Review Article | Open Access
Update on How to Improve the Outcome of Radical Cystectomy: A Systematic Review
Ahmed M. Moeen1, Hassan A Aboul-Ella1
1Assiut Urology and Nephrology Hospital, Assiut University, Assiut 71516, Egypt.
Correspondence: Ahmed M. Moeen ( Assiut Urology and Nephrology Hospital, Assiut University, Assiut 71516, Egypt; Email: moeen3@yahoo.com).
Annals of Urologic Oncology 2022; 5(1): 1-7. https://doi.org/10.32948/auo.2022.05.10
Received: 25 Mar 2022 | Accepted: 21 Apr 2022 | Published online: 19 May 2022
Objective This systematic review tries to put an up to date analysis of the literature on how to improve the outcome of RCX.
Evidence acquisition A systematic literature search in the PubMed and Cochrane databases was performed from 1990 to July 2022 in English language using the keywords ‘‘radical cystectomy’, ‘Enhanced recovery’ and ‘Improved outcome’. Prospective studies were preferred; however, retrospective studies were used when no prospective studies were available.
Evidence synthesis In all, 237 relevant articles were identified and 46 articles were included in this systematic review. RCX may be associated by complications that may reach 70%. Preoperative patient preparation, optimization and counseling are critical. Enhanced recovery after surgery protocols should be adopted. The radicality of surgery is affected by the use of neo-adjuvant and/or adjuvant therapy, timing of surgery, the presence of a well-organized team and the surgeon experience. Ureteral dissection, urethral stump preparation and nerve sparing are three important steps during RCX greatly affecting the function of the following reconstructive step. Close follow up after RCX especially in the first two years is critical.
Conclusions Multiple factors should be followed to achieve good RCX. Regular skilled operative team, high volume surgeon, well equipped operative theater, excellent postoperative care are keys of success.
Key words Radical cystectomy, improved outcome, invasive bladder cancer
Multiple factors affecting these outcomes which includes; the patients’ characteristics, tumor characteristics, previous management, type of RCX, and some technical points. The patients are usually elderly, hard workers in Egypt, having some co-morbid conditions as diabetes, hypertension, and cardio-pulmonary diseases. Some patients are delaying RCX wishing to preserve the bladder. The previous management- as bladder preservation, neo-adjuvant CTH(Chemotherapy), previous pelvic surgery- also are important factors. The different types of RCX, whether an early cystectomy, genital sparing, prostate/seminal sparing and salvage cystectomy, may have different outcomes. Some technical points as lymphadenectomy, urethral stump preparation, intestinal selection and the diversion type are also affecting the outcome (Table 1).
Herein, this systematic review tries to put an up to date analysis of the literature on how to improve the outcome of this kind of advanced surgery.
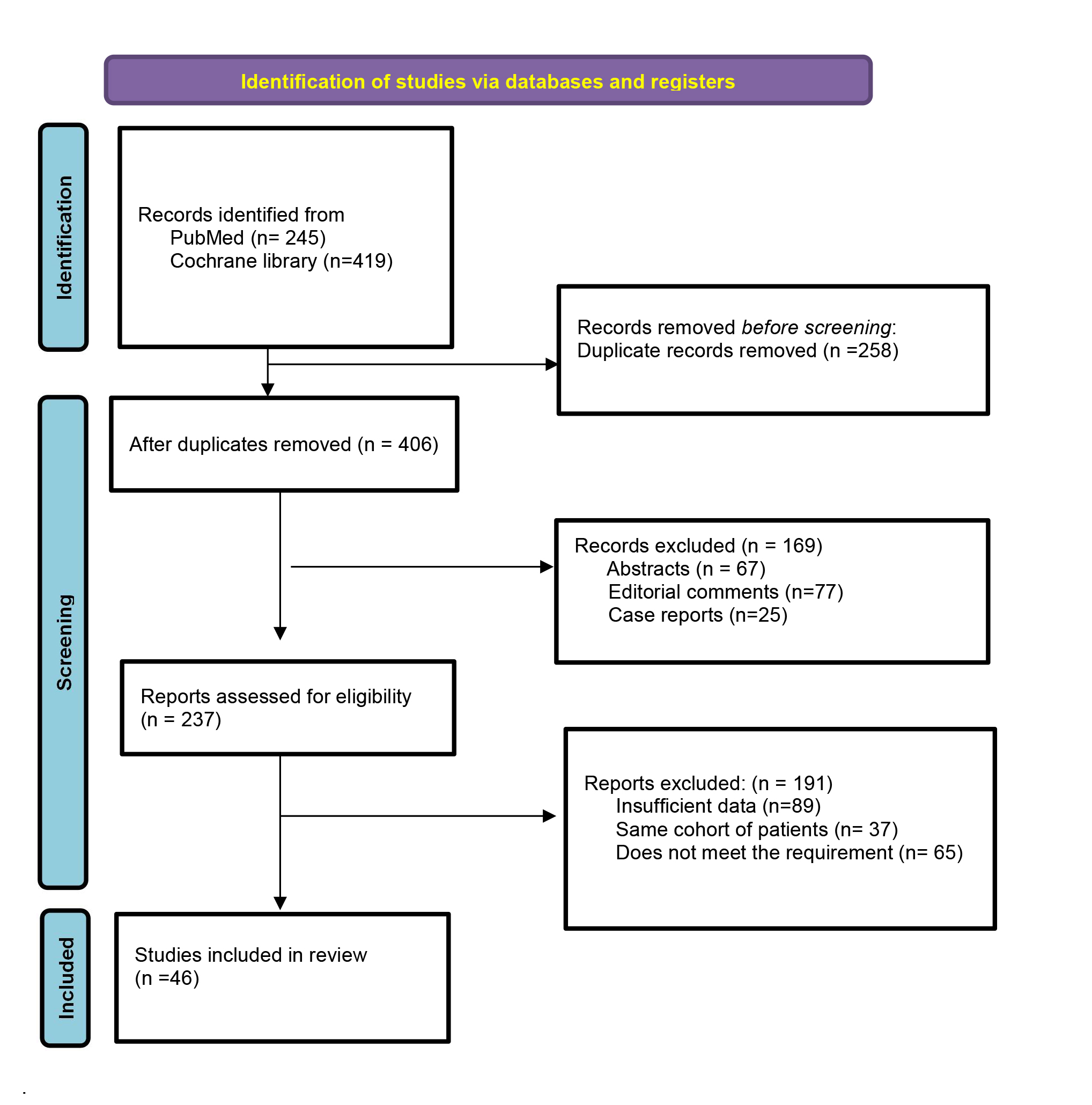 Figure 1. Flow chart for the selection of the studies according to the PRISMA statement.
Figure 1. Flow chart for the selection of the studies according to the PRISMA statement.
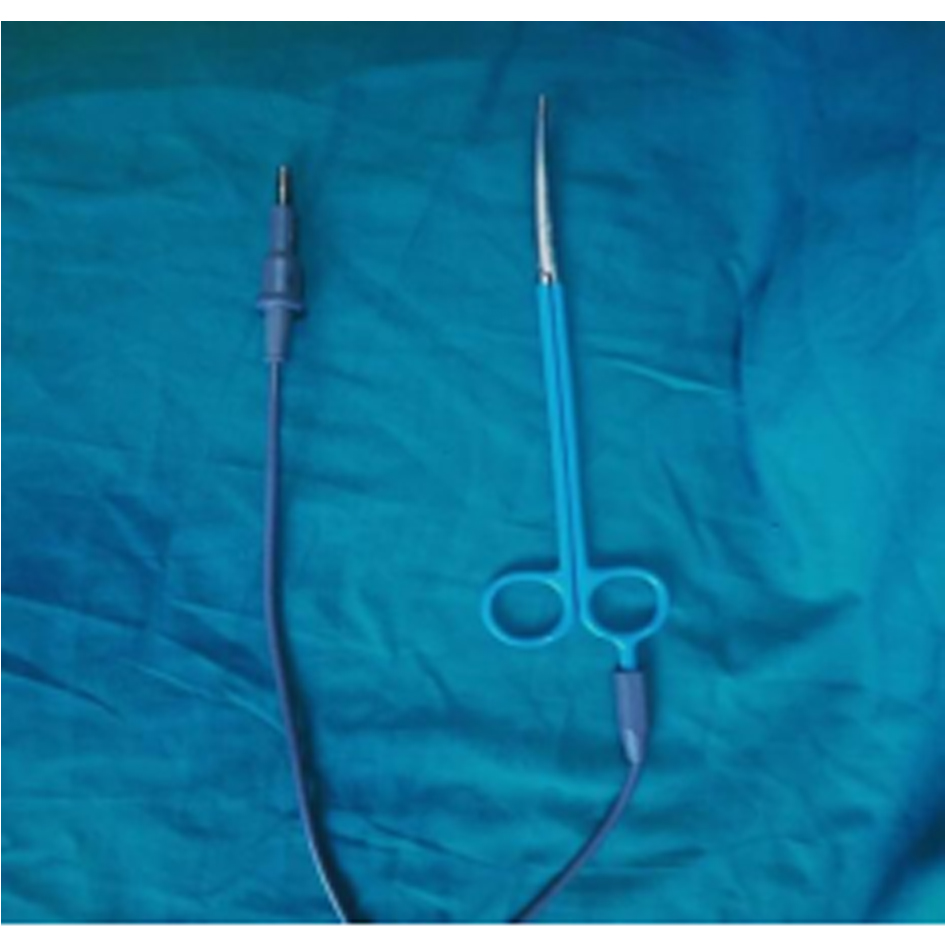
Figure 2. Electro-thermal scissor.
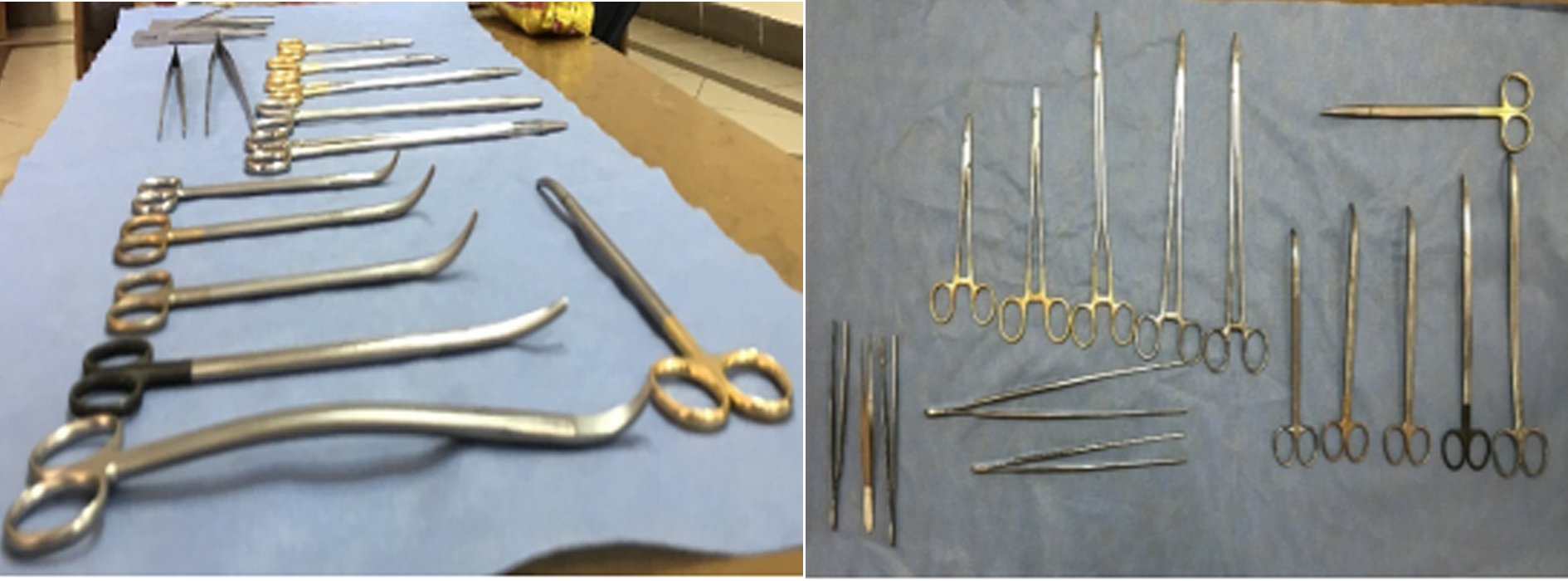 Figure 3. Surgical tray showing instruments of different lengths and curves.
Figure 3. Surgical tray showing instruments of different lengths and curves.
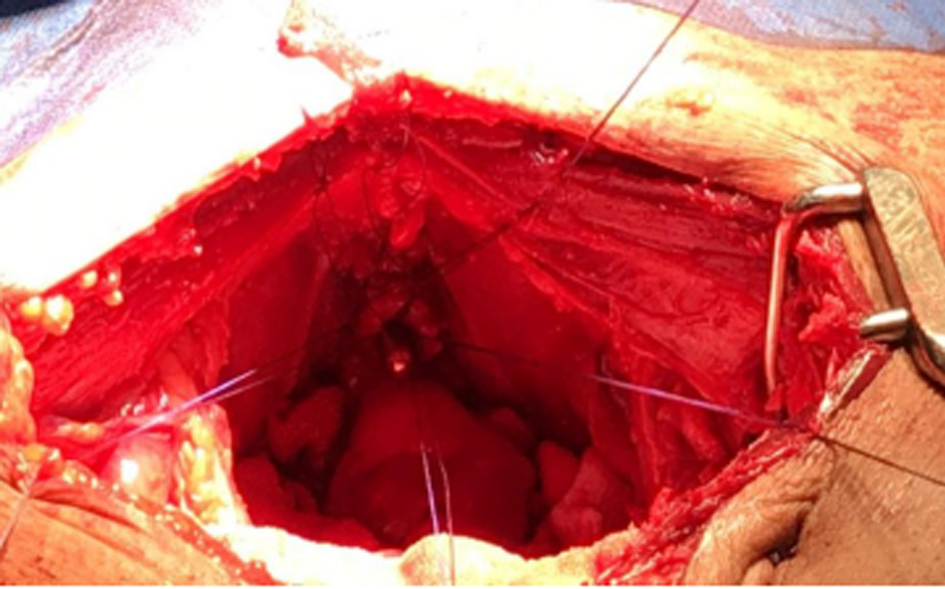 Figure 4. Well-prepared urethral stump with fixed urethral sutures.
Figure 4. Well-prepared urethral stump with fixed urethral sutures.
Eligibility
RCX is indicated for the management of transitional cell carcinoma (T2-T4a, T1 tumors at high risk of progression i.e. high grade, multifocal, CIS and T1 patients failing intravesical therapy). Also, it is performed for aggressive variant as squamous cell carcinoma, adenocarcinoma and the micropapillary carcinoma of the bladder. It is contraindicated in patients with uncorrected cardiac, hepatic, renal or pulmonary diseases. Un-experienced surgeons, unequipped operative theater and inadequate postoperative care are also contraindications for this advanced surgery. So, this surgery is best mastered at high volume centers with high case load performing at least 40-50 RCXs/year [4].
Patients should be adequately counseled about the risks and benefits of this surgery and the quality of life changes after surgery. Also, the patient should be informed that the selected type of urinary diversion may be subjected to intraoperative conversion to another form due to any anatomic, oncologic or anesthetic necessities, although infrequent, is an option [2]. This realistic counseling greatly improve the patient’s expectations.
Preoperatively, detailed history and physical examination should be performed. Imaging studies includes: abdominal ultrasound, enhanced abdomino-pelvic CT, chest x-ray ± chest CT, echocardiography and bone scan in certain patients. Laboratory workup as serum creatinine, complete liver functions, complete blood count and coagulation profile are basics. TUR biopsy from the bladder tumor and abnormal looking urothelium followed by examination under anesthesia then follows. For neobladder, TUR biopsy from prostatic urethra in males/bladder neck in females may be done [5].
Recoverability
In order to achieve a good recovery for this multistep surgery, some precautions, called enhanced recovery after surgery (ERAS), should be adopted. Preoperative medical optimization for any associated comorbid medical conditions should be done first. Bowel preparations can be safely omitted as the ileum is usually utilized for conduit and neobladder reconstruction. Clear fluids till 2 hrs and solid food till 6 hrs before the operation are safe. In order to the decrease the incidence of ileus, long-term sedatives and intravenous fluids overuse should be minimized. A recent randomized trial reported that the usage of intravenous lidocaine infusion with enhanced recovery pathway in patients underwent open RCX improve the gut motility [6]. Deep venous thrombosis (DVT) is not uncommon postoperative morbidity especially in elderly patients, females and after previous pelvic surgery. A well-fitting compressive stocking and low molecular weight heparin in the arms instead of thigh is a good prophylaxis [7]. Prophylactic antimicrobial 1 hr before skin incision and skin preparation with Chlorhexidine-alcohol are important to decrease wound infection. Epidural analgesia is better in relieving pain than opioids for the first 72 hrs. Perioperative fluid management and avoidance of intraoperative hypothermia are critical. Nasogastric tube is optional and not a routine [8]. Early oral diet as soon as the patients is passing flatus and early mobilization to improve the intestinal function and guard against DVT [2, 9].
Radicality
The most critical outcome is the radicality of surgery, which is affected by certain factors. These include, the use of neo-adjuvant and/or adjuvant therapy, timing of surgery, the presence of a well-organized team, some technical points and the surgeon impact.
Neo-adjuvant chemotherapy (NAC) is recommended for T2-T4 Bca. It is beneficial in early treatment of micrometastasis, assessing tumor response in vivo and delivery of the required dose of CTH before the occurrence of extensive fibrosis after surgery. It was shown to improve the survival by 5-8% [10]. However, a delay in RCX about 2 months and exposing some patients to CTH complications are considered limitations and reasons for being underutilized. The South West Oncology Group (SWOG) reported 5-year survival of 43% for the cystectomy group and 57% for the NAC plus surgery group. Also, another study showed 5% survival advantage with neoadjuvant cisplatin-based chemotherapy [11]. These data indicate that, 95% of patients receiving NAC may not benefit, its use is not a routine and needs judgment and is recommended in aggressive variants as micropapillary and small cell types [12].
Adjuvant chemotherapy is recommended for patients with advanced loco-regional disease if NAC was not utilized. It was shown to improve the disease-free survival by 70% at 3 years in the chemotherapy group compared to 46% in the observation group [13]. On the other hand, neo-adjuvant radiotherapy is not recommended especially when RCX and urinary diversion is planned [14].
Timing of RCX is very crucial. A delay more than 3 months was shown to be associated with worse oncological outcomes [15-17]. Therefore, early cystectomy will improve the oncologic outcomes, survival and increase the possibility of having nerve sparing and orthotopic neobladder [18].
Regarding lymphadenectomy, all lymphatic tissues around the common iliac, external iliac, internal iliac, and the obturator group bilaterally should be removed. About 20 pelvic lymph nodes (LN) could represent the standard number of nodes removed. This enables clearance of 80% of positive nodes. Some advocates if frozen section reported no positive LN in the true pelvis, further cranial LN dissection of less importance. If the latter is not performed or identifies positive nodes, the inferior mesenteric artery should be the cranial limit of LN dissection [19-20]. A study in which LN dissection was carried out up to the inferior mesenteric artery, showed that the prognosis of extrapelvic nodal disease is dismal. So, the usefulness of routine superextended LN dissection is questionable [21-22]. Moreover, to increase the yield of LNs, lymphatic tissues should be sent in separate submissions for pathologic evaluation [23].
The term “LN density” which is the ratio of the number of positive nodes to the total number of removed nodes may serve as a prognostic factor. It gives an idea about the extent of node dissection by the total number of nodes and the tumor burden by the number of positive nodes. A ratio of < 20% and 25% had been suggested to have better chance of survival than those with higher ratios [24-25]. However, absence of a fixed proximal extent of lymphadenectomy and standardization of the number of nodes that should be removed are limitations.
The main surgeon greatly affects the oncological outcome, the correct choice and performance of the reconstructive procedure. Also, the presence of a regular skilled operative team shortens the duration of the operation [26]. Herr and colleagues concluded that the quality of RCX is more important than NAC. Also, It was shown that high volume urologists had a margin positive rate of 4% if compared to 14% of low volume ones. Local recurrence developed in 68% of margin positive patients if compared to 6% in margin negative patients. Mortality from RCX at low versus high volume hospitals was 3.1 % versus 0.7%. The overall survival is greatly affected by the surgical margins and the number of LN removed independent of patient age, pathological stage, nodal status and NAC and this is surgeon dependent [27].
Some technical points should be cautiously evaluated as prostatic capsule/seminal sparing and genital sparing RCX. The former initially performed to improve the potency by preserving the neurovascular bundle with a potency rate up to 90 %. But, the incidence of incidental prostatic carcinoma was high in cystoprostatectomy specimens (up to 48%). Moreover, it is associated with 10–15% higher oncological failure rate. So, it is considered by Hautmann a step in the wrong direction [28]. Genital sparing RCX was developed for a selected group of women especially young premenopausal female seeking childbearing [29]. Patients indicated should have an organ-confined disease located above the peritoneal reflection to avoid the oncological failure [30]. Additionally, preserving these organs provides a functional advantage after the orthotopic neobladder by providing a back support to avoid pouch-urethral angulation and chronic retention [10].
Regarding the instruments, in a prospective study evaluating the impact of vessel sealing devices on the outcome of open RCX in comparison with the conventional methods of dissection; RCX was carried out in conventional ligation/clip in a control group (n = 22) or electro-thermal device in the study group (n = 25). The latter was used to divide the posterior and lateral pedicles of bladder. They concluded that; the use of electro-thermal devices reduce the blood loss, saves the operating time and is useful in dividing deep vessels in the pelvis [31]. Additionally, in our practice we, use an electric scissor during lymphadenectomy, this shorten the duration of surgery to a great extent (Figure 2). We think that this saves the fitness of the surgeon for the 2nd step after RCX which is the urinary diversion. Also, long curved scissors of different lengths and curves to fit the different slops of different prostates are important to preserve the maximal functional urethral length to overcome the difficulties of urethro-enteric anastomosis (Figure 3) [32].
The importance of minimally invasive laparoscopic and robotic RCX is addressed in multiple studies. These approaches assume fast recovery, less pain, blood loss and improved cosmosis [33]. However, the higher costs, the length of the procedure which is 2-4 hours more, lower number of retrieved lymph nodes, the unusual sites of metastatic disease that can be explained with hyper Co2 intra-peritoneal pressure and absence of long term results and randomized studies are their limitations [34].
Functionality
During RCX, some functions are lost; others are tried to be maintained and to be restored during urinary diversion. Ureteral dissection, urethral stump preparation and nerve sparing are three important steps during RCX greatly affecting the function of the following reconstructive step. Ureteric dissection should be in well vascularized a-traumatic manner, with no extensive upward dissection, as lengthy as possible, with safety margin, clipped if normal to allows hydro-distention and facilitates future re-implantation and preserve a dry field and limits the absorption through intestinal surface. The left ureter should have a smooth curve if passing to the other side to be reimplanted in the future reservoir. The ureters should be Spatulated and the uretero-enteric anastomosis should be stented and without tension. These precautions improve the uretero-enteric anastomosis and protect the upper urinary tract [32].
Nerve sparing surgery was shown not to improves the sexual function only, but improve the continence also as shown in multiple studies [35-37]. But, this should be done without negatively affecting the oncologic outcome [38].
The urethral stump should be dissected gently in a bloodless manner by careful securing the deep dorsal vein and the santorini’s plexus. Trying to preserve a maximal urethral length and preserving the NVB which will improve the continence also [37]. In our practice, at least six urethral sutures should be fixed to the urethral stump for good stabilization of the neobladder if it will be performed (Figure 4).
In female patients indicated for neobladder reconstruction, no dissection should be done anterior to the urethra along the pelvic floor. The endopelvic fascia should remain undisturbed. The posterior vaginal wall is opened below the cervix. In case of posterior bladder tumor, the anterior vaginal wall should be removed en bloc with the cystectomy specimen. In order to leave a functioning vagina, it should be closed horizontal or side to side and covered by omentum. The omentum serves as a barrier to prevent pouch-vaginal fistula [39]. Additionally, it serves as a back support behind the pouch to avoid the pouch falling back in the wide pelvic cavity and angulation of the poucho-urethral angle. The latter will lead to chronic retention [10].
Availability
Close follow up after RCX especially in the first two years is critical for three main endpoints; assessing the functional outcome, treatment of complications and for early detection and treatment of oncological failures.
RCX has significant peri- and postoperative complications rate up to 50 –64 % [40-42]. The risk of postoperative complications and outcome are affected by several factors, such as performance status, age, preexisting comorbidities and surgeon experience [43-45]. Anyhow, the best functional and oncological results can be achieved if RCX and urinary diversion were performed in a high-volume hospital with at least 40–50 cases annually by high-volume surgeons and an experienced team [46].
To the best of our knowledge, to decrease the postoperative complications, a special attention should be paid to previously operated cases, previous radiotherapy, obese patients, elderly patients, previous bowel resection, ectopic pelvic kidneys, tumor inside diverticulum and transplanted kidney which are technically challenging and may be associated with a higher complication rate.
Ahmed M. Moeen is responsible on studies evaluation, selection, data collection and writing of the manuscript;
Hassan A Aboul-Ella is responsible on studies evaluation, selection and revision of the manuscript.
Competing interests
Dr. Ahmed M. Moeen declares that he has no conflict of interest.
Funding
None.
Ethical Approval
Ethical committee review was not required as the manuscript is a review of the published literature.
- Ferlay J, Parkin DM, Bray F, Pisani P: Global cancer statistics. CA Cancer J Clin 2005, 55(2): 74-108.
- Richard E. Hautmann, Henry Botto, Urs E. Studer: How to Obtain Good Results with Orthotopic Bladder substitution: The 10 Commandments. European urology 2009, supplements 8: 712-717.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009, 62(10): 1006-1012.
- Bernhard Kiss, Fiona C. Burkhard, George N: Thalmann: Open radical cystectomy: still the gold standard for muscle invasive bladder cancer. World J Urol 2016, 34(1): 33-39.
- John P. Stein and Donald G: Skinner: Radical Cystectomy. BJU International 2004, 94(1): 197- 221.
- SM Moeen, AM Moeen: Usage of Intravenous Lidocaine Infusion with Enhanced Recovery Pathway in Patients Scheduled for Open Radical Cystectomy: A Randomized Trial. Pain physician 2019, 22 (2): E71-E80.
- Bradley CT, Brasel KJ, Miller JJ, Pappas SG: Cost-effectiveness of prolonged thromboprophylaxis after cancer surgery. Ann Surg Oncol 2010, 17(1): 31-39.
- Donat SM, Slaton JW, Pisters LL, Swanson DA: Early nasogastric tube removal combined with metoclopramide after radical cystectomy and urinary diversion. J Urol 1999, 162(5): 1599-1602.
- Patel HR, Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, Kassouf W, Müller S, Baldini G et al: Enhanced recovery after surgery: are we ready, and can we afford not to implement these pathways for patients undergoing radical cystectomy? Eur Urol 2014, 65(2): 263-266.
- Ghoneim MA, Abol-Enein H: Management of muscle-invasive bladder cancer: an update. Nat Clin Pract Urol 2008, 5 (9): 501-508.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Neo-adjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patients data. Eur Urol 2005, 48(2): 202-206.
- Siefker-Radtke AO, Dinney CP, Abrahams NA, Moran C, Shen Y, Pisters LL, Grossman HB, Swanson DA, Millikan RE: Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol 2004, 172(2): 481-484.
- Skinner DG, Daniels JR, Russell CA, Lieskovsky G, Boyd SD, Nichols P, Kern W, Sakamoto J, Krailo M, Groshen S: The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol 1991, 145(3): 459-464.
- Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A, Neuzillet Y, et al: European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol 2021, 79(1): 82-104.
- Ghoneim MA, el-Mekresh MM, el-Baz MA, el-Attar IA, Ashamallah A: Radical cystectomy for carcinoma of the bladder: critical evaluation of the results in 1,026 cases. J Urol 1997, 158(2): 393-399.
- Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, Studer UE: Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol 2003, 21(4): 690-696.
- Stein JP and Skinner DJ: Results with radical cystectomy for treating bladder cancer: a reference standard for high grade, invasive bladder cancer. BJU Int 2003, 92(1): 12-17
- Chang SS, Hassan JM, Cookson MS, Wells N, Smith JA Jr: Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol 2003, 170(4 Pt 1): 1085-1087.
- Stein JP, Cai J, Groshen S, Skinner DG: Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol 2003, 170(1): 35-41.
- Weingärtner K, Ramaswamy A, Bittinger A, Gerharz EW, Vöge D, Riedmiller H: Anatomical basis for pelvic lymphadenectomy in prostate cancer: results of an autopsy study and implications for the clinic. J Urol 1996, 156(6): 1969-1971.
- Abol-Enein H, El-Baz M, Abd El-Hameed MA, Abdel-Latif M, Ghoneim MA: Lymph node involvement in patients with bladder cancer treated with radical cystectomy: a patho-anatomical study--a single center experience. J Urol 2004, 172(5 Pt 1): 1818-1821.
- Vieweg J, Gschwend JE, Herr HW, Fair WR: Pelvic lymph node dissection can be curative in patients with node positive bladder cancer. J Urol 1999, 161(2): 449-454.
- Bochner BH, Herr HW, Reuter VE: Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol 2001, 166 (6): 2295-2296.
- Herr HW: Superiority of ratio based lymph node staging for bladder cancer. J Urol 2003, 169(6): 943-945
- Kassouf W, Leibovici D, Munsell MF, Dinney CP, Grossman HB, Kamat AM: Evaluation of the relevance of lymph node density in a contemporary series of patients undergoing radical cystectomy. J Urol 2006, 176(1): 53-57.
- Elting LS, Pettaway C, Bekele BN, Grossman HB, Cooksley C, Avritscher EB, Saldin K, Dinney CP: Correlation between annual volume of cystectomy, professional staffing, and outcomes: a statewide, population-based study. Cancer 2005, 104(5): 975-984.
- Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere White R, Sarosdy MF, Crawford ED: Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol 2004, 22(14): 2781-2789.
- Hautmann RE and Stein JP: Neobladder with prostatic capsule and seminal-sparing cystectomy for bladder cancer: a step in the wrong direction. Urol ClinN Am 2005, 32(2): 177-185
- Ali-El-Dein B, Abdel-Latif M, Mosbah A, Eraky I, Shaaban AA, Taha NM, Ghoneim MA: Secondary malignant involvement of gynecologic organs in radical cystectomy specimens in women: is it mandatory to remove these organs routinely? J Urol 2004, 172(3): 885-887.
- Chang SS, Cole E, Smith JA Jr, Cookson MS: Pathological findings of gynecologic organs obtained at female radical cystectomy. J Urol 2002, 168(1): 147-149.
- Seth A, Panda S, Kumar R, Gupta N, & Dogra P: Impact of Vessel Sealing Device on Outcome of Radical Cystectomy. Urology 2009, 74(4): S107.
- Moeen AM, Safwat AS, Gadelmoula MM, Moeen SM, Behnsawy HM, Shahat AA, Gadelkareem RA, Hameed DA, Hammouda HM: Does the site of the orthotopic neobladder outlet matter? A prospective randomized comparative study. Eur J Surg Oncol 2018, 44(6): 847-852.
- Menon M, Hemal AK, Tewari A, Shrivastava A, Shoma AM, El-Tabey NA, Shaaban A, Abol-Enein H, Ghoneim MA: Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int 2003, 92(3): 232-236.
- Sathianathen NJ, Kalapara A, Frydenberg M, Lawrentschuk N, Weight CJ, Parekh D, Konety BR: Robotic Assisted Radical Cystectomy vs Open Radical Cystectomy: Systematic Review and Meta-Analysis. J Urol 2019, 201(4): 715-720.
- Hekal IA, El-Bahnasawy MS, Mosbah A, El-Assmy A, Shaaban A: Recoverability of erectile function in post-radical cystectomy patients: subjective and objective evaluations. Eur Urol 2009, 55(2): 275-283.
- Kessler TM, Burkhard FC, Studer UE: Clinical indications and outcomes with nerve-sparing cystectomy in patients with bladder cancer. Urol Clin North Am 2005, 32(2): 165-175.
- Furrer MA, Studer UE, Gross T, Burkhard FC, Thalmann GN, Nguyen DP: Nerve-sparing radical cystectomy has a beneficial impact on urinary continence after orthotopic bladder substitution, which becomes even more apparent over time. BJU Int 2018, 121(6): 935-944.
- Pritchett TR, Schiff WM, Klatt E, Lieskovsky G, Skinner DG: The potency-sparing radical cystectomy: does it compromise the completeness of the cancer resection? J Urol 1988, 140(6): 1400-1403.
- Stein JP, Skinner DG: Surgical atlas. Radical cystectomy. BJU Int. 2004, 94(1): 197-221.
- Donat SM, Shabsigh A, Savage C, Cronin AM, Bochner BH, Dalbagni G, Herr HW, Milowsky MI: Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol 2009, 55(1): 177-185.
- Roth B, Birkhauser FD, Zehnder P, Burkhard FC, Thalmann GN, Studer UE: Readaptation of the peritoneum following extended pelvic lymphadenectomy and cystectomy has a significant beneficial impact on early postoperative recovery and complications: results of a prospective randomized trial. Eur Urol 2011, 59(2): 204-210.
- Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, Raj G, Bochner BH, Dalbagni G, Herr HW, Donat SM: Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009, 55(1): 164-174.
- Bolenz C, Ho R, Nuss GR, Ortiz N, Raj GV, Sagalowsky AI, Lotan Y: Management of elderly patients with urothelial carcinoma of the bladder: guideline concordance and predictors of overall survival. BJU Int 2010, 106(9): 1324-1329.
- Mayr R, May M, Martini T, Lodde M, Pycha A, Comploj E, Wieland WF, Denzinger S, Otto W, Burger M, Fritsche HM: Predictive capacity of four comorbidity indices estimating perioperative mortality after radical cystectomy for urothelial carcinoma of the bladder. BJU Int 2012, 110(6 Pt B): E222-E227.
- Roth B, Thalmann GN: Standard Cystectomy fits all: myth or truth. Transl Androl Urol 2015, 4(3): 254-260.
- Hautmann RE, Abol-Enein H, Davidsson T, Gudjonsson S, Hautmann SH, Holm HV, Lee CT, Liedberg F, Madersbacher S, Manoharan M, et al: International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012. ICUD-EAU International Consultation on Bladder Cancer 2012: Urinary diversion. Eur Urol 2013, 63(1): 67-80.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript