Case Report | Open Access
A Diagnostic Twist: Primary Bladder Adenocarcinoma Masquerading as an Ovarian Torsion: A Case Report on Unusual Cystic Presentation
Sonali Kar1, Neeta Verma2, Suren Kumar Das21Department of Pathology, IMS & SUM Hospital, Bhubaneswar, India.
2Department of Urology, IMS & SUM Hospital, Bhubaneswar, India.
Correspondence: Sonali Kar (Department of Pathology, IMS & SUM Hospital, College Building, Kalinga Nagar, K/8, Bhubaneswar, India; Email: sonalikar@soa.ac.in).
Annals of Urologic Oncology 2025, 8(3): 150-155. https://doi.org/10.32948/auo.2025.08.25
Received: 17 Aug 2025 | Accepted: 04 Sep 2025 | Published online: 15 Sep 2025
Key words cystic pelvic mass, ovarian torsion mimic, female bladder cancer, enteric type adenocarcinoma
Ultrasound revealed a well-defined cystic lesion in the suprapubic midline pelvic cavity measuring 9.7x9.6x9.6 cm (volume-480cc) with echogenic debris and peripheral enhancing solid component, showing no vascularity on colour doppler (Figure 1A). Along with it non visualisation of left ovary was suggestive of torsion of left ovarian dermoid cyst.
Given the suspicion of torsion without any further imaging study, the patient underwent emergency explorative laparotomy. A Pfannenstiel incision was made, revealing a cystic mass seem to arise from the dome of bladder (Figure 1B). Both ovaries and fallopian tubes were normal. The cyst ruptured during manipulation exposing solid component inside. Intraoperative frozen from this showed mucin with neoplastic glands in muscle layer suggesting adenocarcinoma. Given its origin from the bladder dome, urachal adenocarcinoma was suspected. Intraoperative cystoscopy also showed solid ulcerating growth in bladder mucosa. Partial cystectomy with extended bilateral pelvic lymphadenectomy along with excision of urachus and umbilicus was done and sent for histopathology study.
Grossly, a ruptured cyst was seen over the bladder along with a solid ulcerating growth inside the mucosa of dome and posterior wall. Cut surface of both lesions contained mucin and debris (Figure 1C). Histopathology revealed a tumour arising from mucosa of bladder showing pure glandular differentiation and lined by mucin producing pseudostratified columnar epithelium with lumen containing mucin and dirty necrosis (Figure 2A, 2B). Tumour penetrates outer half of deep muscularis propria. The mucin dissected the muscle layer (Figure 2C) and formed cyst which sat over the dome of bladder. Background shows feature of chronic cystitis with cystitis cystica (Figure 2D). Thorough sampling revealed no Invasive or non-invasive urothelial carcinoma components or urachal involvement. IHC were positive for CK20, CDX2, SATB2 suggesting adenocarcinoma with intestinal differentiation (Figure 3). Tumour cells were negative for CK7, GATA3, Beta catenin (nuclear reactivity), Pax8 and AMACR (Figure 4), thus confirming it to be primary adenocarcinoma bladder, enteric type after excluding its other differentials. Post-op colonoscopy showed no neoplastic growth.
The patient recovered well post-surgery and remains asymptomatic on regular follow-up.
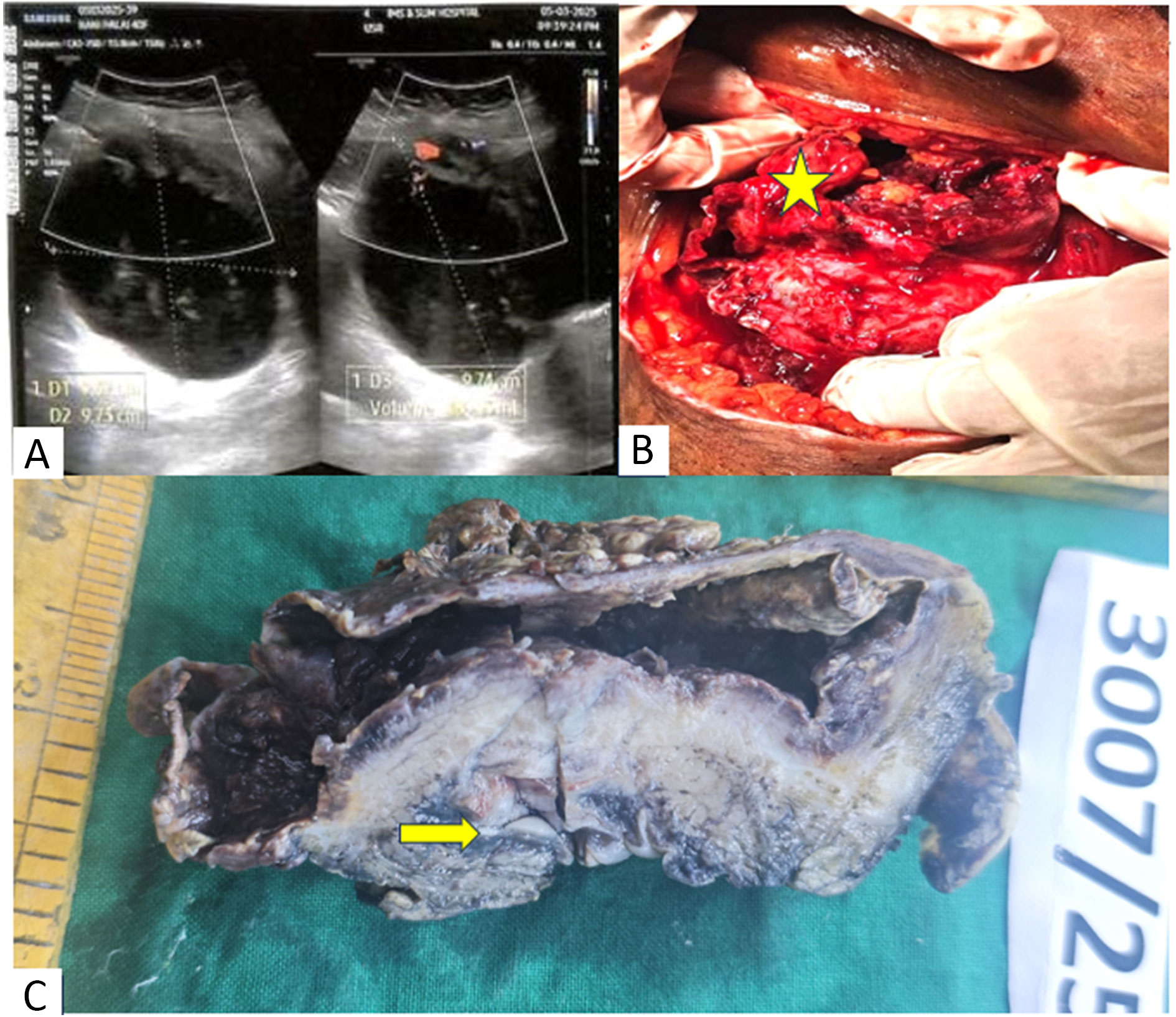 Figure 1. (A) Ultrasound showing suprapubic cystic lesion with lack of doppler blood flow, (B) Intraoperatively a ruptured cyst above bladder dome (yellow star), (C) Gross showing a cyst and mucosal growth (yellow arrow).
Figure 1. (A) Ultrasound showing suprapubic cystic lesion with lack of doppler blood flow, (B) Intraoperatively a ruptured cyst above bladder dome (yellow star), (C) Gross showing a cyst and mucosal growth (yellow arrow).
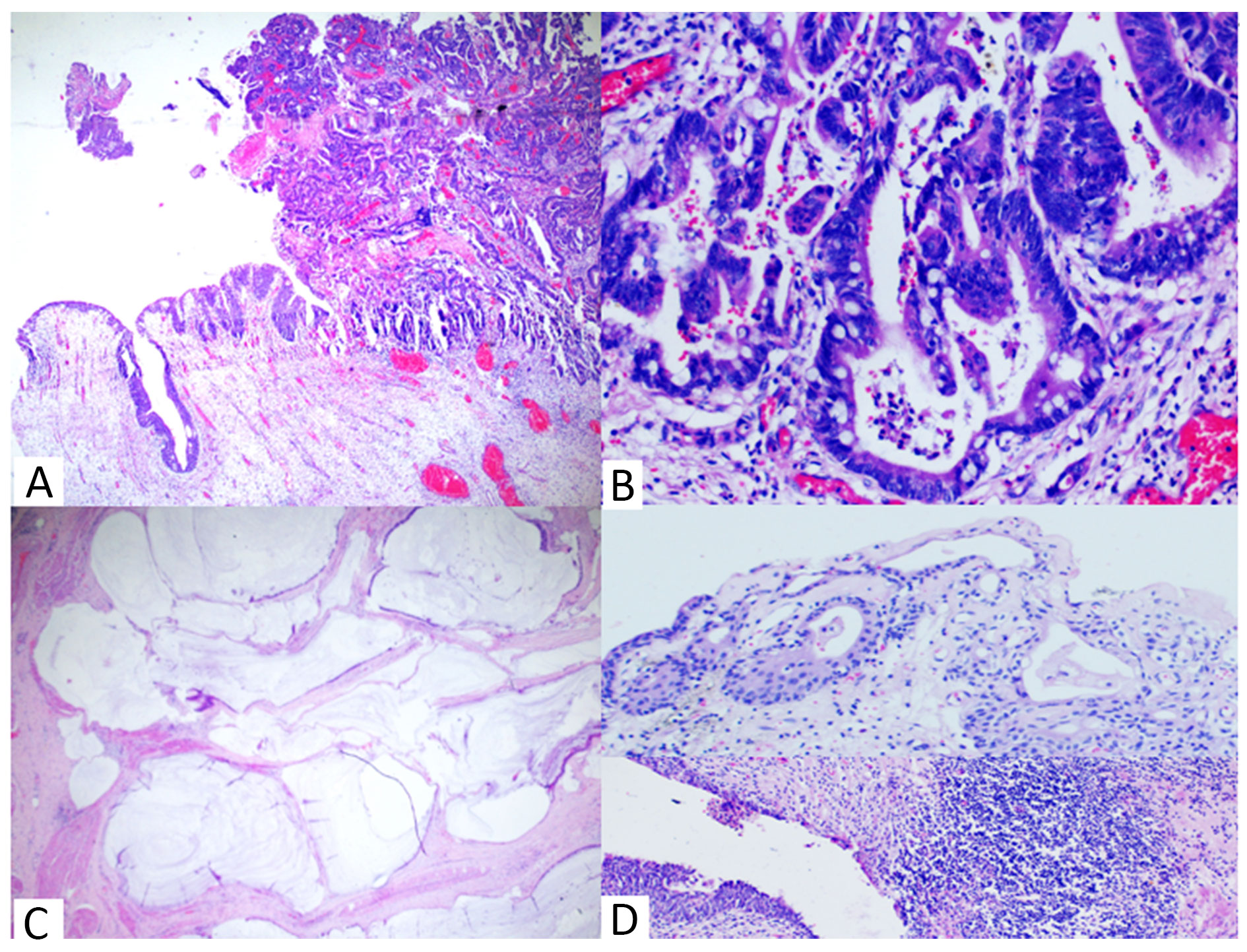 Figure 2. Histopathology (H&E staining) showing. (A) Tumour arising from mucosa (40x), (B) Tumour with Intestinal differentiation & necrotic debris (400x), (C) Mucin in muscle layer (100x), (D) Cystitis cystica and Chronic cystitis (100x).
Figure 2. Histopathology (H&E staining) showing. (A) Tumour arising from mucosa (40x), (B) Tumour with Intestinal differentiation & necrotic debris (400x), (C) Mucin in muscle layer (100x), (D) Cystitis cystica and Chronic cystitis (100x).
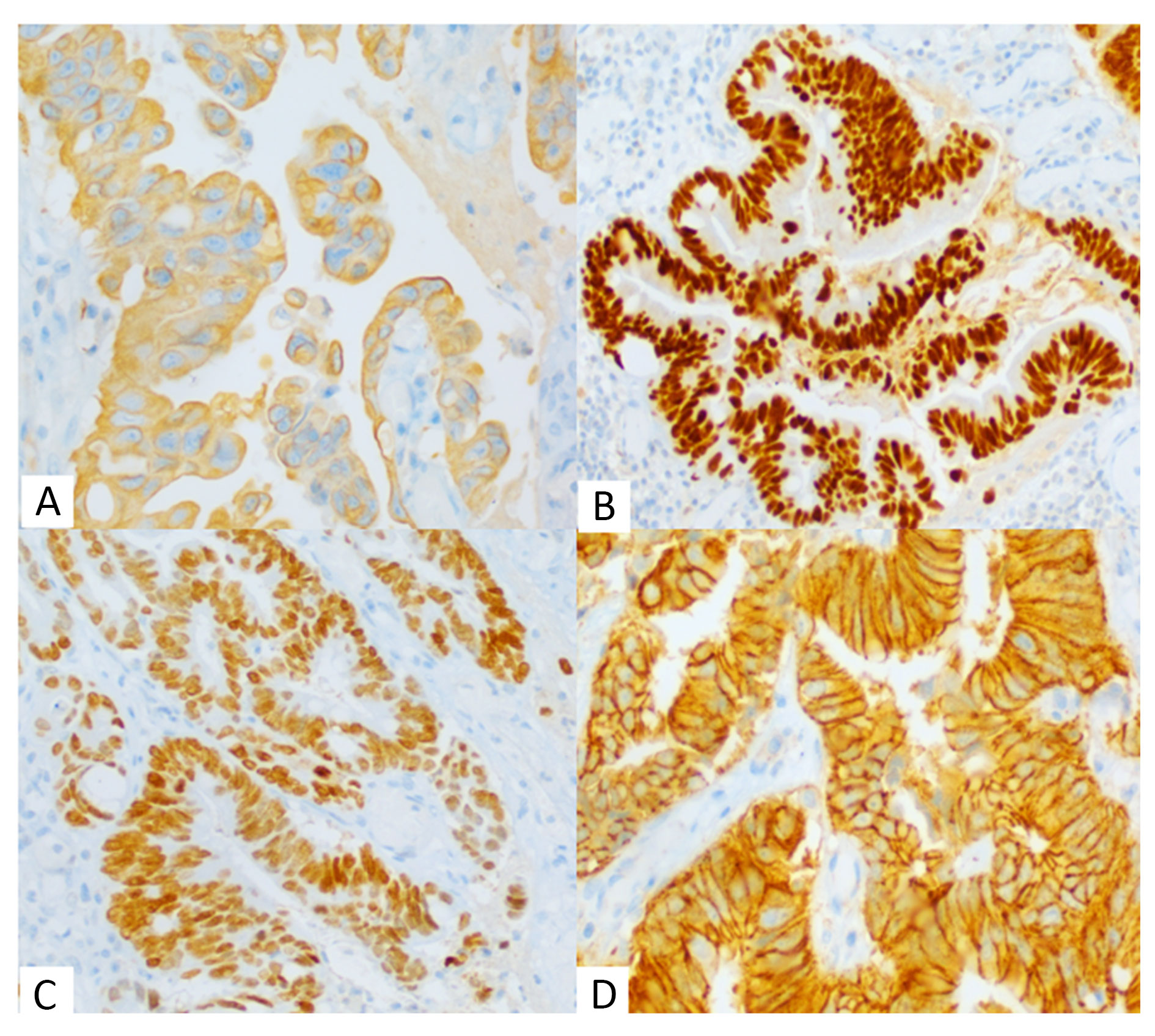 Figure 3. Immunohistochemistry showing tumour cells at 400x. (A) Cytoplasmic positivity for CK20, (B) Nuclear positivity for CDX2, (C) Nuclear positivity for SATB2 & (D) Nuclear negativity for beta catenin.
Figure 3. Immunohistochemistry showing tumour cells at 400x. (A) Cytoplasmic positivity for CK20, (B) Nuclear positivity for CDX2, (C) Nuclear positivity for SATB2 & (D) Nuclear negativity for beta catenin.
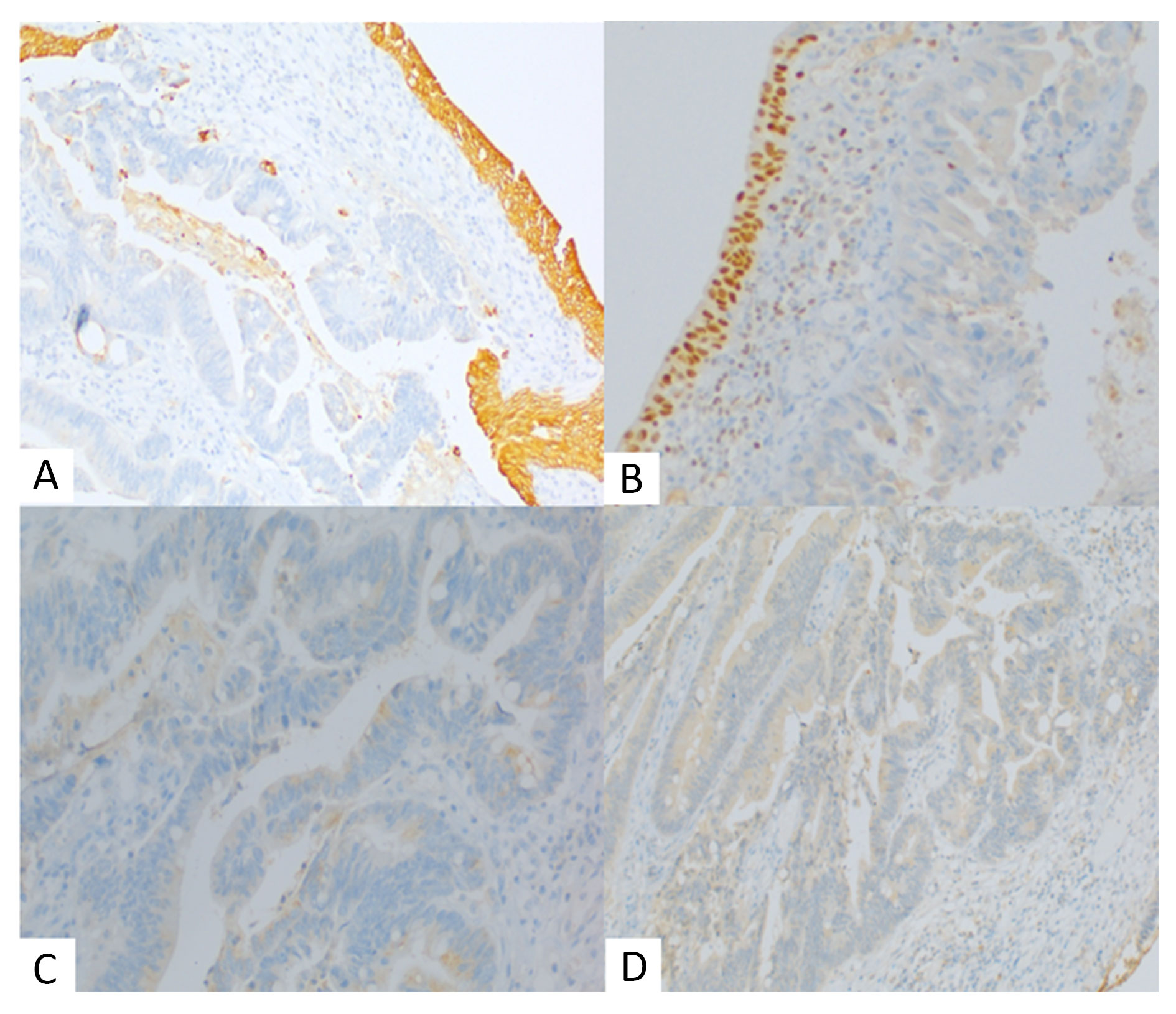 Figure 4. Immunohistochemistry showing tumour cells. (A) Cytoplasmic negativity for CK7 (100x, normal urothelium positive), (B) Nuclear negativity for GATA3 (100x, normal urothelium positive), (C) Negative for AMACR (400x), (D) Negative for PAX8 (100x).
Figure 4. Immunohistochemistry showing tumour cells. (A) Cytoplasmic negativity for CK7 (100x, normal urothelium positive), (B) Nuclear negativity for GATA3 (100x, normal urothelium positive), (C) Negative for AMACR (400x), (D) Negative for PAX8 (100x).
PBA mainly seen in sixth decade with a male predominance [1], but in our case the patient is a 40-yr female. Precursors of this tumour include chronic irritation, cystitis glandularis with intestinal metaplasia, exstrophy and schistosomiasis [1, 3]. Histopathology of our case shows cystitis cystica and chronic inflammation which are likely the trigger. Common symptoms of PBA are haematuria, dysuria, pelvic pain which overlap with other conditions causing diagnostic delay [3, 4]. Ovarian torsion, typically caused by a cyst, presents with sudden abdominal pain & vomiting [5]. Patient in our case had no haematuria but exhibited classic torsion signs. Cystic mass with non-visualisation of left ovary in ultrasound and lack of doppler blood flow further supported the diagnosis of ovarian torsion. Although Computed tomography or magnetic resonance imaging could have provided better characterization and identified bladder involvement, the urgency of suspected ovarian torsion necessitated immediate surgery without further imaging.
However, during surgery, the absence of ovarian involvement and the discovery of a cystic mass arising from the bladder dome prompted a change in diagnosis. Further intraoperative frozen from cystic mass was suggestive of adenocarcinoma. In our case as the cyst was over the dome, which is typical of urachal adenocarcinoma, an intra-op diagnosis of urachal adenocarcinoma was made [6]. A Partial cystectomy with lymph nodes dissection were performed and sent for histopathology.
The definite diagnosis of PBA was established through histopathology, IHC and clinical history. Histopathology was suggestive of adenocarcinoma, lined by mucin secreting cells. The tumour invaded muscle layer with mucin dissecting it and forming a cyst with a thick fibromuscular wall that seem to siting over the dome, which mimic a cystic lesion and ovarian torsion in imaging. We didn’t classify it as mucinous adenocarcinoma due to mucin content being less than < 50% [3]. None of the literature showed this type of features. Kamel K et al. reported a paediatric case mimicking ovarian torsion, later diagnosed histopathologically as complicated urachal cyst [8]. Histomorphology along with positivity for CK20, CDX2 and SATB2 IHC lead to broad diagnosis of adenocarcinoma with intestinal differentiation [9]. In our case CK7 was negative. Eissa et al. found CDX2 positivity in 100%, CK20 in 92%, CK7 in only 15 % of 100 enteric type PBA cases [10]. Similarly, Wang et al. reported CDX2 and SATB2 expression in 100% of bladder malignancy with intestinal differentiation [9].
This adenocarcinoma was confirmed as primary bladder origin after excluding its differentials such as urachal adenocarcinoma, urothelial carcinoma with glandular differentiation, metastatic adenocarcinoma and mullerian origin tumour. Urachal adenocarcinoma arises from urachus, typically at bladder dome and may appear cystic. Histologically it shows glandular differentiation with an intramural tumour epicentre with a sharp demarcation from surface mucosa [3, 6]. In contrast PBA originates from mucosa, mostly from posterior wall and trigone [3, 4]. In our case, a cystic mass at dome with positive frozen section suggested urachal adenocarcinoma. However resected specimen revealed a mucosal origin tumour involving dome and posterior bladder wall with evidence of cystitis cystica, ruling out urachal adenocarcinoma. IHC can’t distinguish between two due to similar marker expression [11]. The most common differential is urothelial carcinoma with glandular differentiation, which must require foci of invasive or non-invasive urothelial carcinoma component and GATA3 positivity [1-3]. In our case, this was excluded due to absence of urothelial carcinoma component despite thorough sampling and negative GATA3 staining. Another key differential was secondary metastasis or direct spread from gastrointestinal or female genital tract. However, the tumour’s mucosal origin with cystitis cystica, negative nuclear reactivity for beta-catenin, negative AMACR and normal colonoscopy rule out possibility of gastrointestinal adenocarcinoma metastasis [11]. Metastasis from female genital tract was also ruled out by Pax 8 negativity and unremarkable imaging. Mullerian origin adenocarcinoma was ruled out based on Pax8, AMACR negativity and the clinical context [11].
Limited data exist on the molecular profiling of PBA [12]. Most of PBA often shares genomic mutation such as KRAS, TP53, APC, PIK3CA with colorectal adenocarcinoma, indicating a common molecular pathway that may guide targeted therapies [2, 12].
Management depends on tumour stage and location. Surgery, including partial or total cystectomy is the mainstay for localized disease, while advanced cases may require chemotherapy or radiotherapy. In this case, the patient underwent partial cystectomy with clear margins and lymph nodes dissection. Prognosis remains poor, with a 5-year survival rate of 40-50%, influenced by stage and histological type [1]. Long-term follow-up is essential due to the potential for recurrence and metastasis.
We acknowledge the cooperation of the patient and support from the staffs of the department.
Ethical policy
Prior informed consent as well as consent for publication was taken from the patient.
Availability of data and materials
No new data were generated during this study.
Author contributions
Study concept and design: Dr. Sonali Kar; Data acquisition and analysis: Dr. Neeta Verma, Dr. Sonali Kar; Drafting of manuscript: Dr. Sonali Kar, Dr. Monali Kar; Critical revision of manuscript: Dr. Suren Kumar Das, Dr. Monali Kar.
Competing interests
The authors declare that they have no competing interests.
Funding
NIL.
- Grignon DJ, Cheville J, Ro JY, Tamboli P: Glandular neoplasm. In: Moch H, Humphrey PA, Ulbright TM, Reuter VE, editors. WHO Classification of Tumours of the Urinary System and Male Genital (5th ed). Lyon: International Agency for Research on Cancer (IARC), 2022, p111-112.
- Santa F, Akgul M, Tannous E, Pacheco RR, Lightle AR, Mohanty SK, Cheng L: Primary adenocarcinoma of the urinary tract and its precursors: Diagnostic criteria and classification. Hum Pathol 2025, 155: 105734.
- Cheng L, Lopez-Beltran A, Montironi R: Bladder pathology. Second ed. Hoboker, NJ: Wiley-Blackwell, 2025.
- Grignon DJ, Ro JY, Ayala AG, Johnson DE, Ordóñez NG: Primary adenocarcinoma of the urinary bladder: A clinicopathologic analysis of 72 cases. Cancer 1991, 67(8): 2165-2172.
- Duan Y, Hoer B, Little A: Twisting Ovaries: Three Cases of Ovarian Torsion. Cureus 2022, 14(10): e30496.
- Paner GP, Lopez-Beltran A, Sirohi D, Amin MB: Updates in the Pathologic Diagnosis and Classification of Epithelial Neoplasms of Urachal Origin. Adv Anat Pathol 2016, 23(2): 71-83.
- Roy S, Smith MA, Cieply KM, Acquafondata MB, Parwani AV: Primary bladder adenocarcinoma versus metastatic colorectal adenocarcinoma: a persisting diagnostic challenge. Diagn Pathol 2012, 7: 151.
- Kamel K, Nasr H, Tawfik S, Azzam A, Elsaid M, Qinawy M, Kamal A, Taher H: Complicated urachal cyst in two pediatric patients: a case report. BMC Pediatr 2023, 23(1): 147.
- Wang HL, Lu DW, Yerian LM, Alsikafi N, Steinberg G, Hart J, Yang XJ: Immunohistochemical distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Am J Surg Pathol 2001, 25(11): 1380-1387.
- Eissa SS, Block N, Khaled HM, Shoman SH, Nassiri M, Nadji M: Primary enteric-type adenocarcinomas of the urinary bladder are histogenetically analogous to colorectal carcinomas: Immunohistochemical evaluation of 109 cases. J Adv Res 2010, 1(2): 151-156.
- Akgul M, MacLennan GT, Cheng L: The applicability and utility of immunohistochemical biomarkers in bladder pathology. Hum Pathol 2020, 98: 32-55.
- Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, Hoadley KA, Groeneveld CS, Al-Ahmadie H, Choi W et al: A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur Urol 2020, 77(4): 420-433.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript