Review Article | Open Access
Research Progress in Focal Treatment of Prostate Cancer
Songtao Cheng1, Shize Wang1, Jing Liu1, Jianlin Huang1, Jiannan Liu1
1Department of Urology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu 610072, China.
Correspondence: Jiannan Liu (Department of Urology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, No. 32, West Section 2, First Ring Road, Chengdu, China, 610072, China; Email: liujiannan1213@163.com).
Annals of Urologic Oncology 2023, 6(2): 79-86. https://doi.org/10.32948/auo.2023.06.24
Received: 09 Jun 2023 | Accepted: 23 Jun 2023 | Published online: 25 Jun 2023
Key words prostate cancer, focal treatment, high intensity focused ultrasound, focal laser ablation, cryotherapy
High intensity focused ultrasound (HIFU) is one of the most commonly used focal treatment modalities. The ultrasound probe is placed in the rectum at the beginning of the procedure. The prostate volume is measured, and an image is created. The doctor marks the treated area on the image. Through HIFU, precise, concentrated ultrasound waves are delivered to the target tissue. Within 2-3s, the temperature of the tissue can be raised to nearly 100 °C and coagulate necrosis can occur [21]. Guillaumier et al. [22] conducted a study, which included the largest clinical trial of HIFU for prostate cancer to date, involving 625 patients with non-metastatic prostate cancer. The 5-year failure-free and overall survival rates for the entire patient cohort were 88% and 99%, respectively. Ganzer et al. [23] followed up 538 patients with local prostate cancer treated with HIFU alone in a single center for nearly 10 years. According to the statistics, the 5-year and 10-year biochemical disease-free survival rates were 81% and 61%, respectively, and the 10-year tumor-specific survival rates were as high as 94% to 97%. In another study, de Castro Abreu et al. [24] followed up 7 patients who underwent HIFU semi-glandular ablation by enhanced transrectal contrast ultrasound (TRUS). The median follow-up after HIFU was 15 (13-20) months, and the prostate volume decreased by 32.5% (range 0-74%), 82% reduction in PSA levels (range 30% to 95%). The median duration of PSA decline was 3 months (range 2 to 12 months). Nahar et al. [25] conducted a study that included a total of 52 patients, and 17% (5/30) of the patients had positive biopsy results 12 months after surgery. After 5 months, Ghai et al. [26] found that residual prostate cancer was detected in 7% (3/44) of the patients at the treatment site. In addition, Albisinni et al. [27] found no statistical difference in the probability of requiring further treatment due to disease progression after HIFU versus RP. These studies all confirmed that HIFU can achieve better tumor control outcomes in the focal treatment of prostate cancer. At the same time, HIFU also has obvious advantages in terms of safety as Guillaumier et al. [22] confirmed that 98% (241/247) of patients achieved complete pad free urine control; Nahar et al. [25] also found that the urinary system function of the patients recovered to the baseline level in 3-6 months, and the sexual function recovered in 12 months. Ghai et al. [26] proposed that the median international index of erectile function at 5 months after surgery, IIEF and international prostate symptom score (IPSS) have no significant differences with baseline prostate symptom score. HIFU has a good treatment success rate, and the safety is guaranteed. At the same time, the operation can be carried out under spinal anesthesia or intravenous anesthesia without incision on the skin, and the patients recover quickly after surgery [21]. However, in Guillaumier et al. [22] found that patient follow-up was insufficient, with only 222 patients receiving puncture biopsy after treatment, and the results obtained were not reliable enough. Therefore, clinical studies with large samples and long-term follow-up are still needed to observe the therapeutic effect of HIFU.
Focal laser ablation
Focal laser ablation (FLA) is treated by inserting a small laser fiber, which is through the perineum or rectum into the tumor and using its discharge to heat up rapidly, causing coagulation necrosis in the controlled area, thereby reducing the risk of damage to adjacent structures [28]. The number of clinical studies on FLA is small, and most of them are small samples. Walser et al. [29] conducted a study, including 120 patients with low - and medium-risk prostate cancer, 17% (20/120) of whitch received further treatment for recurrence after 1 year of rectal FLA treatment [29]. Alhakeem et al. [30] also reached a similar conclusion, with 20.4% (10/49) of patients still having tumors in the treated area after treatment. However, there were some evidence,which were from the United States authoritative Surveillance, Epidemiology, and End Results (SEER) database, showing that the difference in cancer specific mortality (CSM) between FLA and RP is not statistically significant [31] , while RT had a significant advantage in survival benefit [32]. The advantages of FLA were mainly reflected in effectiveness and safety: most patients had no significant differences in IPSS, sexual health inventory in men (SHIM) scores. Besides that, some patients would return to baseline over time even if affected [29]. In addition, FLA can be performed under local anesthesia with less surgical risk.On the one hand, its disadvantages are reflected in the higher requirements for clinicians in terms of technology [33] , on the other hand, there is still a lack of long-term follow-up data.
Cryotherapy
Cryotherapy mainly involves quick freezing, slow thawing, and a repetition of the freeze-thaw cycle. There are two main mechanisms by which cryotherapy destroys tissue: one is cell damage from the cooling and heating cycles; The other is progressive failure of tissue microcirculation and blood vessel stasis. The temperature of frozen tissue should reach -50 ° C for tumor treatment, but there is no consensus on the optimal duration of freezing [34]. Chuang et al. [35] performed negative biopsies on 61 patients who underwent half-side cryotherapy (22/27). Tan et al. [36] used fractional cryoablation, which had 1-5-year fail-free survival rates of 98%, 89%, 84%, 75%, and 75%, respectively. By risk stratification, Mercader et al. [37] found that biochemical progression-free survival rates were 70.2%, 70.3% and 50% in the low, medium and high risk groups, respectively. However, their study used neoadjuvant therapy for 3 months for 5-year treatment, failure free survival rates of 85% ,meanwhile biochemical failure free survival rates of 62% in patients with larger prostate volume [38]. In addition, there was much data, which was from SEER, reflecting that the 10-year tumor-specific survival rates for cryopreservation and surgery were 98.1% and 99.2%, respectively [39]. Freezing was also associated with better outcomes. Besides that, the above studies also analyzed the safety, 95% to 100% of patients can maintain pad free urine control after surgery [36-38]. Shah et al. [40] detected that the probability of IPSS and IIEF scores returning to baseline 12 months after surgery was 78% and 85%, respectively. Through the above analysis, it can be found that cryotherapy has a good oncologic outcome, but the standardized treatment procedures still need to be further explored. Some studies suggest that 3D mapping biopsy, which is before cryotherapy for prostate cancer, can better stratify patients' risk and reduce the failure rate of treatment [41]. At the same time, cryoablation has a certain impact on function, and more exploration is needed in the subsequent use.
Irreversible electroporation
The increase in membrane permeability,that results from the application of a pulsed electric field, is known as electroporation. Electroporation can be reversible or irreversible, depending on different current parameters and cell characteristics. The principle of irreversible electroporation (IRE) stems from it [42]. IRE causes cell death by forming nanopores inside the cell membrane without causing thermal effects. There are some advantages of IRE, for example, it does not affect the therapeutic efficacy through energy dissipation due to heat loss [43]. A preclinical study in dogs showed that collagen structures such as blood vessels and nerves were not damaged after IRE treatment [44]. If this discovery is confirmed, it could encourage the wider use of IRE. Blazevski et al. [45] conducted a clinical trial including 123 patients, with a 3-year failure-free survival rate of 96.75% and a metastasis-free survival rate of 99%. Collettini et al. [46] found that the recurrence rate after 6 months was 17.9% (5/28). In addition, IRE for apex ablation of the prostate also achieved a surprising outcome, with a failure-free survival rate of 90% (36/40) after follow-up of more than 3 years [47]. Within 1 year after IRE, the overall urinary control function of patients was almost back to baseline level, and even 76% to 94% of patients maintained erectile function after surgery [45-47]. IRE has a good oncologic outcome and a low complication rate after treatment. Because it may be selective and can preserve blood vessels, nerves and other tissues, IRE has broad application prospects. The defects of IRE are mainly manifested in the need for general anesthesia and evaluation during treatment [43] , and may lead to arrhythmia in patients during treatment. Nevertheless, synchronizing the pulse frequency with the heart rhythm during treatment can reduce the incidence of arrhythmias [48].
Photodynamic therapy
Photodynamic therapy (PDT) depends on three components: photosensitive compounds, visible light, and oxygen. Photosensitive compounds selectively accumulate in hyperproliferative target cells, which then undergo necrosis and apoptosis under the action of visible light and reactive oxygen species [49]. In vitro experiments, confirmed that the mechanism of photodynamic therapy, which is to change the permeability of mitochondrial membrane and eventually lead to the death of target cells, suggesting that the regulation of mitochondrial membrane permeability-related proteins may be the key to the efficacy of photodynamic therapy [50]. This study focuses on vascular targeted photodynamic therapy (VT-P). Flegar et al. [51] made a comparison between VTP and RP, and the conclusion was not optimistic. However, the follow-up in this study was weak, and there was no matching between VTP and RP patients, at the same time only 79% of patients received MP-MRI and targeted needle biopsy before VTP, resulting in biased results. Compared with active monitoring, VTP still had a greater advantage. After 4 years, the negative rate of biopsy in the VTP group and the active monitoring group was 50% (104/206) and 14 (30/207), respectively. The probability of further treatment after VTP was also lower. There are some side effects of VTP, and Flegar et al. [51] found that 12% of the patients had obstruction of the bladder opening. Grade 3 treatment-related adverse effects occurred in 12% (6/50) of patients included in their study. Besides that, urination and erectile function scores decreased after VTP, and it took longer for patients to return to baseline [52, 53]. VTP is also a promising treatment for localized prostate cancer. However, there are few studies on VTP, and they are small sample studies, with relatively poor oncology outcomes, and more and longer follow-up is needed to determine its efficacy and side effects.
Figure 1 shows the focal therapy strategies in clinical application. Table 1 is a summary on key clinical study of different types of focal treatment for prostate cancer.
|
Table 1. Key clinical study of different types of focal treatment for prostate cancer. |
||||||
|
Research |
Focal ablation modality |
Follow-up number |
Follow-up |
Results |
Functional control |
Reference |
|
Guillaumier 2019 |
HIFU |
625 |
NA |
The 5-year failure-free and overall survival rates were 88% and 99%, respectively. |
98% (241/247) of patients achieved complete pad free urine control. |
[22] |
|
Nahar 2020 |
HIFU |
52 |
NA |
17% (5/30) of patients had positive biopsy results at 12 months. |
Urinary function recovered to baseline level in 3-6 months, and sexual function recovered in 12 months. |
[25] |
|
Ghai 2021 |
HIFU |
44 |
5 months |
Residual cancer at the treatment site was detected in 7% (3/44) of patients at 5 months. |
There was no significant difference between the median IIEF-15 and IPSS scores and baseline data 5 months after surgery. |
[26] |
|
Albisinni 2017 |
HIFU vs RP |
55 |
NA |
There was no statistically significant difference in the need for remedial treatment after surgery. |
NA |
[27] |
|
Walser 2019 |
FLA |
120 |
1 year |
After 1 year, 17% (20/120) of patients were diagnosed with tumor. |
There was no significant difference in IPSS and SHIM scores before and after treatment. |
[29] |
|
Alhakeem 2019 |
FLA |
49 |
NA |
20.4% (10/49) of patients still had tumor in the treated area. |
IPSS scores did not differ from baseline, and SHIM scores declined in the first year but did not differ from baseline at 18 months. |
[30] |
|
Zheng 2019 |
FLA vs RP |
321 pairs |
NA |
FLA had a higher all-cause mortality, but no significant reduction in cancer-specific mortality. |
NA |
[31] |
|
Zhou 2020 |
FLA vs RT |
FLA (n=428) RT (n=93041) |
NA |
RT has obvious advantages in terms of survival benefits. |
NA |
[32] |
|
Chuang 2020 |
Cryotherapy |
61 |
NA |
At 6 months after treatment, 82% (50/61) of the patients were positive. At 18 months after treatment, 82% (22/27) of patients had negative biopsies. |
NA |
[35] |
|
Tan 2021 |
Cryotherapy |
71 |
5 years |
The 1-5-year failure-free survival rates were 98%, 89%, 84%, 75%, and 75%, respectively. |
100% of the patients did not use urine pad, and the IIEF-5 and AUA symptom scores of the patients were decreased after decontamination. |
[36] |
|
Mercader 2020 |
Cryotherapy |
177 |
5 years |
The survival times of low, medium and high risk groups were 70.2%, 70.3% and 50.0%. |
95% of patients maintained complete urine control. |
[37] |
|
Oishi 2019 |
Cryotherapy |
160 |
5 years |
The 5-year survival rate without treatment failure was 85% and the survival rate without biochemical failure was 62%. |
97% of patients maintained pad free urine control, and 73% of patients could achieve full erection after surgery. |
[38] |
|
Guo 2020 |
Cryotherapy vs RP |
Cryotherapy (n=1942) RP (n=5826) |
10 years |
The 10-year tumor specific survival rates of cryotherapy and surgery were 98.1% and 99.2%, respectively. |
NA |
[39] |
|
Shah 2021 |
Cryotherapy |
58 |
12 months |
NA |
The probability of returning to baseline for IPSS and IIEF scores was 78% and 85% at 12 months and 87% and 89% at 24 months. |
[40] |
|
Blazevski 2020 |
IRE |
123 |
3 years |
The 3-year failure-free survival rate was 96.75% and the metastasis-free survival rate was 99%. |
At 24 months after treatment, 98.8% (80/81) of patients did not need to use a urine pad; 76% (40/53) maintained erectile function. |
[45] |
|
Collettini 2019 |
IRE |
28 |
6 months |
The recurrence rate was 17.9%. |
At 12 months after surgery, there was no significant difference in urinary and reproductive function from baseline (P = 0.58 and P = 0.07). |
[46] |
|
Flegar 2021 |
VTP vs RP |
VTP (n=41) RP (n=49) |
4 years |
At 12 months after VTP, 57% (12/21) of the biopsies were negative. At 24 months, 40% (2/5) were negative, and RP100% were negative. |
Patients with VTP and RP retained erectile function 71% and 30%, respectively. |
[51] |
|
Gill 2018 |
VTP vs Active monitoring |
VTP (n=201) Active monitoring (n=206) |
NA |
Negative rates at the most recent biopsy were 50% (104/206) in the VTP group and 14% (30/207) in the active surveillance group. |
NA |
[50] |
|
Tarcey 2020 |
VTP |
50 |
NA |
82% (40/49) of patients had no detectable Gleason grade 2 or higher in their primary lesion at 3 months after treatment. |
After 3 months of treatment, the median IIEF-5 score decreased by 1.0 from the baseline, the median IPSS score decreased by 1.0 from the baseline, and pad less urine control was observed in 100% of patients. |
[53] |
|
Chelly 2020 |
VTP |
82 |
NA |
NA |
Median IIEF-5 score: 3 points lower than baseline at 6 months after treatment, 1 point lower at 1 year and 2 years, no statistically significant difference from baseline at 3, 4, and 5 years. |
[52] |
|
HIFU: High intensity focused ultrasound; IIEF-15: International Index of Erectile Function -15; IPSS: International Prostate Symptom Score; SHIM: Male Sexual Health Questionnaire; AUA: American Urological Association; RP: Radical prostatectomy; FLA: Focal laser ablation; RT: Radical radiotherapy; IRE: irreversible electroporation; VTP: vascular targeted photodynamic therapy; NA: Not available.
|
||||||
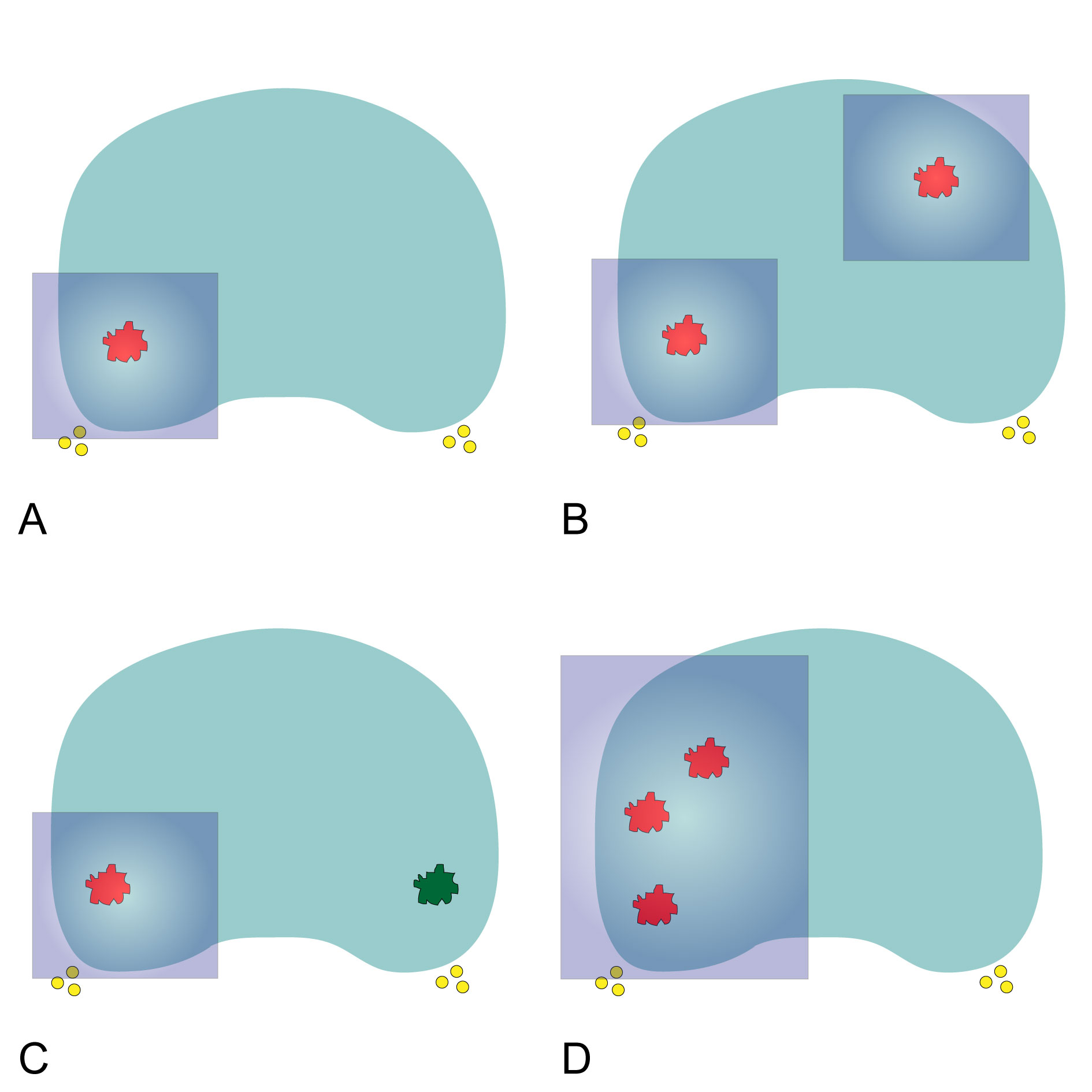 Figure 1. Diagrammatic drawing for focal therapy strategies. A: Lesion-targeted unifocal ablation; B: Lesion-targeted multifocal ablation; C: Lesion-targeted index lesion ablation; D: Region-targeted hemiablation. The red circles represents prostate cancer with clinical significance and the green circles represents prostate cancer with insignificance. The yellow circles represent the neurovascular bundles and the blue rectangle represents the ablation zone.
Figure 1. Diagrammatic drawing for focal therapy strategies. A: Lesion-targeted unifocal ablation; B: Lesion-targeted multifocal ablation; C: Lesion-targeted index lesion ablation; D: Region-targeted hemiablation. The red circles represents prostate cancer with clinical significance and the green circles represents prostate cancer with insignificance. The yellow circles represent the neurovascular bundles and the blue rectangle represents the ablation zone.
Combined with the above discussion, the following types of patients can also be considered for focal treatment: (1) newly detected prostate cancer patients unwilling to accept active monitoring; (2) Wish to maximize the preservation of original function; (3) Old age or can not tolerate general anesthesia surgery. Despite the lack of long-term follow-up data, focal therapy remains a promising treatment option for localized prostate cancer. Nevertheless, a consensus of indications ,as well as a more standard treatment regimen needs to be reached by domestic and international experts before it can be widely used, meanwhile its long-term oncology outcomes and impact on patient quality of life must be thoroughly analyzed.
We thank Dr. Sanjay Gupta (Case Western Reserve University & UH Cleveland Medical Center) for his proofreading for the review.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
STC and SZW wrote the manuscript draft and prepared the figures and tables. JL and JLH edited the final manuscript. JNL approved the final manuscript and came up with the topic.
Competing interests
All authors declare no competing interests.
Funding
This work was funded by Sichuan Provincial People’s Hospital Project (grant number 2022QN01).
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A: Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur Urol 2020, 77(1): 38-52.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J: Cancer statistics in China, 2015. CA Cancer J Clin 2016, 66(2): 115-132.
- Bokhorst LP, Valdagni R, Rannikko A, Kakehi Y, Pickles T, Bangma CH, Roobol MJ: A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur Urol 2016, 70(6): 954-960.
- Tosoian JJ, Mamawala M, Epstein JI, Landis P, Macura KJ, Simopoulos DN, Carter HB, Gorin MA: Active Surveillance of Grade Group 1 Prostate Cancer: Long-term Outcomes from a Large Prospective Cohort. Eur Urol 2020, 77(6): 675-682.
- Ficarra V, Sooriakumaran P, Novara G, Schatloff O, Briganti A, Van der Poel H, Montorsi F, Patel V, Tewari A, Mottrie A: Systematic review of methods for reporting combined outcomes after radical prostatectomy and proposal of a novel system: the survival, continence, and potency (SCP) classification. Eur Urol 2012, 61(3): 541-548.
- Novara G, Ficarra V, Rosen RC, Artibani W, Costello A, Eastham JA, Graefen M, Guazzoni G, Shariat SF, Stolzenburg JU et al: Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol 2012, 62(3): 431-452.
- Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S et al: EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021, 79(2): 243-262.
- Matta R, Chapple CR, Fisch M, Heidenreich A, Herschorn S, Kodama RT, Koontz BF, Murphy DG, Nguyen PL, Nam RK: Pelvic Complications After Prostate Cancer Radiation Therapy and Their Management: An International Collaborative Narrative Review. Eur Urol 2019, 75(3): 464-476.
- Wallis CJD, Glaser A, Hu JC, Huland H, Lawrentschuk N, Moon D, Murphy DG, Nguyen PL, Resnick MJ, Nam RK: Survival and Complications Following Surgery and Radiation for Localized Prostate Cancer: An International Collaborative Review. Eur Urol 2018, 73(1): 11-20.
- Ahmed HU: The index lesion and the origin of prostate cancer. N Engl J Med 2009, 361(17): 1704-1706.
- Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, Chen L, Ewing CM, Eisenberger MA, Carducci MA et al: Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 2009, 15(5): 559-565.
- Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A et al: Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017, 389(10071): 815-822.
- Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, Decaussin-Petrucci M, Dubreuil-Chambardel M, Magaud L, Remontet L et al: Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol 2019, 20(1): 100-109.
- Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, Tempany CM, Choyke PL, Cornud F, Margolis DJ et al: Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 2019, 76(3): 340-351.
- Lee DJ, Ahmed HU, Moore CM, Emberton M, Ehdaie B: Multiparametric magnetic resonance imaging in the management and diagnosis of prostate cancer: current applications and strategies. Curr Urol Rep 2014, 15(3): 390.
- Gupta RT, Kauffman CR, Polascik TJ, Taneja SS, Rosenkrantz AB: The state of prostate MRI in 2013. Oncology (Williston Park) 2013, 27(4): 262-270.
- Felker ER, Raman SS, Lu DSK, Tuttle M, Margolis DJ, ElKhoury FF, Sayre J, Marks LS: Utility of Multiparametric MRI for Predicting Residual Clinically Significant Prostate Cancer After Focal Laser Ablation. AJR Am J Roentgenol 2019, 213(6): 1253-1258.
- De Visschere PJ, Naesens L, Libbrecht L, Van Praet C, Lumen N, Fonteyne V, Pattyn E, Villeirs G: What kind of prostate cancers do we miss on multiparametric magnetic resonance imaging? Eur Radiol 2016, 26(4): 1098-1107.
- Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, Taneja SS, Thoeny H, Villeirs G, Villers A: Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol 2015, 68(6): 1045-1053.
- Barkin J: High intensity focused ultrasound (HIFU). Can J Urol 2011, 18(2): 5634-5643.
- Guillaumier S, Peters M, Arya M, Afzal N, Charman S, Dudderidge T, Hosking-Jervis F, Hindley RG, Lewi H, McCartan N et al: A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur Urol 2018, 74(4): 422-429.
- Ganzer R, Fritsche HM, Brandtner A, Bründl J, Koch D, Wieland WF, Blana A: Fourteen-year oncological and functional outcomes of high-intensity focused ultrasound in localized prostate cancer. BJU Int 2013, 112(3): 322-329.
- de Castro Abreu AL, Ashrafi AN, Gill IS, Oishi M, Winter MW, Park D, Duddalwar V, Stern MC, Palmer SL, Aron M et al: Contrast-Enhanced Transrectal Ultrasound for Follow-up After Focal HIFU Ablation for Prostate Cancer. J Ultrasound Med 2019, 38(3): 811-819.
- Nahar B, Bhat A, Reis IM, Soodana-Prakash N, Becerra MF, Lopategui D, Venkatramani V, Patel R, Madhusoodanan V, Kryvenko ON et al: Prospective Evaluation of Focal High Intensity Focused Ultrasound for Localized Prostate Cancer. J Urol 2020, 204(3): 483-489.
- Ghai S, Finelli A, Corr K, Chan R, Jokhu S, Li X, McCluskey S, Konukhova A, Hlasny E, van der Kwast TH et al: MRI-guided Focused Ultrasound Ablation for Localized Intermediate-Risk Prostate Cancer: Early Results of a Phase II Trial. Radiology 2021, 298(3): 695-703.
- Albisinni S, Aoun F, Bellucci S, Biaou I, Limani K, Hawaux E, Peltier A, van Velthoven R: Comparing High-Intensity Focal Ultrasound Hemiablation to Robotic Radical Prostatectomy in the Management of Unilateral Prostate Cancer: A Matched-Pair Analysis. J Endourol 2017, 31(1): 14-19.
- Colin P, Mordon S, Nevoux P, Marqa MF, Ouzzane A, Puech P, Bozzini G, Leroux B, Villers A, Betrouni N: Focal laser ablation of prostate cancer: definition, needs, and future. Adv Urol 2012, 2012: 589160.
- Walser E, Nance A, Ynalvez L, Yong S, Aoughsten JS, Eyzaguirre EJ, Williams SB: Focal Laser Ablation of Prostate Cancer: Results in 120 Patients with Low- to Intermediate-Risk Disease. J Vasc Interv Radiol 2019, 30(3): 401-409.
- Al-Hakeem Y, Raz O, Gacs Z, Maclean F, Varol C: Magnetic resonance image-guided focal laser ablation in clinically localized prostate cancer: safety and efficacy. ANZ J Surg 2019, 89(12): 1610-1614.
- Zheng X, Jin K, Qiu S, Han X, Liao X, Yang L, Wei Q: Focal Laser Ablation Versus Radical Prostatectomy for Localized Prostate Cancer: Survival Outcomes From a Matched Cohort. Clin Genitourin Cancer 2019, 17(6): 464-469.
- Zhou X, Jin K, Qiu S, Jin D, Liao X, Tu X, Zheng X, Li J, Yang L, Wei Q: Comparative Effectiveness of Radiotherapy versus Focal Laser Ablation in Patients with Low and Intermediate Risk Localized Prostate Cancer. Sci Rep 2020, 10(1): 9112.
- Ahdoot M, Lebastchi AH, Turkbey B, Wood B, Pinto PA: Contemporary treatments in prostate cancer focal therapy. Curr Opin Oncol 2019, 31(3): 200-206.
- Gage AA, Baust J: Mechanisms of tissue injury in cryosurgery. Cryobiology 1998, 37(3): 171-186.
- Chuang R, Kinnaird A, Kwan L, Sisk A, Barsa D, Felker E, Delfin M, Marks L: Hemigland Cryoablation of Clinically Significant Prostate Cancer: Intermediate-Term Followup via Magnetic Resonance Imaging Guided Biopsy. J Urol 2020, 204(5): 941-949.
- Tan WP, Chang A, Sze C, Polascik TJ: Oncological and Functional Outcomes of Patients Undergoing Individualized Partial Gland Cryoablation of the Prostate: A Single-Institution Experience. J Endourol 2021, 35(9): 1290-1299.
- Mercader C, Musquera M, Franco A, Alcaraz A, Ribal MJ: Primary cryotherapy for localized prostate cancer treatment. Aging Male 2020, 23(5): 1460-1466.
- Oishi M, Gill IS, Tafuri A, Shakir A, Cacciamani GE, Iwata T, Iwata A, Ashrafi A, Park D, Cai J et al: Hemigland Cryoablation of Localized Low, Intermediate and High Risk Prostate Cancer: Oncologic and Functional Outcomes at 5 Years. J Urol 2019, 202(6): 1188-1198.
- Guo XX, Liu SJ, Wang M, Hou HM, Wang X, Zhang ZP, Liu M, Wang JY: Comparing the Oncological Outcomes of Cryoablation vs. Radical Prostatectomy in Low-Intermediate Risk Localized Prostate Cancer. Front Oncol 2020, 10: 1489.
- Shah TT, Peters M, Miah S, Eldred-Evans D, Yap T, Hosking-Jervis F, Dudderidge T, Hindley RG, McCracken S, Greene D et al: Assessment of Return to Baseline Urinary and Sexual Function Following Primary Focal Cryotherapy for Nonmetastatic Prostate Cancer. Eur Urol Focus 2021, 7(2): 301-308.
- Barqawi A, Pessoa RR, Al-Musawi M, MacDermott T, O'Donnell CI: The Impact of Performing a 3D Mapping Biopsy Prior to Primary Cryotherapy for the Treatment of Prostate Cancer. Urology 2020, 144: 171-176.
- Kiełbik A, Szlasa W, Saczko J, Kulbacka J: Electroporation-Based Treatments in Urology. Cancers (Basel) 2020, 12(8): 2208.
- Valerio M, Ahmed HU, Emberton M: Focal Therapy of Prostate Cancer Using Irreversible Electroporation. Tech Vasc Interv Radiol 2015, 18(3): 147-152.
- Tsivian M, Polascik TJ: Bilateral focal ablation of prostate tissue using low-energy direct current (LEDC): a preclinical canine study. BJU Int 2013, 112(4): 526-530.
- Blazevski A, Amin A, Scheltema MJ, Balakrishnan A, Haynes AM, Barreto D, Cusick T, Thompson J, Stricker PD: Focal ablation of apical prostate cancer lesions with irreversible electroporation (IRE). World J Urol 2021, 39(4): 1107-1114.
- Collettini F, Enders J, Stephan C, Fischer T, Baur ADJ, Penzkofer T, Busch J, Hamm B, Gebauer B: Image-guided Irreversible Electroporation of Localized Prostate Cancer: Functional and Oncologic Outcomes. Radiology 2019, 292(1): 250-257.
- O'Neill CH, Martin RCG, 2nd: Cardiac synchronization and arrhythmia during irreversible electroporation. J Surg Oncol 2020, 122(3): 407-411.
- Luksiene Z: Photodynamic therapy: mechanism of action and ways to improve the efficiency of treatment. Medicina (Kaunas) 2003, 39(12): 1137-1150.
- Xu DD, Leong MML, Wong FL, Lam HM, Hoeven R: Photodynamic therapy on prostate cancer cells involve mitochondria membrane proteins. Photodiagnosis Photodyn Ther 2020, 31: 101933.
- Gill IS, Azzouzi AR, Emberton M, Coleman JA, Coeytaux E, Scherz A, Scardino PT: Randomized Trial of Partial Gland Ablation with Vascular Targeted Phototherapy versus Active Surveillance for Low Risk Prostate Cancer: Extended Followup and Analyses of Effectiveness. J Urol 2018, 200(4): 786-793.
- Flegar L, Buerk B, Proschmann R, Propping S, Groeben C, Baunacke M, Herout R, Huber J, Thomas C, Borkowetz A: Vascular-targeted Photodynamic Therapy in Unilateral Low-risk Prostate Cancer in Germany: 2-yr Single-centre Experience in a Real-world Setting Compared with Radical Prostatectomy. Eur Urol Focus 2022, 8(1): 121-127.
- Chelly S, Maulaz P, Bigot P, Azzouzi AR, Lebdai S: Erectile function after WST11 vascular-targeted photodynamic therapy for low-risk prostate cancer treatment. Asian J Androl 2020, 22(5): 454-458.
- van den Bos W, Muller BG, Ahmed H, Bangma CH, Barret E, Crouzet S, Eggener SE, Gill IS, Joniau S, Kovacs G et al: Focal therapy in prostate cancer: international multidisciplinary consensus on trial design. Eur Urol 2014, 65(6): 1078-1083.
- Donaldson IA, Alonzi R, Barratt D, Barret E, Berge V, Bott S, Bottomley D, Eggener S, Ehdaie B, Emberton M et al: Focal therapy: patients, interventions, and outcomes--a report from a consensus meeting. Eur Urol 2015, 67(4): 771-777.
- Muller BG, van den Bos W, Brausi M, Fütterer JJ, Ghai S, Pinto PA, Popeneciu IV, de Reijke TM, Robertson C, de la Rosette JJ et al: Follow-up modalities in focal therapy for prostate cancer: results from a Delphi consensus project. World J Urol 2015, 33(10): 1503-1509.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript