Review Article | Open Access
The Concomitant Use of Angiotensin System Inhibitors Predicts Favorable Prognosis for Renal Cell Carcinoma Patients
Shuenqin Hu1, Xiuying Li21Department of Gynecology and Obstetrics, the First Affiliated Hospital of Kunming Medical University, Kunming, China.
2Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, China.
Correspondence: Xiuying Li (Faculty of Life Science and Technology, Kunming University of Science and Technology, Kunming, Yunnan, 650093, China; E-mail: xiuyingli2013@kust.edu.cn).
Annals of Urologic Oncology 2025, 8(2): 68-75. https://doi.org/10.32948/auo.2025.08.18
Received: 03 Aug 2025 | Accepted: 18 Aug 2025 | Published online: 28 Aug 2025
Methods We conducted a comprehensive search of electronic databases, including PubMed and Web of Science, to identify relevant studies according to the predefined inclusion and exclusion criteria. The pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using either random-effects or fixed-effects models, as appropriate.
Results Favourable overall survival (OS) was observed in patients using ASIs compared with non-users (hazard ratio [HR] = 0.68, 95% CI: 0.57–0.81; p = 0.000). In the subgroup analysis, OS benefits were evident across treatment agents, the time window of ASI use, and in patients with metastatic renal cell carcinoma (mRCC). Moreover, progression-free survival (PFS) and disease-specific survival (DSS) benefits were observed in ASI users compared to non-users (HR = 0.74, 95% CI: 0.62–0.87; p = 0.000). Additionally, consistent DSS and PFS advantages were noted across treatment agents, mRCC, and the time window for ASI use.
Conclusion Our findings indicate that concomitant use of ASIs is significantly associated with improved survival outcomes in patients with RCC. Further high-quality studies are required to validate these conclusions.
Key words renal cell carcinoma, angiotensin system inhibitors, meta-analysis
A substantial body of preclinical evidence suggests that ASIs possess strong immunomodulatory activities and play an important role in shaping the immunosuppressive tumor microenvironment [3-5]. Several clinical trials and retrospective studies have indicated that the use of ASIs can enhance the efficacy of antiangiogenic agents, immune checkpoint inhibitors (ICIs), and chemotherapy in patients with cancer [3, 6, 7]. However, other retrospective studies have reported no significant association between ASI use and overall survival [8, 9]. Previous meta-analyses have primarily examined the relationship between ASI use and the risk of developing renal cancer [10, 11]. To date, only one meta-analysis based on two studies with very limited sample size has investigated the prognosis of ASI use versus non-use in patients with RCC [12].
Renal cancer is widely recognized as a significant public health problem. The morbidity and mortality rates are increasing, and the prognosis remains poor. Therefore, development of new and effective therapeutic strategies is critical. Renal cell carcinoma (RCC) is the most common form of renal cancer, and approximately 25–30% of RCC patients are diagnosed at a locally advanced or metastatic stage [13]. Furthermore, recurrence occurs in 30–40% of patients, even after successful treatment of localized disease [14]. Given these challenges, the relationship between ASI use and prognosis in RCC patients warrants further investigation. Therefore, we performed this meta-analysis using all available studies to assess the prognostic value of ASI use in RCC patients.
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [15]. The Web of Science and PubMed electronic databases were searched for eligible studies published until 15 April 2025. The search terms used were: (angiotensin II OR angiotensin-converting enzyme OR renin-angiotensin OR losartan OR valsartan OR candesartan OR eprosartan OR telmisartan OR captopril OR enalapril OR fosinopril) AND (renal) AND (cancer OR carcinoma OR neoplasm OR tumor) AND (patient). The reference lists of relevant studies and previous systematic reviews were manually screened to identify additional eligible publications.
Inclusion and exclusion criteria
Articles written in English that evaluated the survival outcomes of patients with RCC treated with ASIs versus those not receiving ASIs were included. The most comprehensive and recent study was selected when overlapping data appeared in the multiple studies. The outcomes of interest were defined as hazard ratios (HRs) with 95% confidence intervals (CIs) for disease-specific survival (DSS), progression-free survival (PFS), and overall survival (OS). The exclusion criteria were as follows: (1) preclinical studies, letters, meeting abstracts or summaries, reviews, case reports, or comments; (2) studies in which data for statistical analysis were not accessible; and (3) duplicate publications.
Data extraction and quality assessment
Data extraction was independently performed by two investigators based on predefined inclusion and exclusion criteria, and any disagreements were resolved through discussion. The extracted data included the first author’s name, country, publication year, cancer stage, time window of ASI use, total number of patients, number of patients using ASIs, treatment agents, and follow-up duration. This meta-analysis focused on two primary outcomes: The first was long-term survival, defined as overall survival (OS), and the second was short-term survival, including progression-free survival (PFS) and disease-specific survival (DSS). To minimise the influence of confounding factors, multivariate results were preferentially used for meta-analysis when both univariate and multivariate results were available. The Newcastle–Ottawa Scale (NOS) [16], which comprises three domains (outcome assessment, comparability, and selection), was used to evaluate the quality of the included studies. Studies with NOS scores greater than 6 were considered high quality.
Statistical analysis
Pooled hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for OS and DSS/PFS were calculated to compare outcomes between ASI users and non-users. HR < 1 indicated a beneficial effect of ASI use, whereas HR > 1 indicated a detrimental effect of ASI use. The fixed-effects model (Mantel–Haenszel method) was applied when no significant heterogeneity was detected, and the random-effects model (DerSimonian–Laird method) was used in the presence of significant heterogeneity. Heterogeneity among studies was assessed using Higgins’ I² statistic [17] and Cochran’s Q test [18]; a chi-square p-value < 0.10 or I² > 50% was considered indicative of heterogeneity. Publication bias was evaluated using Egger’s and Begg’s tests, with p-values > 0.05 suggesting no publication bias. Sensitivity analyses were conducted by sequentially removing the individual studies to assess the robustness of the pooled results. Potential sources of heterogeneity were further examined through subgroup analyses based on treatment agents, metastatic RCC (mRCC), and time window of ASI use. Meta-analysis was performed using STATA software (version 12.0), with a p-value < 0.05 considered statistically significant.
Initially, 1,924 papers related to the search terms were identified after removing duplicates. Of these, 1,905 papers were excluded after reviewing their titles and abstracts according to the exclusion criteria. The remaining 19 studies were retrieved for full-text review, and 10 of these were excluded for the following reasons: five studies explored the relationship between antihypertensive treatment and the risk of cancer; four studies assessed the concomitant use of various antihypertensive medications (including angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, calcium-channel blockers, beta-blockers, diuretics, or any antihypertensive medications) and cancer outcomes, but did not specifically evaluate the prognostic association between ASI use and RCC; and one study investigated the potential clinical benefit of combining PD-1/L1 inhibitors with renin–angiotensin system inhibitors in patients with metastatic renal cell carcinoma but lacked the necessary data for analysis [19]. Ultimately, nine studies were included in this meta-analysis [8, 20-27]. All nine studies were retrospective in nature. The studies by McKay, Fiala, and Nuzzo each included two cohorts [22, 24, 27], which were treated as two separate reports in the analysis. The literature search process is illustrated in Figure 1.
Study characteristics
All eligible studies will be published between 2011 and 2022. A total of 8,688 patients were included (range, 127-4,736 per study), of whom 8,131 were assessed for OS and 7,824 for DSS/PFS. Among them, 2,595 patients with RCC received ASIs, whereas 6,093 did not. All the included studies were hospital-based retrospective analyses. Of the nine studies, seven focused on metastatic renal cell carcinoma (mRCC). The time window for ASI use was reported either before or at baseline. Baseline was defined as ASI use at the time of treatment initiation and before referral to treatment initiation prior to the diagnosis of the tumour. Baseline characteristics and quality assessments of the included studies are presented in Table 1.
Pooled OS and DSS/PFS
Some studies have reported a significant positive effect of concomitant ASI use on DSS/PFS and OS in patients with RCC, whereas others have drawn the opposite conclusion. A meta-analysis was performed to address these conflicting results. The pooled HR for OS comparing ASI users and non-users was calculated based on eight studies involving 8,131 patients. Owing to the significant heterogeneity among the studies (I² = 72.7%, p = 0.000), a random-effects model was applied to estimate the combined HR and 95% CI. The results indicated that OS was significantly improved in patients with RCC using ASIs compared to those not using ASIs (HR = 0.68, 95% CI: 0.57–0.81; p = 0.000) (Figure 2a).
The pooled HR for DSS/PFS comparing ASI users with non-users was calculated based on seven studies comprising 7,824 patients. Compared with patients who did not use ASIs, the results indicated that ASI use reduced the risk of disease progression, with a pooled HR for DSS/PFS of 0.74 (95% CI: 0.62–0.87; p = 0.000) (Figure 2b).
Subgroup analysis
To explore the potential sources of heterogeneity, subgroup analyses were conducted based on treatment agents, time window of ASI use, and mRCC status. A survival benefit for OS was observed with concomitant use of ASIs and sunitinib or ICIs. Patients who used ASIs at baseline or both before and at baseline showed a significantly improved OS. In patients with mRCC, ASI use was also associated with significantly better OS (Table 2). Furthermore, ASI use was consistently associated with improved DSS/PFS across subgroups (Table 3).
Publication bias analysis
Egger’s test and Begg’s test were performed to assess the presence of potential publication bias in this meta-analysis. For OS analysis, Begg’s test (Figure 3a) and Egger’s test (Figure 3b) indicated the existence of publication bias. After removing the study by Penttilä P [20], the publication bias disappeared, suggesting that this study may have been the source of bias.
Publication bias was also detected in the subgroup analysis based on the time window of ASI use. To adjust the pooled estimates, a Trim and Fill analysis was performed [28], which indicated that two studies were likely missing. However, the overall effect estimate remained unchanged after adjustment. For the DSS/PFS analysis, the Begg’s test (Figure 4a) and Egger’s test (Figure 4b) demonstrated no evidence of publication bias.
Sensitivity analysis
To assess the robustness of the results, each study was subjected to sensitivity analysis to evaluate its influence on the overall findings. The analysis demonstrated that the OS (Figure 5a) and DSS/PFS (Figure 5b) in ASI users were stable. The sequential one-by-one exclusion of individual studies did not significantly alter the pooled HRs or 95% CIs. These findings suggest that the results of this meta-analysis are robust.
|
Table 1. Baseline characteristics of included studies. |
||||||||
|
Author |
Year |
Stage |
Study period |
No. ASIs/ Total patients |
Treatment agents |
Time window of ASIs use |
Follow-up (months) |
QS |
|
Penttilä P [19] |
2017 |
M |
2006-2014 |
126/303 |
sunitinib or pazopanib |
baseline and before |
55.3 |
7 |
|
Keizman D [20] |
2011 |
M |
2004-2010 |
44/127 |
sunitinib |
baseline |
NA |
7 |
|
Sorich MJ [8] |
2015 |
M |
NA |
385/1545 |
sunitinib or pazopanib |
baseline |
36 |
8 |
|
McKay RR [21] |
2015 |
M |
2003-2013 |
1487/4736 |
Sunitinib/sorafenib/axitinib/temsirolimus/IFNa/ bevacizumab |
baseline |
NA |
9 |
|
Izzedine H [22] |
2015 |
M |
2004-2013 |
105/213 |
sunitinib |
baseline and before |
43.2 |
8 |
|
Fiala O [23] |
2021 |
M |
2007-2020 |
172/343 |
sunitinib or pazopanib |
baseline and before |
19.9 |
7 |
|
Kostine M [24] |
2021 |
A |
2015-5017 |
83/635 |
ICIs |
baseline and before |
24 |
6 |
|
Miyajima A [25] |
2015 |
G1-G3 |
1996-2009 |
104/557 |
surgery |
baseline and before |
79.2 |
7 |
|
Nuzzo PV (1) [26] |
2022 |
M |
2015-2019 |
30/100 |
ICIs |
baseline and before |
45.6 |
7 |
|
Nuzzo PV (2) [26] |
2022 |
M |
2015-2019 |
59/129 |
ICIs |
baseline and before |
27.6 |
7 |
|
ICIs: immune checkpoint inhibitors; NA: not available. Data source of Nuzzo PV (1) came from Dana-Farber Cancer Institute. Data source of Nuzzo PV (2) came from University of California San Diego. M: metastatic; A: advanced. QS: Quality score. |
||||||||
|
Stratified analysis |
No. of study |
Pooled HR (95%CI) |
p |
Effects model |
Heterogeneity |
||
|
I2 (%) |
Ph |
||||||
|
Total |
8 |
0.68(0.57-0.81) |
0.000 |
Random |
72.7 |
0.000 |
|
|
Deleted the study of Penttilä P |
7 |
0.75(0.65-0.87) |
0.000 |
Random |
56.9 |
0.013 |
|
|
Treatment agents |
Sunitinib or pazopanib |
3 |
0.68(0.45-1.02) |
0.064 |
Random |
85.8 |
0.000 |
|
Sunitinib |
2 |
0.50(0.34-0.74) |
0.000 |
Fixed |
47.1 |
0.169 |
|
|
ICIs |
2 |
0.56(0.37-0.84) |
0.002 |
Fixed |
21.5 |
0.280 |
|
|
mRRC |
Total |
7 |
0.67(0.56-0.81) |
0.000 |
Random |
75.4 |
0.000 |
|
Before and baseline |
4 |
0.51(0.34-0.78) |
0.002 |
Random |
79.8 |
0.000 |
|
|
Baseline |
3 |
0.82(0.75-0.89) |
0.000 |
Fixed |
45.7 |
0.137 |
|
|
The time window of ASIs use |
Before and baseline |
5 |
0.54(0.37-0.78) |
0.001 |
Random |
75.9 |
0.000 |
|
Baseline |
3 |
0.82(0.75-0.89) |
0.000 |
Fixed |
45.7 |
0.137 |
|
|
Stratified analysis |
No. of study |
Pooled HR (95%CI) |
p |
Effects model |
Heterogeneity |
||
|
I2 (%) |
Ph |
||||||
|
Total |
7 |
0.74(0.62-0.87) |
0.000 |
Random |
76.1 |
0.000 |
|
|
Treatment agents |
Sunitinib or pazopanib |
3 |
0.66(0.46-0.94) |
0.020 |
Random |
84.4 |
0.000 |
|
Sunitinib |
2 |
0.54(0.40-0.74) |
0.000 |
Fixed |
0.0 |
0.940 |
|
|
mRRC |
Total |
6 |
0.73(0.61-0.86) |
0.000 |
Random |
77.4 |
0.000 |
|
Before and baseline |
3 |
0.57(0.38-0.86) |
0.008 |
Random |
81.0 |
0.001 |
|
|
Baseline |
3 |
0.88(0.82-0.94) |
0.000 |
Fixed |
41.8 |
0.161 |
|
|
The time window of ASIs use |
Before and baseline |
4 |
0.63(0.42-0.96) |
0.033 |
Random |
79.4 |
0.001 |
|
Baseline |
3 |
0.88(0.82-0.94) |
0.000 |
Fixed |
41.8 |
0.161 |
|
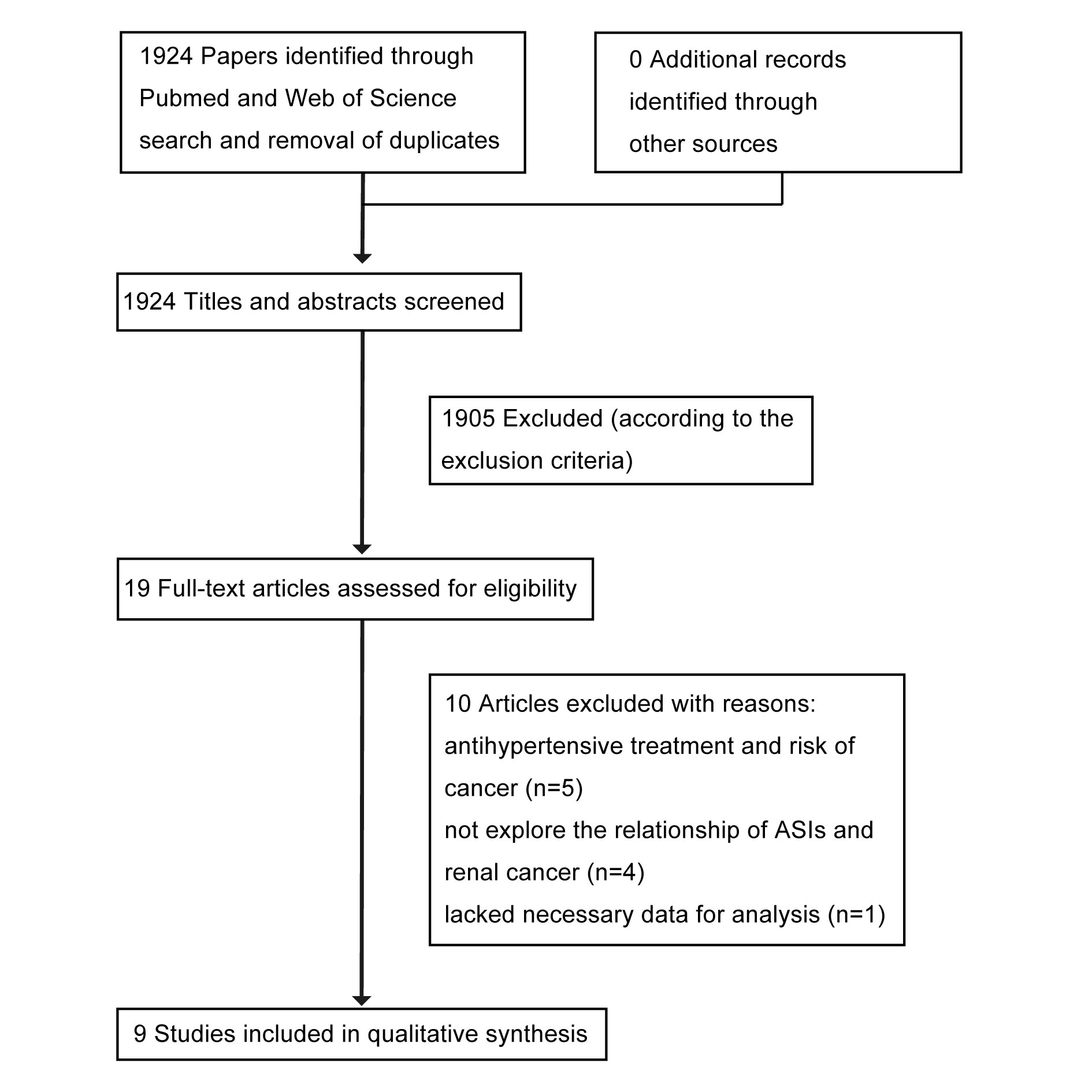 Figure 1. Flow chart summarizing the selection process of studies in this meta-analysis.
Figure 1. Flow chart summarizing the selection process of studies in this meta-analysis.
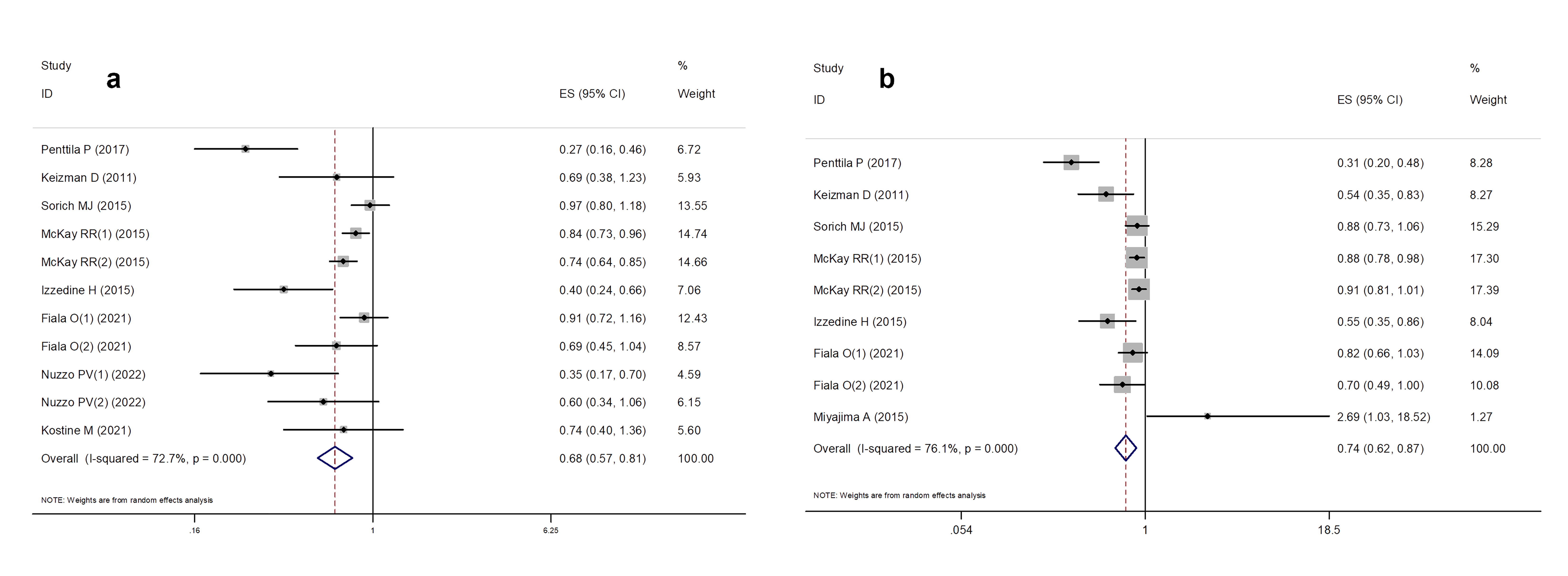 Figure 2. Forest plot of meta-analysis results. (a) the association between OS and ASIs use; (b) the association between DFS/PFS and ASIs use.
Figure 2. Forest plot of meta-analysis results. (a) the association between OS and ASIs use; (b) the association between DFS/PFS and ASIs use.
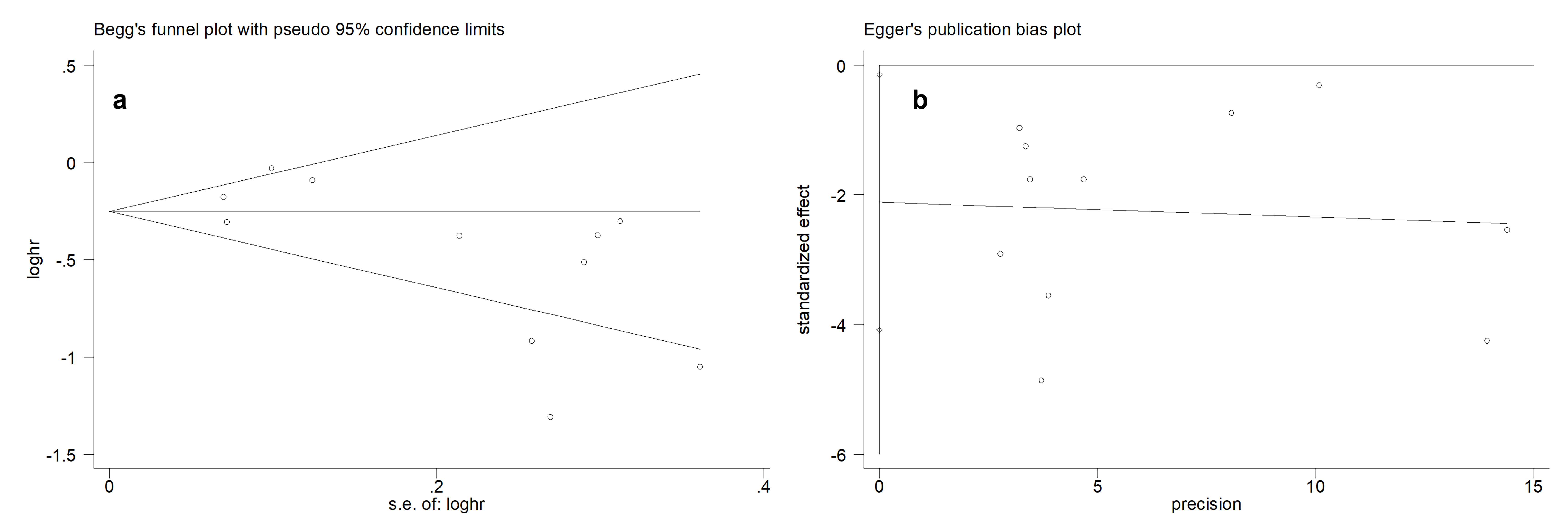 Figure 3. Publication bias test for OS. (a) Begg’s funnel plot. (b) Egger’s publication bias plot.
Figure 3. Publication bias test for OS. (a) Begg’s funnel plot. (b) Egger’s publication bias plot.
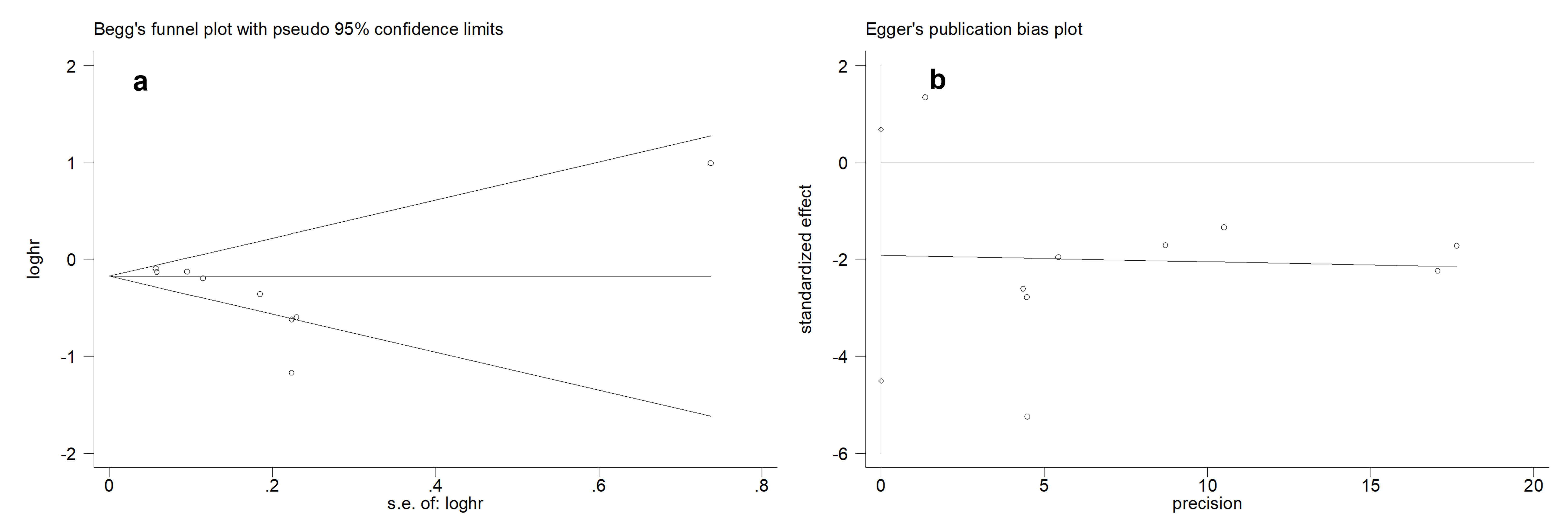 Figure 4. Publication bias test for DSS/PFS. (a) Begg’s funnel plot. (b) Egger’s publication bias plot.
Figure 4. Publication bias test for DSS/PFS. (a) Begg’s funnel plot. (b) Egger’s publication bias plot.
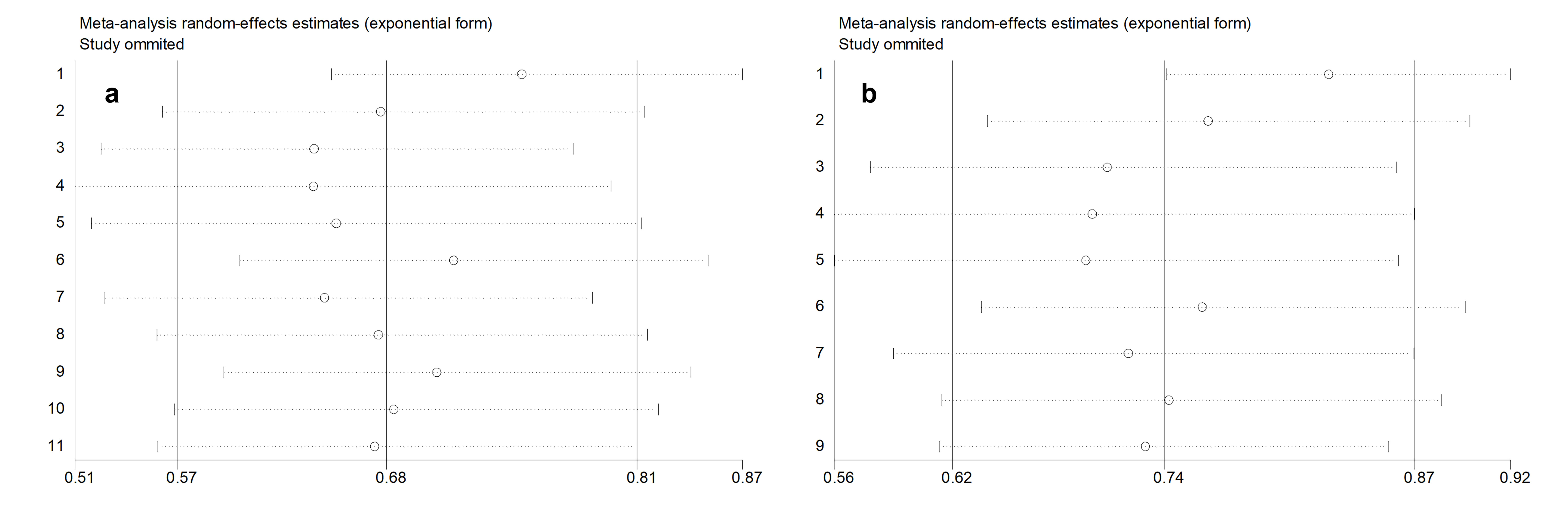 Figure 5. Sensitivity analysis for OS (a) and DFS/PFS (b).
Figure 5. Sensitivity analysis for OS (a) and DFS/PFS (b).
Our findings indicate that concomitant ASI use is associated with improved survival in RCC patients. Subgroup analyses further revealed that patients with RCC may benefit from the combined use of ASIs with immune checkpoint inhibitors (ICIs) or sunitinib. Notably, Kichenadasse et al. reported no survival benefit from the concomitant use of ASIs and atezolizumab in patients with RCC [29], which differs from our results. Possible explanations for this discrepancy include the following: (1) in our subgroup analysis of ICIs, the ICI category included atezolizumab among other agents; (2) the number of patients in the study by Kichenadasse et al. was limited; and (3) the study was a phase II clinical trial, whereas all studies included in our analysis were retrospective.
Compared to ASI-free patients, our results indicated that ASI use reduced the risk of disease progression in patients with RA. Furthermore, ASI use was consistently associated with favourable DSS/PFS regardless of the treatment agent, cancer stage, or time window of ASI use. Few studies have examined the role of ASIs specifically in patients with mRCC; however, our findings suggest that patients with mRCC may also benefit from ASI use.
Given the increasing morbidity and mortality associated with malignant tumors, the disease imposes a substantial financial burden. [30, 31]. Drug development is widely recognized as increasingly expensive and time-consuming, and the efficacy of monotherapy for solid tumors is often limited, highlighting the need to explore combination treatment strategies for solid tumors. Consequently, repurposing existing drugs offers a promising approach to accelerate therapeutic innovations. ASIs are widely prescribed in clinical practice for cardiovascular and chronic kidney diseases. Clarifying their impact on survival outcomes in RCC provides an evidence-based rationale for their potential concomitant use in clinical oncology.
All studies included in this meta-analysis were retrospective in design, which introduced potential sources of bias including selection, detection, publication, recall, and confounding biases. Consistent with this finding, heterogeneity was observed among the included studies. Although subgroup and sensitivity analyses were conducted, heterogeneity persisted. Certain confounding factors, such as the specific type and dosage of the ASIs, may have influenced the pooled conclusions. Unfortunately, such detailed information was not available in the included studies.
None.
Ethical policy
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
XYL conceptualized, designed, and conducted the research and drafted the manuscript. SQH contributed to the manuscript revision and figure preparation.
Competing interests
The authors declare no conflicts of interest related to the publication of this paper.
Funding
None.
- Crea F: The challenge of cancer therapy-related cardiac dysfunction: facts and perspectives. Eur Heart J 2024, 45(41): 4355-4358.
- Alcala K, Mariosa D, Smith-Byrne K, Nasrollahzadeh Nesheli D, Carreras-Torres R, Ardanaz Aicua E, Bondonno NP, Bonet C, Brunström M, Bueno-de-Mesquita B, et al: The relationship between blood pressure and risk of renal cell carcinoma. Int J Epidemiol 2022, 51(4): 1317-1327.
- George AJ, Thomas WG, Hannan RD: The renin–angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer 2010, 10(11): 745-759.
- Nakamura K, Yaguchi T, Ohmura G, Kobayashi A, Kawamura N, Iwata T, Kiniwa Y, Okuyama R, Kawakami Y: Involvement of local renin-angiotensin system in immunosuppression of tumor microenvironment. Cancer Sci 2018, 109(1): 54-64.
- Vallejo Ardila DL, Walsh KA, Fifis T, Paolini R, Kastrappis G, Christophi C, Perini MV: Immunomodulatory effects of renin-angiotensin system inhibitors on T lymphocytes in mice with colorectal liver metastases. J Immunother Cancer 2020, 8(1): e000487.
- Pinter M, Jain RK: Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci Transl Med 2017, 9(410): eaan5616.
- Wegman-Ostrosky T, Soto-Reyes E, Vidal-Milla´n S, Sa´nchez-Corona J: The renin-angiotensin system meets the hallmarks of cancer. J Renin ngiotensin Aldosterone Syst 2015, 16(2): 227-233.
- Sorich MJ, Kichenadasse G, Rowland A, Woodman RJ, Mangoni AA: Angiotensin system inhibitors and survival in patients with metastatic renal cell carcinoma treated with VEGF-targeted therapy: A pooled secondary analysis of clinical trials. Int J Cancer 2016, 138(9): 2293-2299.
- Nakai Y, Isayama H, Sasaki T, Takahara N, Saito K, Takeda T, Umefune G, Saito T, Takagi K, Watanabe T: No survival benefit from the inhibition of renin-angiotensin system in biliary tract cancer. Anticancer Res 2016, 36(9): 4965-4970.
- Xie YX, Xu Peng, Wang M, Zheng Y, Tian T, Yang S, Deng Y, Wu Y, Zhai Z, Hao Q: Antihypertensive medications are associated with the risk of kidney and bladder cancer: a systematic review and meta-analysis. Aging (Albany NY) 2020, 12(2): 1545-1562.
- Asgharzadeh F, Hashemzehi M, Moradi-Marjaneh R, Hassanian SM, Ferns GA, Khazaei M, Avan A: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers as therapeutic options in the treatment of renal cancer: A meta-analysis. Life Sci 2020, 242: 117181.
- Shen J, Hou H, Liang B, Guo X, Chen L, Yang Y, Wang Y: Effect of reninangiotensin-aldosterone system inhibitors on survival outcomes in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol 2023, 14: 1155104.
- Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, Rawla P, Barsouk A: Epidemiology of renal cell carcinoma. World J Oncol 2020, 11(3): 79-87.
- Young M, Jackson-Spence F, Beltran L, Day E, Suarez C, Bex A, Powles T, Szabados B: Renal cell carcinoma. Lancet 2024, 404(10451): 476-491.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group: Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015, 350: g7647.
- Stang A: Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010, 25(9): 603-605.
- Higgins JPT, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stat Med 2002, 21(11): 1539-1558.
- Cochran WG: The role of mathematics in the medical sciences. N Engl J Med 1961, 265: 176.
- Fortune K, Ali S, Masur J, Viscuse P, Devitt M, Dreicer R, Skelton WP 4th: Impact of renin-angiotensin system inhibitors on response to PD1/L1 inhibitors in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2025; 23(1): 102256.
- Penttilä P, Rautiola J, Poussa T, Peltola K, Bono P: Angiotensin inhibitors as treatment of sunitinib pazopanib-induced hypertension in metastatic renal cell carcinoma. Clin Genitourin Cancer 2017, 15(3): 384-390.
- Keizman D, Huang P, Eisenberger MA, Pili R, Kim JJ, Antonarakis ES, Hammers H, Carducci MA: Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. Eur J Cancer 2011, 47(13): 1955-1961.
- McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik OP, Sabbisetti VS, Bhatt RS, Simantov R, Choueiri TK: Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res 2015; 21(11): 2471-2479.
- Izzedine H, Derosa L, Le Teuff G, Albiges L, Escudier B: Hypertension and angiotensin system inhibitors: impact on outcome in sunitinib-treated patients for metastatic renal cell carcinoma. Ann Oncol 2015, 26(6): 1128-1133.
- Fiala O, Ostašov P, Rozsypalová A, Hora M, Šorejs O, Šustr J, Bendová B, Trávníček I, Filipovský J, Fínek J, et al: Impact of concomitant cardiovascular medication on survival of metastatic renal cell carcinoma patients treated with sunitinib or pazopanib in the first line. Target Oncol 2021, 16(5): 643-652.
- Kostine M, Mauric E, Tison A, Barnetche T, Barre A, Nikolski M, Rouxel L, Dutriaux C, Dousset L, Prey S, et al: Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur J Cancer 2021, 157: 474-484.
- Miyajima A, Yazawa S, Kosaka T, Tanaka N, Shirotake S, Mizuno R, Kikuchi E, Oya M: Prognostic impact of renin–angiotensin system blockade on renal cell carcinoma after surgery. Ann Surg Oncol 2015; 22(11): 3751-3759.
- Nuzzo PV, Adib E, Weise N, Curran C, Stewart T, Freeman D, Nassar AH, Abou Alaiwi S, Bakouny Z, McGregor BA, et al: Impact of renin-angiotensin system inhibitors on outcomes in patients with metastatic renal cell carcinoma treated with immune-checkpoint inhibitors. Clin Genitourin Cancer 2022, 20(4): 301-306.
- Duval S, Tweedie R: A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000, 95: 89-98.
- Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Sorich MJ, Hopkins AM: Effect of concomitant ues of antihiypertensives and immune check point inhibitors on cancer outcomes. J Hypertens 2021, 39(7): 1274-1281.
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74(3): 229-263.
- Carrera PM, Kantarjian HM, Blinder VS: The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin 2018, 68(2): 153-165.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript