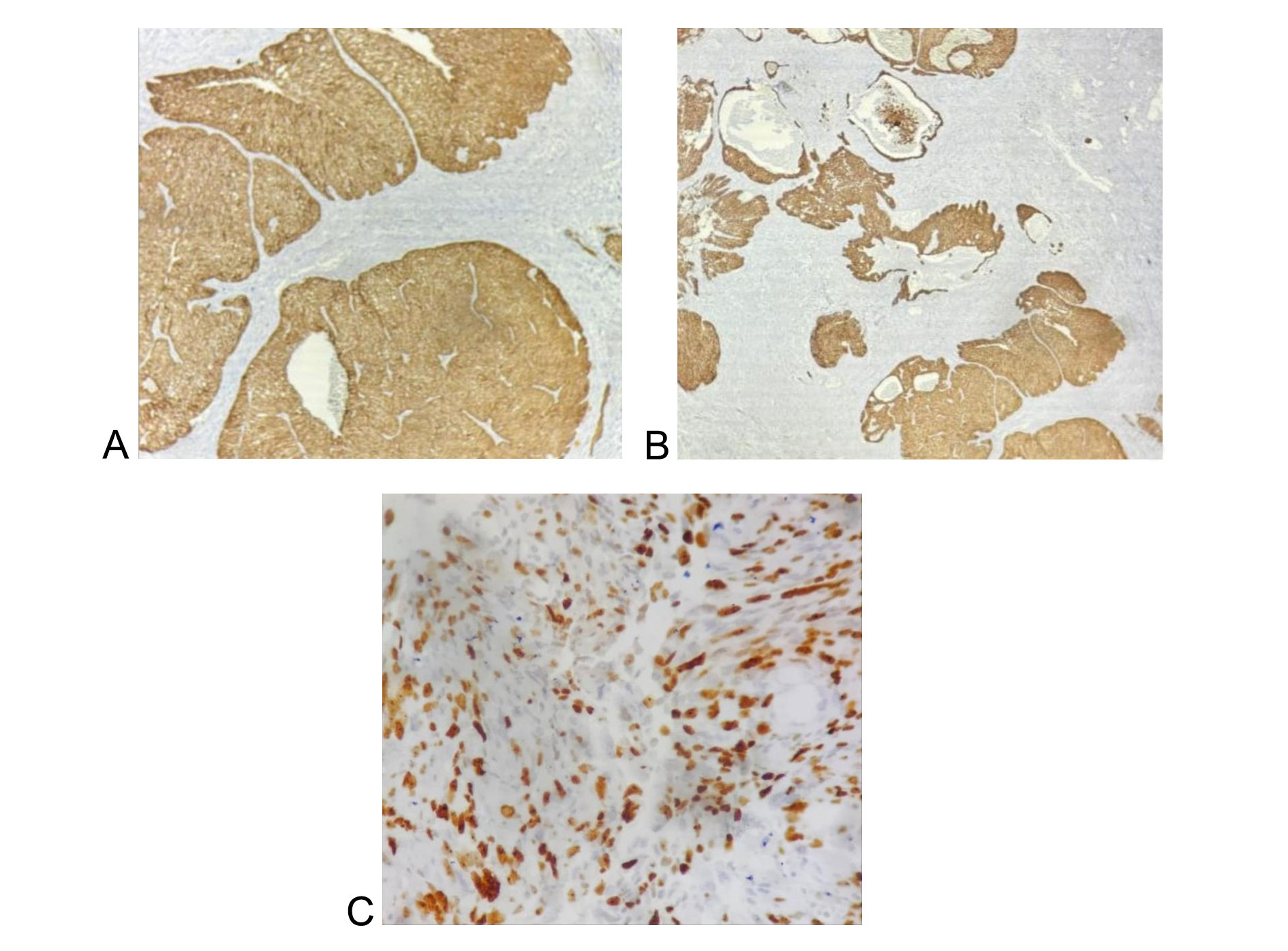 Figure 2. Immunohistochemistry of adenoid cystic (basal cell) carcinoma of the prostate. (A) Tumour cells are showing diffuse and strong positivity for HMWCK (400X), (B) Tumour cells are showing diffuse and strong positivity for Bcl2 (100X), (C) Tumour cells are showing high Ki67 proliferative index (60-65%) (400X).
Figure 2. Immunohistochemistry of adenoid cystic (basal cell) carcinoma of the prostate. (A) Tumour cells are showing diffuse and strong positivity for HMWCK (400X), (B) Tumour cells are showing diffuse and strong positivity for Bcl2 (100X), (C) Tumour cells are showing high Ki67 proliferative index (60-65%) (400X).
Shibuya et al. reported a mean patient age of 65.3 ± 13.6 years [9], which is consistent with the age of our patient (69 years). One of the most important differential diagnoses of prostatic BCC is basal cell hyperplasia. Histopathological evaluation is pivotal in this distinction, as carcinoma demonstrates infiltrative growth, architectural complexity, cytological atypia, increased mitotic activity, necrosis, a desmoplastic stromal response, and a high Ki-67 proliferative index.
Basal cell carcinoma of the prostate exhibits considerable morphological heterogeneity. It may resemble salivary gland ACC with a cribriform architecture or present as a basaloid carcinoma analogous to cutaneous basal cell carcinoma [10, 11]. Some tumors show a single predominant histologic pattern, whereas others demonstrate a combination of basaloid and adenoid cystic components. Solid and basaloid growth patterns have been associated with more aggressive biological behavior [1, 9]. In a clinicopathological evaluation of 29 cases, Ali et al. described small solid nests with peripheral palisading and identified the adenoid cystic pattern as the predominant architecture [12], findings also supported by Shibuya et al. [9]. McKenney et al. reported extensive intraglandular hyalinization and infiltrative growth with extraprostatic extension and perineural invasion in basaloid carcinomas [13]. In the present case, both solid growth with necrosis and basaloid architecture were observed, suggesting a potentially aggressive phenotype.
Unlike conventional prostatic adenocarcinoma, ACC/BCC of the prostate typically presents with normal PSA levels. This observation in our patient is consistent with multiple published reports [9, 14-18]. Although previously regarded as an indolent tumor, recent studies have demonstrated variable clinical behavior. Shibuya et al. reported disease progression, including recurrence and metastasis, in 37% of patients [9], underscoring the malignant potential of this entity.
Treatment strategies for basal cell carcinoma of the prostate have included surgery, radiotherapy, chemotherapy, and hormonal therapy. Owing to the rarity of this entity, management recommendations are largely extrapolated from small case series and retrospective reviews. In a comprehensive review and discussion by Currò et al. [5], radical surgical excision was favored for organ-confined disease, as it provides optimal local control and allows accurate pathological staging. Radiotherapy has been proposed as an alternative in non-surgical candidates or in cases with locally advanced disease; however, long-term outcome data remain limited. Chemotherapy and androgen deprivation therapy have shown inconsistent responses and are generally reserved for metastatic or refractory disease.
In the present case, radical prostatectomy was selected due to localized disease, absence of distant metastasis, normal PSA levels, and the presence of high-risk histological features, including a solid basaloid growth pattern, necrosis, perineural invasion, and a markedly elevated Ki-67 proliferative index. These features have been associated with aggressive behavior and an increased risk of recurrence in published series. The patient was placed on close postoperative surveillance, consistent with recommendations by Currò et al., given reports of delayed local recurrence and distant metastasis even after apparently complete resection. Surveillance includes periodic clinical assessment and radiological imaging rather than PSA-based monitoring, as PSA levels are typically uninformative in basal cell carcinoma of the prostate [9, 18].
Systemic chemotherapy has been the most frequently employed treatment modality, with highly variable outcomes. Several cases demonstrated disease progression despite docetaxel-based or platinum-based regimens, even after only two treatment cycles. In contrast, occasional responses have been reported, including significant regression of pulmonary metastases with etoposide after failure of docetaxel-based therapy and prolonged survival following docetaxel treatment in select patients [19]. Table 1 depicts the brief literature review of the case reports published in the recent literature.
|
Table 1. The brief literature review of the case reports published in the recent literature. |
|||
|
Author (year) |
Age |
PSA (ng/ml) |
Treatment |
|
Ryan P. et al (2022) [19] |
72 |
1.2ng/mL |
Sunitinib |
|
Trinh JQ. et al (2023) [1] |
80 |
NA- |
Combination chemotherapy (carboplatin and paclitaxel) |
|
Glavinov MS. et al (2024) [20] |
63 |
0,657 |
Radiotherapy (RT) |
|
He, L.et al (2021) [21] |
92 |
<0.05 |
TURP plus RT |
|
Ridai, S.et al (2021) [22] |
40 |
3.5 |
TURP plus concurrent CT (Cisplatin)-RT |
|
Dong, S.et al (2020) [23]
|
62
|
Normal range |
Radical Prostatectomy (RP) + RT |
|
Julka, P.K.et al (2020) [24] |
79 |
NA |
TURP plus CT (Carboplatin Paclitaxel) then ADT (Degarelix) |
|
Shibuya, T.et al (2018) [25] |
68 |
Normal range |
RP |
|
Bernhardt, D. et al (2018) [26]
|
Case 1: 65 Case 2: 44 |
Normal range
|
TURP plus RP plus RT as photon IMRT plus C12 heavy ion boost |
|
Zhang, M. et al (2016) [27] |
73 |
1.9 |
Pelvic exenteration |
|
Simper, N.B. (2015) [28] |
57–97 |
NA |
TURP (6 pts), Pelvic exenteration (1 pt), RP (2 pts) |
|
Bishop, J.A.(2015) [29] |
65–86 |
NA |
TURP |
|
NA: Not applicable; TURP: Transurethral resection of the prostate; CT: Chemotherapy; RP: Radical prostatectomy; RT: Radiotherapy; ADT: Androgen deprivation therapy. |
|||
None.
Ethical policy
The study was done in accordance with the Declaration of Helsinki. Informed patient consent was taken.
Availability of data and materials
That data is available from the corresponding author on request.
Author contributions
Shabana Andleeb Ansari: conceptualisation, data curation, formal analysis, writing original draft; Mohd. Mubashir Ali Khan: conceptualisation, formal analysis, review and writing; Azmat Kamal Ansari: conceptualisation, resources, data curation, supervision, review and editing; Beant Kaur: conceptualisation, formal analysis, review, writing and editing; Nagma Parveen: conceptualisation, formal analysis, review, writing and editing.
Competing interests
The authors declare that they have no competing interests.
Funding
None.
- Trinh JQ, Lele SM, Teply BA: A case of metastatic adenoid cystic (basal cell) carcinoma of the prostate: systemic therapy for a rare disease. Prostate 2023; 83(8): 814‐819.
- Ahuja A, Das P, Kumar N, Saini AK, Seth A, Ray R: Adenoid cystic carcinoma of the prostate: case report on a rare entity and review of the literature. Pathol Res Pract 2011, 207(6): 391-394.
- Woida FM, Ribeiro-Silva A: Adenoid cystic carcinoma of the Bartholin gland: An overview. Arch Pathol Lab Med 2007, 131(5): 796-798.
- Ramakrishnan R, Chaudhry IH, Ramdial P, Lazar AJ, McMenamin ME, Kazakov D, Brenn T, Calonje E: Primary cutaneous adenoid cystic carcinoma: A clinicopathologic and immunohistochemical study of 27 cases. Am J Surg Pathol 2013, 37(10): 1603-1611.
- Cozzi S, Bardoscia L, Najafi M, Botti A, Blandino G, Augugliaro M, Manicone M, Iori F, Giaccherini L, Sardaro A et al: Adenoid cystic carcinoma/basal cell carcinoma of the prostate: overview and update on rare prostate cancer subtypes. Curr Oncol 2022, 29(3): 1866‐1876.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71(3): 209‐249.
- Siegel RL, Miller KD, Fuchs HE, Jemal A: Cancer statistics, 2022. CA Cancer J Clin 2022, 72(1): 7‐33.
- Humphrey PA: Histological variants of prostatic carcinoma and their significance. Histopathology 2012, 60(1): 59‐74.
- Shibuya T, Takahashi G, Kan T: Basal cell carcinoma of the prostate: a case report and review of the literature. Mol Clin Oncol 2019, 10(1): 101‐104.
- Ayyathurai R, Civantos F, Soloway MS and Manoharan M: Basal cell carcinoma of the prostate: Current concepts. BJU Int 2007, 99(6): 1345-1349.
- Begnami MD, Quezado M, Pinto P, Linehan WM and Merino M: Adenoid cystic/basal cell carcinoma of the prostate: Review and update. Arch Pathol Lab Med 2007, 131(4): 637-640.
- Ali TZ, Epstein JI: Basal cell carcinoma of the prostate: A clinicopathologic study of 29 cases. Am J Surg Pathol 2007, 31(5): 697-705.
- McKenney JK, Amin MB, Srigley JR, Jimenez RE, Ro JY, Grignon DJ, Young RH: Basal cell proliferations of the prostate other than usual basal cell hyperplasia: A clinicopathologic study of 23 cases, including four carcinomas, with a proposed classification. Am J Surg Pathol 2004, 28(10): 1289-1298.
- Denholm SW, Webb JN, Howard GC, Chisholm GD: Basaloid carcinoma of the prostate gland: Histogenesis and review of the literature. Histopathology 1992, 20(2): 151-155.
- Bohn OL, Rios-Luna NP, Navarro L, Duran-Peña A, Sanchez-Sosa S: Basal cell carcinoma of the prostate gland: A case report and brief review of the basal cell proliferations of the prostate gland. Ann Diagn Pathol 2010, 14(5): 365-368.
- Schmid HP, Semjonow A, Eltze E, Wörtler K, Hertle L: Late recurrence of Adenoid cystic carcinoma of the prostate. Scand J Urol Nephrol 2002, 36(2): 158-159.
- Komura K, Inamoto T, Tsuji M, Ibuki N, Koyama K, Ubai T, Azuma H, Katsuoka Y: Basal cell carcinoma of the prostate: Unusual subtype of prostatic carcinoma. Int J Clin Oncol 2010, 15(6): 594-600.
- Tuan J, Pandha H, Corbishley C, Khoo V: Basaloid carcinoma of the prostate: A literature review with case report. Indian J Urol 2012, 28(3): 322-324.
- Ryan P, Kelly C, Shanahan S, Jordan E, Keane J, Daly P: Adenoid cystic carcinoma of the prostate–a rare case of genitourinary malignancy. Urol Case Rep 2022, 42: 102025.
- Glavinov MS, Krsteska B, Stojmenova V, Petrovska T, Jovanovic R. Adenoid cystic/basal-cell carcinoma of the prostate following high-grade urothelial bladder cancer: a case report. Oxf Med Case Reports 2024, 2024(5): omae050.
- He L, Metter C, Margulis V, Kapur P: A review leveraging a rare and unusual case of basal cell carcinoma of the prostate. Case Rep Pathol 2021, 2021: 5520581.
- Ridai S, Moustakbal C, Lachgar A, Jouhadi H, Benider A, Regragui M, Marnissi F: Prostatic basal cell carcinoma treated by chemoradiation with weekly cisplatine: Case report and literature review. Afr J Urol 2021, 27: 79.
- Dong S, Liu Q, Xu Z, Wang H: An unusual case of metastatic basal cell carcinoma of the prostate: A case report and literature review. Front Oncol 2020, 10: 859.
- Julka PK, Verma A, Gupta S, Gupta K, Rathod R: Adenoid cystic carcinoma of the prostate: An unusual subtype of prostate cancer. J Transl Genet Genom 2020, 4: 455-463.
- Shibuya T, Takahashi G, Kan T: Basal cell carcinoma of the prostate: A case report and review of the literature. Mol Clin Oncol 2019, 10(1): 101-104.
- Bernhardt D, Sterzing F, Adeberg S, Herfarth K, Katayama S, Foerster R, Hoerner-Rieber J, König L, Debus J, Rieken S: Bimodality treatment of patients with pelvic adenoid cystic carcinoma with photon intensitymodulated radiotherapy plus carbon ion boost: A case series. Cancer Manag Res 2018, 10: 583-588.
- Zhang M, Pettaway C, Vikram R, Tamboli P: Adenoid cystic carcinoma of the urethra/Cowper’s gland with concurrent high-grade prostatic adenocarcinoma: A detailed clinicopathologic case report and review of the literature. Hum Pathol 2016, 58: 138-144.
- Simper NB, Jones CL, MacLennan GT, Montironi R, Williamson SR, Osunkoya AO, Wang M, Zhang S, Grignon DJ, Eble JN: Basal cell carcinoma of the prostate is an aggressive tumor with frequent loss of PTEN expression and overexpression of EGFR. Hum Pathol 2015, 46(6): 805-812.
- Bishop JA, Yonescu R, Epstein JI, Westra WH: A subset of prostatic basal cell carcinomas harbor the MYB rearrangement of adenoid cystic carcinoma. Hum Pathol 2015, 46(8): 1204-1208.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript