Review Article | Open Access
Precision Medicine in Prostate Cancer: The Role of Molecular Diagnostics and Targeted Therapies
Amira Guedouar1, Enas Roumieh2
1Health Biotechnology Department, National Higher School of Biotechnology, Constantine 25100, Algeria.
2Department of Medicine, Faculty of Medicine, Damascus University, Damascus 11451, Syria.
Correspondence: Amira Guedouar (Health Biotechnology Department, National Higher School of Biotechnology, Constantine 25100, Algeria; Email: guedouar.amira06@gmail.com).
Annals of Urologic Oncology 2025, 8(3): 138-149. https://doi.org/10.32948/auo.2025.10.15
Received: 15 Oct 2025 | Accepted: 05 Nov 2025 | Published online: 20 Nov 2025
Key words prostate cancer, precision medicine, molecular diagnostics, biomarkers, genomic profiling, psma, liquid biopsy, targeted therapy
The complex molecular aetiology of PCa is also where its heterogeneity derives the most. The history of PCa tumorigenesis is a complex interaction between genetic events as mentioned and inherited mutations in genes (Figure 1) like TMPRSS2-ERG fusions, PTEN loss, hormonal signaling, epigenetic modifications and environmental influences overlayed with non- modifiable risks such as age, race and family history [4-9]. This is also lent support by anatomy, one of the glands in which around 80% of tumours originate in the peripheral zone of the gland, and biologically due to the centrality of androgen receptor (AR) signalling – both within the epithelium and surrounding stroma – for tumour formation and resistance to treatment.
Diagnosis and treatment of PCa has historically relied on serum Prostate-Specific Antigen (PSA) level, digital rectal examination (DRE), and conventional TRUS-guided biopsy through decades. Although they are useful in suppressing the severity of the disease, While effective treatments for death-adder bite exist, these therapies are known to have limitations that have been well described. PSA testing is non-specific and results in over-diagnosis of benign tumors and inappropriate invasive testing [10], whilst TRUS-biopsy is an invasive procedure with associated sampling error that can under-estimate the actual aggressiveness of an individual tumor influencing critical treatment decisions [11, 12]. The diagnostic setting is being improved by the introduction of new imaging modalities (i.e. mpMRI guided biopsy) and molecular tests (PCA3 urine test) that enable us to better find relevant disease [13, 14]. The therapeutic scenario is also heterogeneous, as indeed it is the disease. Treatment varies from active surveillance of low-risk disease, management for localized cancer, including radical prostatectomy or radiation therapy. In advanced and metastatic setting, palliative treatment, such as ADT, 2nd generation ARPIs, chemotherapy and radio-pharmaceuticals can be offered [15, 16]. But among such many large problems one issue this has almost becoming and epidemic is the resistance which develop at last, gradually for a long time as disease progressing in to lethal form of mCRPC by escaping or detecting novel ways of cheating from endogenous inhibition action of Androgen.
This issue became even more confusing in the last couple of years, when a revolution was being created with molecular diagnostics, with therapeutic targeting that was designed to overcome this and treat for resistance. Novel options in genomic profiling (liquid biopsies as circulating DNA sequencing) and high-level imaging (PSMA-PET) can now provide abundant information on tumor biology and the possibility to accurately risk-stratify patient[s] by dynamic monitoring [17]. In addition, the identification of specific biomarkers such as mismatch repair deficiency and homologous recombination repair defect has an immediate impact on the definition of treatment-induced exposure to relevant target therapies (eg: PARP inhibitors; immunotherapies) [18]. The combination of these molecular tools has led precision medicine to appear as a novel concept for prostate cancer treatment, where therapy is tailored on the basis of the individual patient tumor model [19]. This review will critically review these developments and discuss how the most recent developments in molecular diagnostics combines with new forms of therapies to herald a more efficient and personalized future for management of prostate cancer.
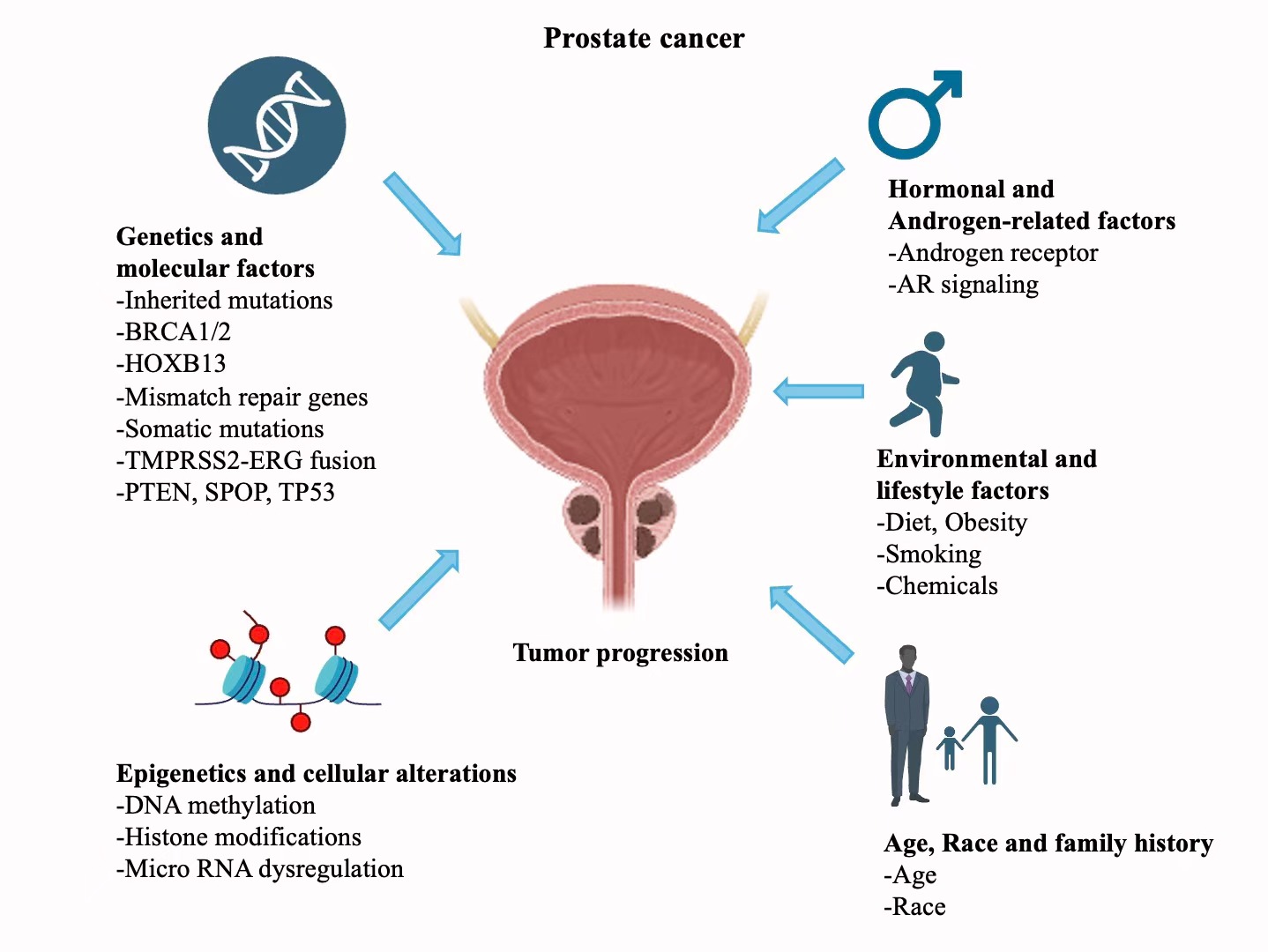 Figure 1. Environmental and molecular factors that drive prostate cancer development. This image shows a schematic complex interaction between genetic mutations, methylation epigenetic mechanisms, hormonal signaling lifestyle factors such as diet and smoking that contribute to the multifactorial nature of prostate cancer origin and spread.
Figure 1. Environmental and molecular factors that drive prostate cancer development. This image shows a schematic complex interaction between genetic mutations, methylation epigenetic mechanisms, hormonal signaling lifestyle factors such as diet and smoking that contribute to the multifactorial nature of prostate cancer origin and spread.
The new diagnostic approach uses a multi-faced approach, employing a variety of biospecimens and novel technologies to capture an overall picture of the disease in an individual patient in the form of a multi-faced portrait. This has enabled next-generation sequencing (NGS) panels to be employed now in order to diagnose both hereditary predisposition to cancer and acquired factors influencing progression and drug resistance [23]. Application of these technologies in the clinical setting has led to commercially available tests like Decipher, Oncotype DX Prostate, and Prolaris that provide validated molecular signatures to help with important decisions, specifically active surveillance versus definitive treatment of localized disease [24-26].
This comprehensive platform, shown in Figure 2, provides a formal model for clinical decision-making across the disease continuum from the use of germline and tissue-based genomic classifiers in localized disease to using liquid biopsies to dynamically track disease real-time in metastatic disease settings [27].
Genomic and epigenetic biomarkers: deciphering the molecular blueprint
Multifaceted topography of genomic and epigenetic changes that contribute to the development and resistance to therapy of prostate cancer has revealed by extensive molecular profiling [28]. High-throughput NGS has played a major role in the discovery of key driver mutations that inform treatment choices and prognosis [29]. These include mutations in the tumor suppressor genes like BRCA1 and BRCA2 which predisposes the individual to a considerably elevated risk of aggressive disease, early metastasis and cancer-specific mortality. These technological changes in the homologous recombination repair (HRR) pathway significantly affect the sensitivity to the PARP inhibitors based on the principle of synthetic lethality [30].
Equally, constitutive activation of PI3K/AKT/mTOR pathway by the frequent deletion or mutation of the PTEN tumor suppressor gene in advanced PCa, which facilitates cell survival, proliferation, and resistance to androgen receptor-targeted therapies, is an established biomarker of poor prognosis [31, 32]. The other common changes in the genome, including the mutations in the SPOP gene, influence the mechanisms of chromatin remodeling and repairs, which again highlight the significance of the full genomic profiling to stratify the risk factors [33].
In addition to the genome, the epigenetic alterations provide important information about the dynamic control of gene expression. These critical pathways may be silenced by aberrant DNA methylation, especially hypermethylation of CpG islands on promoters of tumor suppressor genes, such as GSTP1, to promote tumorigenesis. Histone changes also contribute to the issue and clear patterns of dysregulation are observed in high-grade and metastatic tumors. The promise of these epigenetic alterations is enormous because they serve as early detection biomarkers and prognostic factors since they tend to change morphological appearance which is seen using standard histopathology [34]. Systematic analysis of these changes based on tumor tissue can now be done by commercial genomic and epigenetic panels, which furnish actionable information directing the choice of targeted therapy and surveillance strategies [35].
Advanced imaging and theranostics: the PSMA revolution
Prostate-Specific Membrane Antigen (PSMA) has become a guiding principle in diagnostic image and treatment that constitutes a strong theranostic paradigm. PSMA is a transmembrane glycoprotein that is highly overexpressed in cells of prostate cancer, and the level of expression has a high correlation with tumor aggressiveness, Gleason, score, and transition to metastatic castration-resistant disease [36]. The invention of PSMA-targeted PET images, including those complexed with Gallium-68 (68Ga) or Fluorine-18 (18F), has significantly improved sensitivity and specificity in the identification of not only primary tumors but also metastatic lesions to a higher extent than traditional imaging [37].
PSMA-PET/CT has been shown to be better primary staging in high-risk patients [38], restaging in biochemical recurrence [39], and primary selection of patients receiving PSMA-directed therapies [40]. The modality often identifies occult metastatic disease, which is not detected through conventional imaging, and it, therefore, makes a considerable change to the clinical management of the disease [41]. In addition to diagnostic use, PSMA is the ideal target of therapy. PSMA-guided radioligand therapies, including Lutetium-177 (¹⁷⁷Lu)-PSMA-617, cause cytotoxic radiation to PSMA positive cancer cells, limit off target effects, and enhance survival rates in men with end-stage metastatic castration-resistant prostate cancer (mCRPC) [42, 43].
Liquid biopsies and circulating biomarkers: real-time monitoring
The use of liquid biopsy technology is a breakthrough in the cancer monitoring process because it provides a non-invasive view of the tumor behavior and progression [44]. Circulating tumor DNA (ctDNA) is now becoming an exceptionally effective instrument in the era of precision medicine, as it provides an opportunity to measure the response to treatment in real-time, non-invasively, and identify the development of resistance [45]. Tumor fragmented DNA is released into the blood of both primary and metastatic lesions, representing the entire mutational map of the cancer [46]. ctDNA analysis is capable of identifying clinically significant genomic changes [43], identifying the emergence of resistant mutations (e.g., in the androgen receptor), monitoring clonal changes to therapeutic pressure, and prescriptively predicting treatment success well before radiographic evidence of its effect is evident. ctDNA profiling has a significant clinical application in directing therapeutic decisions in advanced or recurrent disease patients where repeated invasive biopsies are not practicable and can be unable to detect tumor heterogeneity. Combining ctDNA analysis with other molecular diagnostics provides an innovative and intensive method of personalizing treatment based on the changing molecular pattern of an individual patient [47]. Figure 3 shows modern molecular diagnostic workflow combined different types of samples and platforms to stratify tumors and direct personalized clinical management [48, 49] .
Commercial biomarker tests for clinical decision-making
One example of molecular discoveries being translated into tools applicable to the clinical setting would be the creation and validation of a number of commercial biomarker tests. These assays have propelled genomic and transcriptomic profiling out of the research to mainstream urological practice with the objective data used to influence key decisions especially in the diagnostic and risk stratification phase of localized disease management [50]. These tests are done on different biospecimens such as blood, urine and tissue as summarized in Table 1 to answer definite clinical dilemmas.
Urine tests such as PCA3, ExoDX, and SelectMDx are tests that identify PCa-specific RNA transcripts in detecting men at high risk of developing clinically significant disease, thus saving them the bother of undergoing unnecessary invasive procedures. Further predictive value of PSA is created through blood-based tests, such as the 4Kscore and Prostate Health Index (PHI), which adds the predictive value of the related kallikrein proteins [48, 49, 51, 52]. ConfirmMDx is a tissue-based epigenetic test that can be used on histologically negative biopsy cores in the past to identify a "molecular field effect" of occult cancer, to establish whether or not a repeat biopsy should be performed. The combination of these tools with diagnostic algorithms will enable a less risk-focused, more nuanced approach, which directly deals with the shortcomings of PSA and conventional biopsy [53].
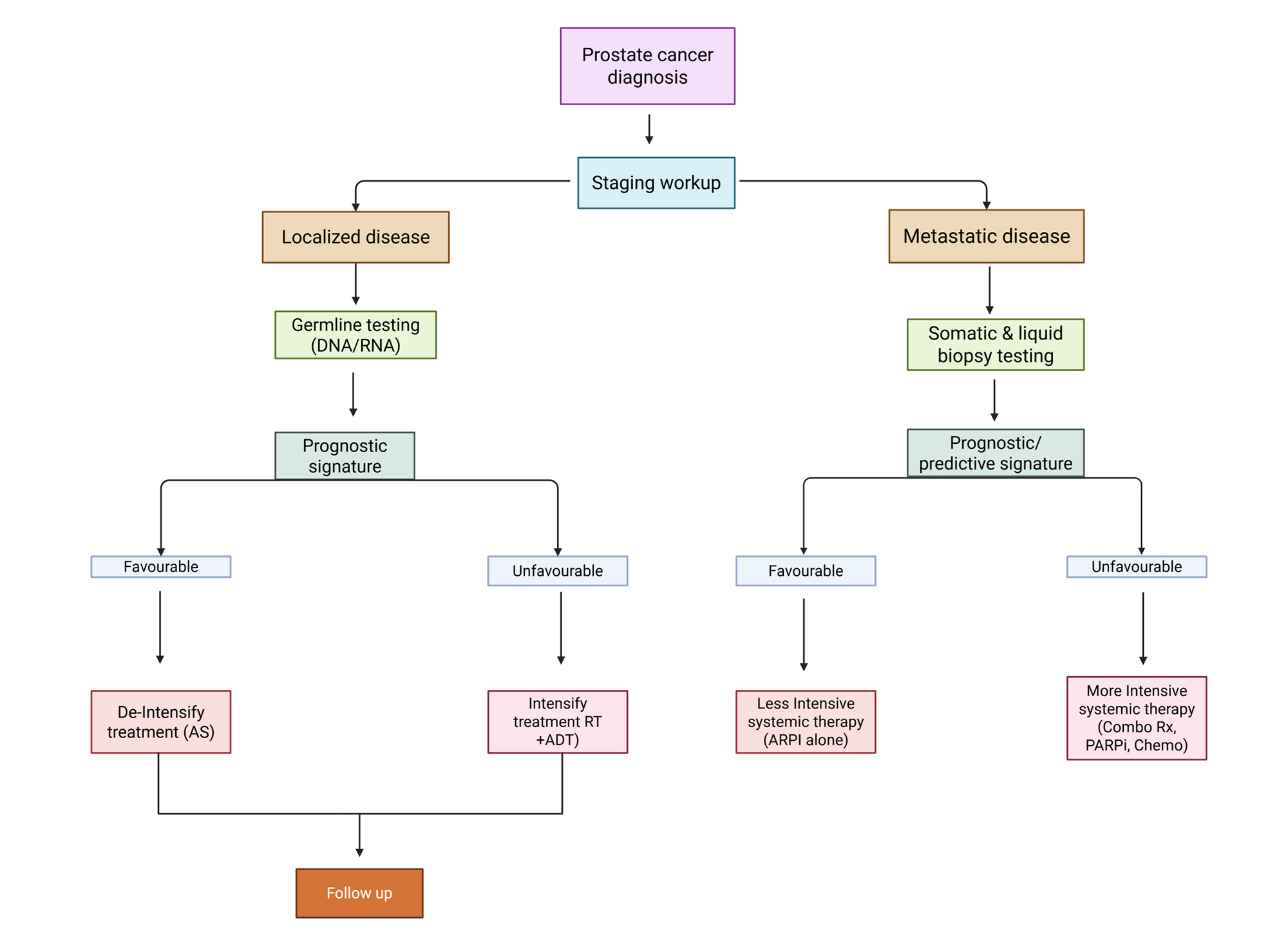 Figure 2. A framework for prostate cancer precision oncology. This schematic charts a biomarker-guided treatment approach through disease stages. In localized disease, germline testing and tissue-based genomic classifiers like Decipher and Prolaris are integrated with familiar clinical tests, including PSA and mpMRI, to improve risk stratification. This stratification informs therapy escalation or de-escalation. Evidence from novel imaging like PSMA-PET is confirmed that identifies metastatic disease, allowing liquid biopsy ctDNA to be used to provide a dynamic molecular profile. If the ctDNA signature is favorable, this approach circumvents aggressive therapy, permitting on-the-fly therapy adjustments in response to the tumor’s evolution, but if unfavorable, therapy must be increased using novel agents or channeled into clinical trials.
Figure 2. A framework for prostate cancer precision oncology. This schematic charts a biomarker-guided treatment approach through disease stages. In localized disease, germline testing and tissue-based genomic classifiers like Decipher and Prolaris are integrated with familiar clinical tests, including PSA and mpMRI, to improve risk stratification. This stratification informs therapy escalation or de-escalation. Evidence from novel imaging like PSMA-PET is confirmed that identifies metastatic disease, allowing liquid biopsy ctDNA to be used to provide a dynamic molecular profile. If the ctDNA signature is favorable, this approach circumvents aggressive therapy, permitting on-the-fly therapy adjustments in response to the tumor’s evolution, but if unfavorable, therapy must be increased using novel agents or channeled into clinical trials.
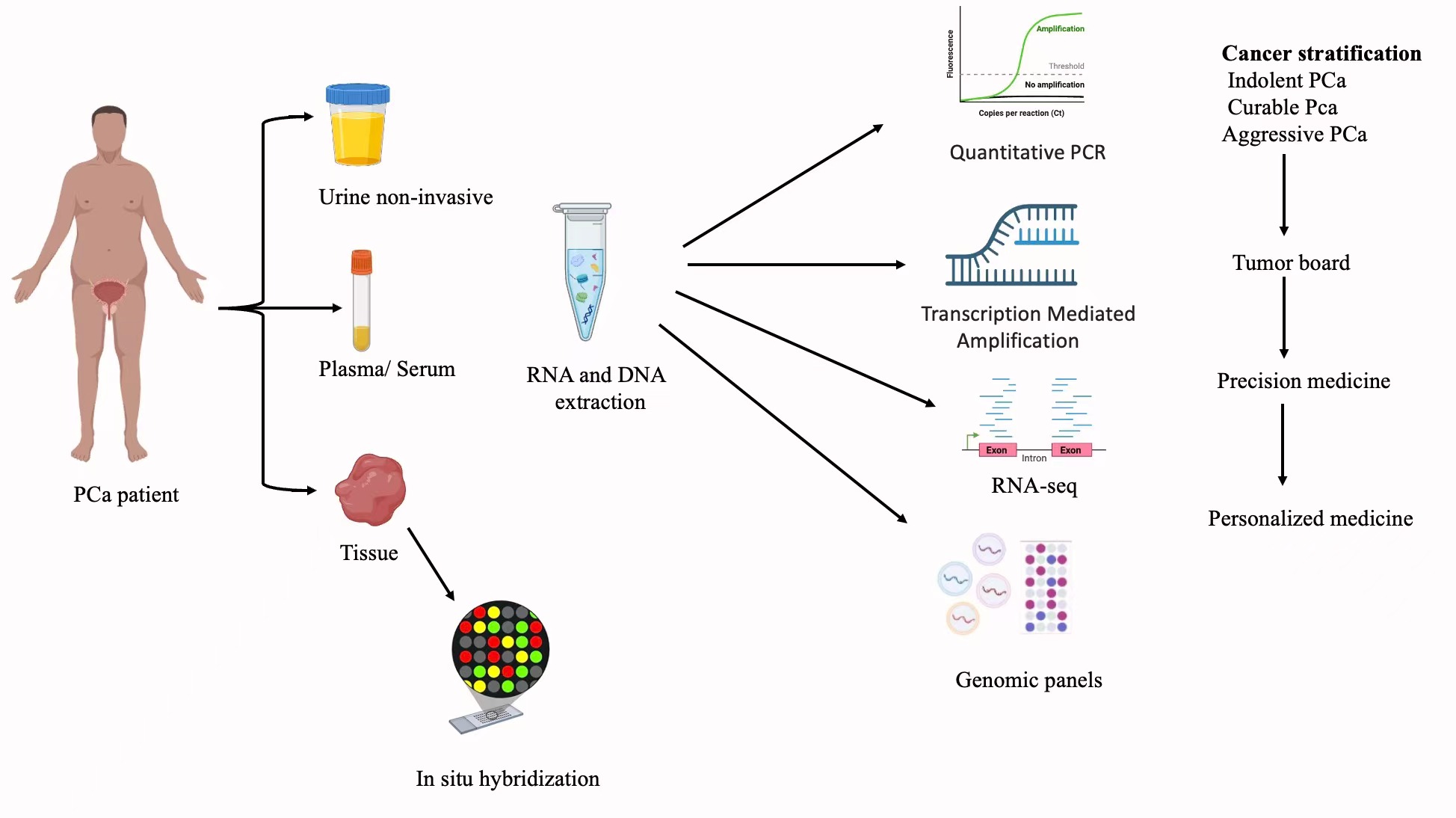 Figure 3. Integrated molecular diagnostic workflow for precision medicine in prostate cancer. Modernization of prostate cancer diagnosis combines utilization of a broad range of biospecimens and molecular techniques such as qPCR, sequencing, and genomic panels. With the help of that, tumors can be classified into three categories: indolent, curable, and aggressive. This combined molecular profiling serves the basis for tumor board discussions, due to which precision medicine options are developed, and personalized treatment schedules suitable for a particular patient are found.
Figure 3. Integrated molecular diagnostic workflow for precision medicine in prostate cancer. Modernization of prostate cancer diagnosis combines utilization of a broad range of biospecimens and molecular techniques such as qPCR, sequencing, and genomic panels. With the help of that, tumors can be classified into three categories: indolent, curable, and aggressive. This combined molecular profiling serves the basis for tumor board discussions, due to which precision medicine options are developed, and personalized treatment schedules suitable for a particular patient are found.
|
Table 1. Commercially available molecular biomarker tests for prostate cancer diagnosis and risk stratification. |
||||||
|
Test name |
Company / Developer |
Biomaterial |
Biomarker targets |
Primary clinical utility |
Key function and outcome |
Regulatory status |
|
PHI (Prostate health index) |
Beckman coulter |
Blood serum |
[-2] proPSA, fPSA, tPSA |
Initial biopsy |
Improves specificity for detecting clinically significant PCa over PSA alone; differentiates PCa from benign conditions. |
FDA approved |
|
4Kscore |
OPKO health |
Blood plasma |
tPSA, fPSA, intact PSA, hK2 |
Initial & Repeat biopsy |
Predicts probability of high-grade (Gleason ≥7) PCa; helps avoid unnecessary biopsies in men with elevated PSA. |
CLIA certified |
|
PCA3 (Prostate cancer antigen 3) |
Hologic |
Post-DRE urine |
PCA3 mRNA (non-coding RNA) |
Repeat biopsy |
Overexpressed in >90% of PCa; reduces unnecessary repeat biopsies following a negative initial biopsy. |
FDA approved |
|
ExoDX prostate (IntelliScore) |
Bio-Techne |
Urine |
Exosomal RNA (ERG, PCA3, SPDEF) |
Initial & Repeat biopsy |
Risk score for predicting high-grade PCa upon initial biopsy; minimizes unnecessary procedures. |
CLIA certified |
|
SelectMDx |
MDxHealth |
Post-DRE urine |
mRNA (HOXC6, DLX1), PSA |
Initial & Repeat biopsy |
Identifies men at high risk for clinically significant PCa; used to decide on the need for initial biopsy. |
CLIA certified |
|
TMPRSS2-ERG |
Various labs |
Post-DRE urine / Tissue |
TMPRSS2-ERG Gene Fusion |
Initial biopsy |
Specific marker for PCa; presence associated with higher tumor volume and aggressiveness. |
Laboratory developed test (LDT) |
|
MiPS (Mi-prostate score) |
Michigan laboratories |
Post-DRE urine |
PCA3, TMPRSS2-ERG mRNA, PSA |
Initial biopsy |
Combines serum PSA with urinary RNA markers to predict risk of high-grade PCa. |
CLIA certified |
|
ConfirmMDx |
MDxHealth |
FFPE tissue |
DNA Methylation (GSTP1, APC, RASSF1) |
Repeat biopsy |
Detects an "epigenetic field effect"; identifies men with a negative biopsy who are at low risk for occult PCa, reducing unnecessary repeat biopsies. |
CLIA certified |
|
Abbreviations: CLIA: Clinical Laboratory Improvement Amendments; DRE: Digital Rectal Examination; fPSA: free PSA; FFPE: Formalin-Fixed Paraffin-Embedded; hK2: human Kallikrein 2; PCa: Prostate Cancer; tPSA: total PSA; TMPRSS2: transmembrane protease, serine 2; ERG: ETS-related gene; PCA3: prostate cancer antigen 3; HOXC6: homeobox C6; DLX1: distal-less homeobox 1; RASSF1: RAS association domain family member 1; APC: adenomatous polyposis coli; GSTP1: glutathione S-transferase pi 1. |
||||||
Biomarker-guided therapies
The most impactful advancements in prostate cancer treatment are those based on molecular profiles. A major challenge in advanced disease is resistance to therapies that target the androgen receptor (AR). Figure 4 illustrates that castration-resistant prostate cancer (CRPC) cells acquire a wide range of escape mechanisms, including AR splice variants (e.g., AR-V7), androgen production within tumors, amplification of the AR gene, and activation by various ligands [23, 58-63]. AR-V7 in circulating tumor cells (CTCs) has emerged as a reliable liquid biopsy biomarker to guide treatment selection in identifying a group with a lower likelihood of response to next-generation AR-targeted therapies, potentially necessitating taxane chemotherapy sooner [58].
Inhibitors of poly (ADP-ribose) polymerase (PARP), such as olaparib and rucaparib, should be considered among the most effective therapies guided by biomarkers. These are agents that exploit the idea of synthetic lethality in tumors with homologous recombination repair (HRR) deficiencies, particularly in those with mutations in the BRCA1/2 gene [23, 63]. Recent endorsements have broadened to encompass a larger spectrum of HRR genes beyond BRCA1/2, and combined strategies using PARP inhibitors with AR-directed treatment are also showing considerable survival benefits in both metastatic castration-resistant prostate cancer (mCRPC) and metastatic castration-sensitive prostate cancer (mCSPC), establishing a new first-line treatment option for a specific group of patients. Regulatory authorizations for several PARP inhibitors in this group characterized by specific molecular traits have been granted thanks to pivotal clinical trials, establishing it as the new standard of care and demonstrating the essential value of extensive genomic profiling [64, 65].
The arsenal for prostate cancer also features immunotherapies, which rely significantly on specific biomarkers. Checkpoint inhibitors like pembrolizumab have shown impressive results, but primarily within a small percentage of tumors exhibiting high microsatellite instability (MSI-H) or a high tumor mutational burden (TMB-H). This has led to tissue-agnostic FDA approvals that highlight a treatment strategy based on molecular phenotype rather than the tissue of origin [66]. These include bispecific T-cell engagers (BiTEs) that focus on PSMA and CD3, along with chimeric antigen receptor (CAR) T-cell therapies aimed at PSMA and NKG2D ligands, both showing initial efficacy in early-phase clinical trials of heavily treated mCRPC.
Targeted and PSMA-directed therapies
The use of prostate-specific membrane antigen (PSMA)-based therapies ranks among the most transformative advances in prostate cancer patient management and generated a new theranostic paradigm. Taking advantage of the promise for diagnosis by PSMA-PET, radioligand therapy uses agents such as Lutetium-177 (177Lu)-PSMA-617 to target radiotherapy to tumor environments and cells over-expressing PSMA [67]. A paradigm-changing phase III study (VISION, TheraP) has shown significantly better overall survival and radiographic progression-free survival in a heavily pretreated mCRPC population, culminating in worldwide regulatory approval. The efficacy of 177Lu-PSMA-617 in the mCRPC prechemo space has now been established by recent studies (PSMAFore), and is under study in metastatic disease hormone sensitive. It is this modality that confers the maximal tumour cell kill and minimum systemic toxicity [43]. The future of RLT lies in the development of more powerful alpha-emitting radionuclides, new PSMA-targeting vectors and tailored dosimetry for a better therapeutic index.
Besides PSMA, other popper agents that have been explored may contribute to the potential for overcoming resistance. Pathway inhibitors of the PI3K/AKT/mTOR pathways, frequently activated by PTEN loss, such as ipatasertib as AKT inhibitors combined with abiraterone have shown benefit in PTEN deficient tumors (major mechanism of resistance when AR is blocked) [68, 69]. There is also growing treatment related gap in t-NEPC, a lethal AR-indifferent state of cancer that needs to be filled by approaches encompassing dysregulated factors such as EZH2 and AURKA commonly observed in this terminal mode of disease [70, 71].
Combination Strategies and Future Directions
The therapeutic combination is considered as the key in order to make treatment more effective, to avoid resistance and to tackle various tumor heterogeneity [72]. BIG ranks high among such priorities; CO AR-guided therapies combined with PARP, AKT or strong biological synergy within a given tumor — e.g., chemotherapy. Novel practice-changing trials show that AR-pathway inhibitor early intensification plus docetaxel or PARP inhibitor improves survival in mCSPC patients and support this treatment approach. These strategies are designed to overcome androgenic-dependent tumor growth and at the same time set up a resistance escape route, resulting in a more effective anti-tumor response [68, 73-75]. Treatment that is adjusted to the individual patient, based on real-time monitoring of molecular information from circulating Tumor DNA (ctDNA), are adaptive treatment schedules that might optimize personalized therapy.
Longitudinal liquid biopsy would facilitate exploration of treatment cycling toward clinical relevance and manipulation of dynamic tumor biology. Having said this, and despite the promising data generated in such a rapidly expanding field, substantial obstacles remain for both researchers and clinicians in the form of intra-tumor heterogeneity, cancer cell plasticity and lineage switching [76-78], as well as cost and complexity profiles of molecularly targeted therapy. Hence, the future of research should aim for universal detection of biomarkers, multimodal strategic therapy combinations (e.g., RLT plus immunotherapy or PARP inhibitors) and construction of scalable and fair precision medicine model to adapt the dynamic molecular profile in each patient's disease [79].
|
Table 2. Spectrum of treatment-related adverse effects in prostate cancer management. |
||
|
Treatment modality |
Common short-term adverse effects |
Common long-term adverse effects |
|
Active surveillance/watchful waiting |
Haematuria; dysuria; erectile dysfunction; unexplained weight loss |
Disease progression; skeletal-related events; cancer-specific mortality |
|
Surgery (Radical prostatectomy) |
Erectile dysfunction; urinary incontinence |
Persistent erectile dysfunction; chronic urinary incontinence |
|
Radiotherapy |
Gastrointestinal irritation (diarrhoea, rectal bleeding); genitourinary symptoms (urgency, haematuria); fatigue |
Chronic bowel dysfunction; erectile dysfunction; persistent urinary symptoms; secondary malignancies |
|
Androgen deprivation therapy |
Hot flushes; fatigue; reduced libido; cognitive changes; bone pain flare |
Osteoporosis; sarcopenia; metabolic syndrome; weight gain; persistent sexual dysfunction |
|
Novel AR-targeted agents |
|
|
|
・Enzalutamide |
Fatigue; cognitive impairment; falls; fractures |
Persistent cognitive effects; osteoporosis |
|
・Abiraterone |
Fluid retention; hypertension; hypokalaemia; hepatotoxicity |
Cardiovascular complications |
|
Chemotherapy (Taxanes) |
Myelosuppression; peripheral neuropathy; fatigue; alopecia; nausea |
Chronic neuropathy; persistent fatigue; oedema |
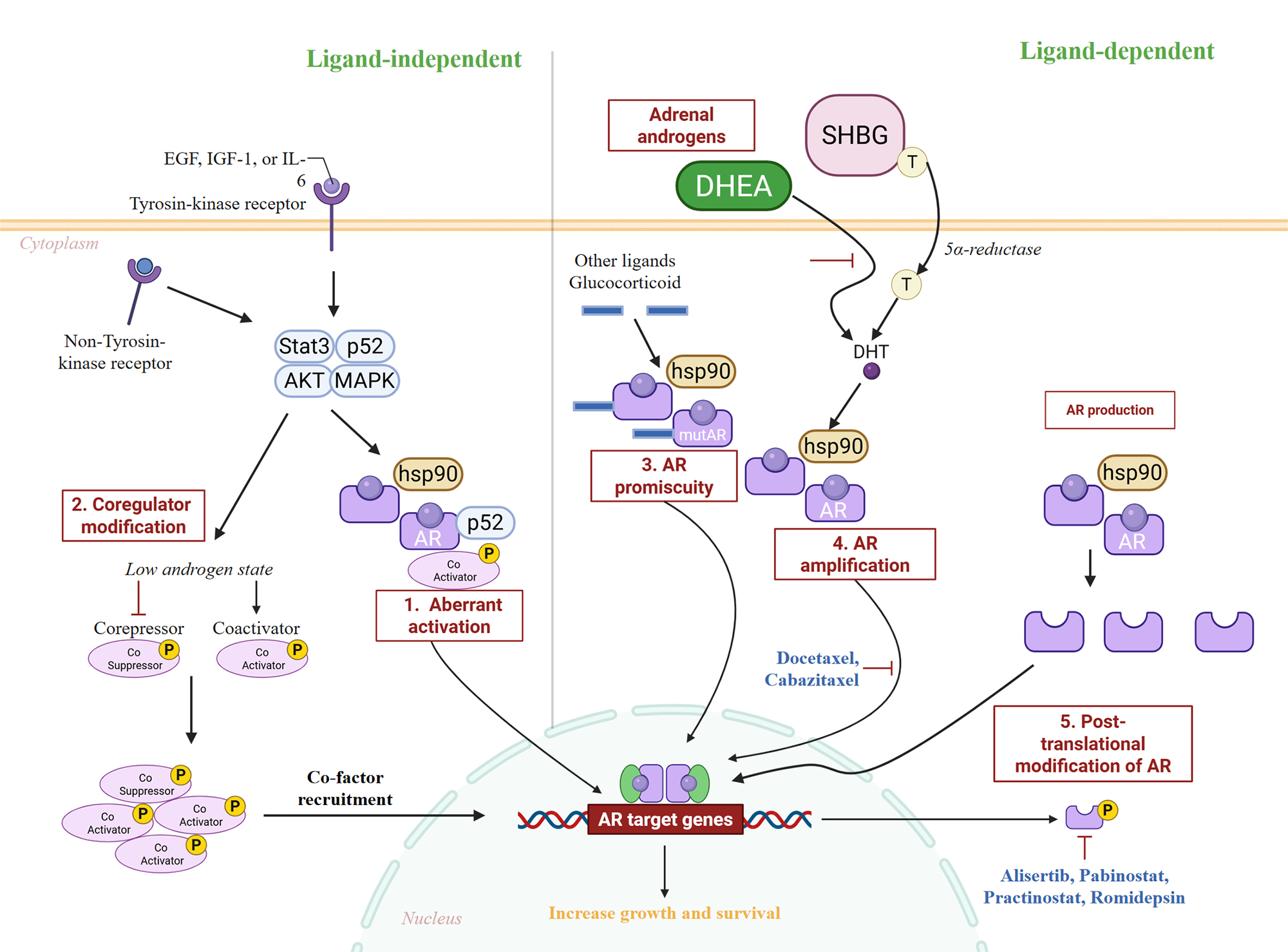 Figure 4. Mechanisms of therapy-induced AR adaptation in prostate cancer. Key molecular adaptations of the AR pathway that facilitate resistance to androgen deprivation therapy. The model highlights how prostate tumors bypass androgen blockade through alternative activation of wild-type AR (e.g., via growth factors, intratumoral androgens), structural alterations to AR itself (e.g., splice variants, mutations), and genomic changes (e.g., amplification). For each mechanism (1-5), corresponding investigational or approved therapeutic interventions are shown.
Figure 4. Mechanisms of therapy-induced AR adaptation in prostate cancer. Key molecular adaptations of the AR pathway that facilitate resistance to androgen deprivation therapy. The model highlights how prostate tumors bypass androgen blockade through alternative activation of wild-type AR (e.g., via growth factors, intratumoral androgens), structural alterations to AR itself (e.g., splice variants, mutations), and genomic changes (e.g., amplification). For each mechanism (1-5), corresponding investigational or approved therapeutic interventions are shown.
Resistance to therapy is a major problem in treatment of patients with prostate cancer. Even with new targeted therapies, immunotherapy and rational combinations, tumors often develop resistance in numerous ways (adaptive) [3, 72]. Changes in the androgen receptor A. Receptor gene amplifications, LBD mutations of the receptor (e.g., F876L) or expression of constitutively active AR splice variants (e.g., AR-V7) can lead to resistance against both traditional androgen deprivation and modern AR-targeted treatments [58, 82]. In addition, the induction and phosphorylation of other survival and growth signaling pathways, including PI3K/AKT/mTOR, MAPK or Wnt/β-catenin, allow tumor cells to acquire resistance during subsequent cycles of initial inhibitory treatment while they proliferate and survive [82, 83]. Additional layers of complexity in therapeutic approaches related to epigenetic plasticity and phenotypic switching, including transformation towards an aggressive treatment-emergent neuroendocrine prostate cancer (t-NEPC) phenotype also frequently occur in rapidly fatal disease [70, 77]. The development of such complex resistance mechanisms highlights the immediate necessity for real-time molecular surveillance within hospital practice to allow rapid recognition of adaptive shifts and prompt adaptation of treatment paradigms [77, 84]. Circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) tests are useful for detecting resistance mutations and monitoring tumor evolution non-invasively. Yet, technical limitations concerning low and high-frequency detection, logistics in sample handling as well as acceptable test price prohibit their broad use in clinical routine applications still [85, 86].
Apart from tumor heterogeneity and resistance to drug, the reliable verification and practical application of new type of biomarker are the main obstacles for precision medicine. Discovery phase studies have identified several highly promising biomarkers, across all the genomic -omics (genomic, epigenomic, proteomic, metabolomic) however to become robust useful tools in a clinical setting the discovery-phase findings need widespread prospective multicenter validation trials [87, 88]. This approach is essential to demonstrating the test’s analytical validity, clinical sensitivity and specificity, prognostic value and – most importantly- predictive value that can guide decisions about treatment in different populations of patients [89]. In the absence of robust validation, novel markers could be overestimated with regard to their clinical value or a practice may be adopted that does not significantly benefit patients. Furthermore, harmonization of assay-related methods, QC procedures and interpretative criteria is critical for enabling uniformity and consistency between various laboratories and clinical areas [90, 91]. Regulation of companion diagnostics, payer reimbursement strategies, and the harmonization of advanced molecular data with existing clinical decision making processes are a few other critical issues that need to be addressed in an organized fashion to overcome barriers for the integration and use of biomarker-based precision medicine.
In the near future, different strategies and technology developers have a great deal of promise to address those limitations. Creating large, comprehensive panels of integrated biomarkers from multiple omics—genomic, transcriptomic, epigenetic, proteomic and imaging—will yield a more complete picture of the biology of tumors and lead to better risk stratification and informed decisions [92, 93]. Such an “adaptive” or “N-of-1” approach to therapy, wherein real-time molecular data on individual lesions obtained from liquid biopsies is hierarchically integrated into its current treatment, has the potential for improving outcomes over long-term periods by dynamically tailoring interventions to the cancer’s evolving local milieu, effectively shifting from a static to dynamic treatment paradigm [94, 95]. Moreover, recent advances in machine learning and artificial intelligence (AI) can enable processing of high-dimensional complex datasets, discovery of hidden predictive patterns, and generation of prospective clinical decision support to an extent that has not previously been possible [96, 97]. This will be crucial, and academia - industry - regulatory alliances will all need to cooperate in a spirit of partnership if the field is to advance biomarker qualification; promote innovative clinical trial designs, including consideration of adaptive designs; increase access for patients who might benefit from beneficial treatments/diagnostics whether they have unmet medical needs or not [98, 99].
In the end, overcoming the obstacles of tumor heterogeneity, treatment resistance, and biomarker validation is critical to delivering on the possibilities promised by precision medicine in prostate cancer [83, 100]. Although it is an exciting and considerable progress, future development would rely on ongoing scientific discovery and translating sophisticated molecular knowledge into practice with prudence [80, 101]. By addressing these issues collectively, the field can strive for truly personalized care that optimizes survival while minimizing treatment-related toxicity and maximizing quality of life for the patient with prostate cancer [19, 102].
But barriers to the full implementation of precision medicine are formidable. Tumor heterogeneity of an extraordinary manner and adaptability potential of cancer cells are the main reasons for unavoidable drug resistance. New biomarkers will only be integrated as validated clinical tools through rigorous prospective validation and standardisation. Dismantling these silos with integrated multi-omics profiling, interpretation of complex information by artificial intelligence, and building capacity for adaptive clinical trials based on liquid biopsy in the context of personalized medicine is the future in clinical genomics. To ensure these novel approaches are accessible to all, academia, industry and regulators need to collaborate. Amidst all these challenges, this field can work towards a distant future where treatment for prostate cancer is truly personalized to achieve the maximal efficacy and minimal toxicity and ultimately with improved long-term outcomes in our patients.
None.
Ethical policy
Non applicable.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
Amira Guedouar contributed to the conception of the review, literature screening, data synthesis, and drafting of the manuscript.Enas Roumieh participated in literature retrieval, verification of extracted information, and critical revision of the final manuscript.
Competing interests
The author declares no competing interests.
Funding
None.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Kirby M, Hirst C, Crawford ED: Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011, 65(11): 1180-1192.
- Watson PA, Arora VK, Sawyers CL: Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 2015, 15(12): 701-711.
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R et al: Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005, 310(5748): 644-648.
- Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, Lotan TL: Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol 2018, 15(4): 222-234.
- Heinlein CA, Chang C: Androgen receptor in prostate cancer. Endocr Rev 2004, 25(2): 276-308.
- Pernar CH, Ebot EM, Wilson KM, Mucci LA: The Epidemiology of Prostate Cancer. Cold Spring Harb Perspect Med 2018, 8(12): a030361.
- Hjelmborg JB, Scheike T, Holst K, Skytthe A, Penney KL, Graff RE, Pukkala E, Christensen K, Adami HO, Holm NV et al: The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev 2014, 23(11): 2303-2310.
- Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, Gillessen S, Van der Kwast T, Bristow RG: Prostate cancer. Nat Rev Dis Primers 2021, 7(1): 9.
- Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, Carroll P, Etzioni R: Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014, 65(6): 1046-1055.
- Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, Bloom J, Gurram S, Siddiqui M, Pinsky P et al: MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med 2020, 382(10): 917-928.
- Surasi DSS, Chapin B, Tang C, Ravizzini G, Bathala TK: Imaging and Management of Prostate Cancer. Semin Ultrasound CT MR 2020, 41(2): 207-221.
- Burk KS, Naik S, Lacson R, Tuncali K, Lee LK, Tempany C, Cole AP, Trinh Q-D, Kibel AS, Khorasani R: MRI-Targeted, Systematic, or Combined Biopsy for Detecting Clinically Significant Prostate Cancer. J Am Coll Radiol 2023, 20(7): 687-695.
- McKone EL, Sutton EA, Johnson GB, Phillips RM: Application of Advanced Imaging to Prostate Cancer Diagnosis and Management: A Narrative Review of Current Practice and Unanswered Questions. J Clin Med 2024, 13(2): 446.
- Kachanov M, Volk AE, Falkenbach F, Möllring A, Hauke J, Rading K, Frommolt P, Becker K, Graefen M, Schmutzler RK et al: Evaluation of Different National Comprehensive Cancer Network Clinical Practice Guidelines in Prostate Cancer for Germline Genetic Testing in Localized and Locally Recurrent Prostate Cancer. Eur Urol Focus 2025, S2405-4569(25): 00151-8.
- Martin SK, Vaughan TB, Atkinson T, Zhu H, Kyprianou N: Emerging biomarkers of prostate cancer (Review). Oncol Rep 2012, 28(2): 409-417.
- Mullane SA, Van Allen EM: Precision medicine for advanced prostate cancer. Curr Opin Urol 2016, 26(3): 231-239.
- de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D et al: Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020, 382(22): 2091-2102.
- Tannock IF, Hickman JA: Limits to Personalized Cancer Medicine. N Engl J Med 2016, 375(13): 1289-1294.
- Cucchiara V, Cooperberg MR, Dall'Era M, Lin DW, Montorsi F, Schalken JA, Evans CP: Genomic Markers in Prostate Cancer Decision Making. Eur Urol 2018, 73(4): 572-582.
- Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS et al: Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019, 17(5): 479-505.
- Giri VN, Beebe-Dimmer JL: Familial prostate cancer. Semin Oncol 2016, 43(5): 560-565.
- Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, Cieslik M, Benelli M, Robinson D, Van Allen EM et al: Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019, 116(23): 11428-11436.
- Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, Bergstralh EJ, Kollmeyer T, Fink S, Haddad Z et al: Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE 2013, 8(6): e66855.
- Knezevic D, Goddard AD, Natraj N, Cherbavaz DB, Clark-Langone KM, Snable J, Watson D, Falzarano SM, Magi-Galluzzi C, Klein EA et al: Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics 2013, 14: 690.
- Cuzick J, Berney DM, Fisher G, Mesher D, Møller H, Reid JE, Perry M, Park J, Younus A, Gutin A et al: Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer 2012, 106(6): 1095-1099.
- Eggener SE, Rumble RB, Armstrong AJ, Morgan TM, Crispino T, Cornford P, van der Kwast T, Grignon DJ, Rai AJ, Agarwal N et al: Molecular Biomarkers in Localized Prostate Cancer: ASCO Guideline. J Clin Oncol 2020, 38(13): 1474-1494.
- Armenia J, Wankowicz SAM, Liu D, Gao J, Kundra R, Reznik E, Chatila WK, Chakravarty D, Han GC, Coleman I et al: The long tail of oncogenic drivers in prostate cancer. Nat Genet 2018, 50(5): 645-651.
- Fraser M, Sabelnykova VY, Yamaguchi TN, Heisler LE, Livingstone J, Huang V, Shiah YJ, Yousif F, Lin X, Masella AP et al: Genomic hallmarks of localized, non-indolent prostate cancer. Nature 2017, 541(7637): 359-364.
- Lord CJ, Ashworth A: PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355(6330): 1152-1158.
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS et al: Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003, 4(3): 209-221.
- Ferraldeschi R, Nava Rodrigues D, Riisnaes R, Miranda S, Figueiredo I, Rescigno P, Ravi P, Pezaro C, Omlin A, Lorente D et al: PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol 2015, 67(4): 795-802.
- Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N et al: Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 2012, 44(6): 685-689.
- Börno ST, Fischer A, Kerick M, Fälth M, Laible M, Brase JC, Kuner R, Dahl A, Grimm C, Sayanjali B et al: Genome-wide DNA methylation events in TMPRSS2-ERG fusion-negative prostate cancers implicate an EZH2-dependent mechanism with miR-26a hypermethylation. Cancer Discov 2012, 2(11): 1024-1035.
- Partin AW, Van Neste L, Klein EA, Marks LS, Gee JR, Troyer DA, Rieger-Christ K, Jones JS, Magi-Galluzzi C, Mangold LA et al: Clinical validation of an epigenetic assay to predict negative histopathological results in repeat prostate biopsies. J Urol 2014, 192(4): 1081-1087.
- Perner S, Hofer MD, Kim R, Shah RB, Li H, Möller P, Hautmann RE, Gschwend JE, Kuefer R, Rubin MA: Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol 2007, 38(5): 696-701.
- Eiber M, Fendler WP, Rowe SP, Calais J, Hofman MS, Maurer T, Schwarzenboeck SM, Kratowchil C, Herrmann K, Giesel FL: Prostate-Specific Membrane Antigen Ligands for Imaging and Therapy. J Nucl Med 2017, 58(Suppl 2): 67s-76s.
- Fendler WP, Weber M, Iravani A, Hofman MS, Calais J, Czernin J, Ilhan H, Saad F, Small EJ, Smith MR et al: Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2019, 25(24): 7448-7454.
- Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N: Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2016, 70(6): 926-937.
- Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, Pattison DA, Tan TH, Kirkwood ID, Ng S et al: [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 2021, 397(10276): 797-804.
- Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, Nguyen HG, Reiter RE, Rettig MB, Okamoto S et al: Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol 2019, 5(6): 856-863.
- Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, Baum RP, Kulkarni HR, Schmidt M, Drzezga A et al: German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med 2017, 58(1): 85-90.
- Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G et al: Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2021, 385(12): 1091-1103.
- Heitzer E, Haque IS, Roberts CES, Speicher MR: Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 2019, 20(2): 71-88.
- Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R, Rosenfeld N: Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017, 17(4): 223-238.
- Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM et al: Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014, 6(224): 224.
- Tomlins SA, Day JR, Lonigro RJ, Hovelson DH, Siddiqui J, Kunju LP, Dunn RL, Meyer S, Hodge P, Groskopf J et al: Urine TMPRSS2:ERG Plus PCA3 for Individualized Prostate Cancer Risk Assessment. Eur Urol 2016, 70(1): 45-53.
- McKiernan J, Donovan MJ, O'Neill V, Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A, Andriole G et al: A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol 2016, 2(7): 882-889.
- Van Neste L, Hendriks RJ, Dijkstra S, Trooskens G, Cornel EB, Jannink SA, de Jong H, Hessels D, Smit FP, Melchers WJ et al: Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score. Eur Urol 2016, 70(5): 740-748.
- Loeb S, Catalona WJ: The Prostate Health Index: a new test for the detection of prostate cancer. Ther Adv Urol 2014, 6(2): 74-77.
- Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, Concepcion R, David RD, Deck KB, Dumbadze I et al: A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol 2015, 68(3): 464-470.
- Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, Slawin KM, Marks LS, Loeb S, Broyles DL et al: A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol 2011, 185(5): 1650-1655.
- Crocetto F, Musone M, Chianese S, Conforti P, Digitale Selvaggio G, Caputo VF, Falabella R, Del Giudice F, Giulioni C, Cafarelli A et al: Blood and urine-based biomarkers in prostate cancer: Current advances, clinical applications, and future directions. J Liq Biopsy 2025, 9: 100305.
- Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, Blazeby JM, Peters TJ, Holding P, Bonnington S et al: Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 2016, 375(15): 1425-1437.
- Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Potosky AL, Stanford JL, Stroup AM et al: Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013, 368(5): 436-445.
- Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL, Penson DF, Rosario DJ, Tombal B, Smith MR: Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015, 67(5): 825-836.
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H et al: Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 2018, 378(15): 1408-1418.
- Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL et al: AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014, 371(11): 1028-1038.
- Heemers HV, Tindall DJ: Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 2007, 28(7): 778-808.
- Gioeli D, Paschal BM: Post-translational modification of the androgen receptor. Mol Cell Endocrinol 2012, 352(1-2): 70-78.
- Kregel S, Chen JL, Tom W, Krishnan V, Kach J, Brechka H, Fessenden TB, Isikbay M, Paner GP, Szmulewitz RZ et al: Acquired resistance to the second-generation androgen receptor antagonist enzalutamide in castration-resistant prostate cancer. Oncotarget 2016, 7(18): 26259-26274.
- Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C et al: Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013, 155(6): 1309-1322.
- Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N et al: DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015, 373(18): 1697-1708.
- Karantanos T, Corn PG, Thompson TC: Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013, 32(49): 5501-5511.
- Graff JN, Alumkal JJ, Drake CG, Thomas GV, Redmond WL, Farhad M, Cetnar JP, Ey FS, Bergan RC, Slottke R et al: Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget 2016, 7(33): 52810-52817.
- Hummel HD, Kufer P, Grüllich C, Seggewiss-Bernhardt R, Deschler-Baier B, Chatterjee M, Goebeler ME, Miller K, de Santis M, Loidl W et al: Pasotuxizumab, a BiTE(®) immune therapy for castration-resistant prostate cancer: Phase I, dose-escalation study findings. Immunotherapy 2021, 13(2): 125-141.
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ et al: μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 2010, 78(4): 756-766.
- de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, Arranz Arija JA, Shih KC, Radavoi GD, Xu N et al: Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res 2019, 25(3): 928-936.
- Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, Massard C, Matsubara N, Alekseev B, Parnis F et al: Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2021, 398(10295): 131-142.
- Dardenne E, Beltran H, Benelli M, Gayvert K, Berger A, Puca L, Cyrta J, Sboner A, Noorzad Z, MacDonald T et al: N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30(4): 563-577.
- Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbé DP, Gomez EC, Wang J et al: Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355(6320): 78-83.
- Chandrasekar T, Yang JC, Gao AC, Evans CP: Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol 2015, 4(3): 365-380.
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A et al: Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017, 377(4): 352-360.
- Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM et al: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015, 373(8): 737-746.
- Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, Jassem J, Fléchon A, Redfern C et al: Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2018, 19(7): 975-986.
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, Brewer DS, Kallio HML, Högnäs G, Annala M et al: The evolutionary history of lethal metastatic prostate cancer. Nature 2015, 520(7547): 353-357.
- Beltran H, Hruszkewycz A, Scher HI, Hildesheim J, Isaacs J, Yu EY, Kelly K, Lin D, Dicker A, Arnold J et al: The Role of Lineage Plasticity in Prostate Cancer Therapy Resistance. Clin Cancer Res 2019, 25(23): 6916-6924.
- Halabi S, Dutta S, Tangen CM, Rosenthal M, Petrylak DP, Thompson IM, Jr., Chi KN, Araujo JC, Logothetis C, Quinn DI et al: Overall Survival of Black and White Men With Metastatic Castration-Resistant Prostate Cancer Treated With Docetaxel. J Clin Oncol 2019, 37(5): 403-410.
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P et al: Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017, 35(1): 40-47.
- Haffner MC, Zwart W, Roudier MP, True LD, Nelson WG, Epstein JI, De Marzo AM, Nelson PS, Yegnasubramanian S: Genomic and phenotypic heterogeneity in prostate cancer. Nat Rev Urol 2021, 18(2): 79-92.
- Boutros PC, Fraser M, Harding NJ, de Borja R, Trudel D, Lalonde E, Meng A, Hennings-Yeomans PH, McPherson A, Sabelnykova VY et al: Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat Genet 2015, 47(7): 736-745.
- Crumbaker M, Khoja L, Joshua AM: AR Signaling and the PI3K Pathway in Prostate Cancer. Cancers (Basel) 2017, 9(4): 34.
- Sawyers CL: The cancer biomarker problem. Nature 2008, 452(7187): 548-552.
- Wyatt AW, Gleave ME: Targeting the adaptive molecular landscape of castration-resistant prostate cancer. EMBO Mol Med 2015, 7(7): 878-894.
- Heidrich I, Ačkar L, Mossahebi Mohammadi P, Pantel K: Liquid biopsies: Potential and challenges. Int J Cancer 2021, 148(3): 528-545.
- Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, Bivona T, Diehn M, Dive C, Dziadziuszko R et al: Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J Thorac Oncol 2021, 16(10): 1647-1662.
- Ioannidis JPA, Bossuyt PMM: Waste, Leaks, and Failures in the Biomarker Pipeline. Clin Chem 2017, 63(5): 963-972.
- Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, Vergara IA, Davicioni E, Erho N, Ghadessi M et al: The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet 2013, 45(11): 1392-1398.
- Simon RM, Paik S, Hayes DF: Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009, 101(21): 1446-1452.
- Roy S, Coldren C, Karunamurthy A, Kip NS, Klee EW, Lincoln SE, Leon A, Pullambhatla M, Temple-Smolkin RL, Voelkerding KV et al: Standards and Guidelines for Validating Next-Generation Sequencing Bioinformatics Pipelines: A Joint Recommendation of the Association for Molecular Pathology and the College of American Pathologists. J Mol Diagn 2018, 20(1): 4-27.
- Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, Lindeman N, Lockwood CM, Rai AJ, Schilsky RL et al: Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol 2018, 36(16): 1631-1641.
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P et al: Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017, 35(1): 40-47.
- Spratt DE, Zumsteg ZS, Feng FY, Tomlins SA: Translational and clinical implications of the genetic landscape of prostate cancer. Nat Rev Clin Oncol 2016, 13(10): 597-610.
- Letai A, Bhola P, Welm AL: Functional precision oncology: Testing tumors with drugs to identify vulnerabilities and novel combinations. Cancer Cell 2022, 40(1): 26-35.
- Bos MK, Angus L, Nasserinejad K, Jager A, Jansen MPHM, Martens JWM, Sleijfer S: Whole exome sequencing of cell-free DNA - A systematic review and Bayesian individual patient data meta-analysis. Cancer Treat Rev 2020, 83: 101951.
- Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, Cui C, Corrado G, Thrun S, Dean J: A guide to deep learning in healthcare. Nat Med 2019, 25(1): 24-29.
- Litjens G, Sánchez CI, Timofeeva N, Hermsen M, Nagtegaal I, Kovacs I, Hulsbergen - van de Kaa C, Bult P, van Ginneken B, van der Laak J: Deep learning as a tool for increased accuracy and efficiency of histopathological diagnosis. Scientific Reports 2016, 6(1): 26286.
- Woodcock J, LaVange LM: Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N Engl J Med 2017, 377(1): 62-70.
- Collins FS, Varmus H: A new initiative on precision medicine. N Engl J Med 2015, 372(9): 793-795.
- He W, McCoy MD, Riggins RB, Beckman RA, Yeang C-H: Personalized cancer treatment strategies incorporating irreversible and reversible drug resistance mechanisms. NPJ Syst Biol Appl 2025, 11(1): 70.
- Ma L, Guo H, Zhao Y, Liu Z, Wang C, Bu J, Sun T, Wei J: Liquid biopsy in cancer: current status, challenges and future prospects. Signal Transduct Target Ther 2024, 9(1): 336.
- Mateo J, Bono JSd, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Agarwal N, Olmos D, Thiery-Vuillemin A et al: Olaparib for the Treatment of Patients With Metastatic Castration-Resistant Prostate Cancer and Alterations in BRCA1 and/or BRCA2 in the PROfound Trial. J Clin Oncol 2024, 42(5): 571-583.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript