Review Article | Open Access
Research Progress of New Urine Markers in the Diagnosis of Bladder Cancer
Rose Lamichhane11Hubei University of Technology, Hongshan District, Wuhan City 430068, Hu Bei Province, P.R. China.
Correspondence: Rose Lamichhane (Hubei University of Technology, No.28, Nanli Road, Hongshan District, Wuhan City 430068, Hu Bei Province, P.R. China; Email: rose_agnuss@outlook.com).
Annals of Urologic Oncology 2024, 7(1): 1-9. https://doi.org/10.32948/auo.2024.02.03
Received: 11 Jan 2024 | Accepted: 31 Jan 2024 | Published online: 06 Feb 2024
Key words urine markers, bladder cancer, new type, diagnosis, research progress
Previous tests for MCM5 in urine were complex, expensive, and impractical. However, the recently developed adx bladder (Arquer Diagnostics, Sunderland) test, a commercial MCM5 enzyme-linked immunosorbent test, is capable of detecting MCM5 in urine mud in BCa hematuria patients [10]. A meta-analysis of 5,114 patients showed that MCM5 predicted BCa with an overall sensitivity and specificity of 66% and 72%, respectively. Among them, subgroup analysis using adx bladder detection technology showed that the sensitivity and specificity of the diagnosis of BCa were 61% and 67%, respectively [11]. Therefore, the urine MCM5 test has moderate diagnostic accuracy in diagnosing BCa. ADX bladder is a simpler ELISA based method for detecting MCM5 in urine and may provide a new urine marker detection method for the initial diagnosis of BCa, but more clinical studies with big datasets are needed to further determine its diagnostic value in BCa.
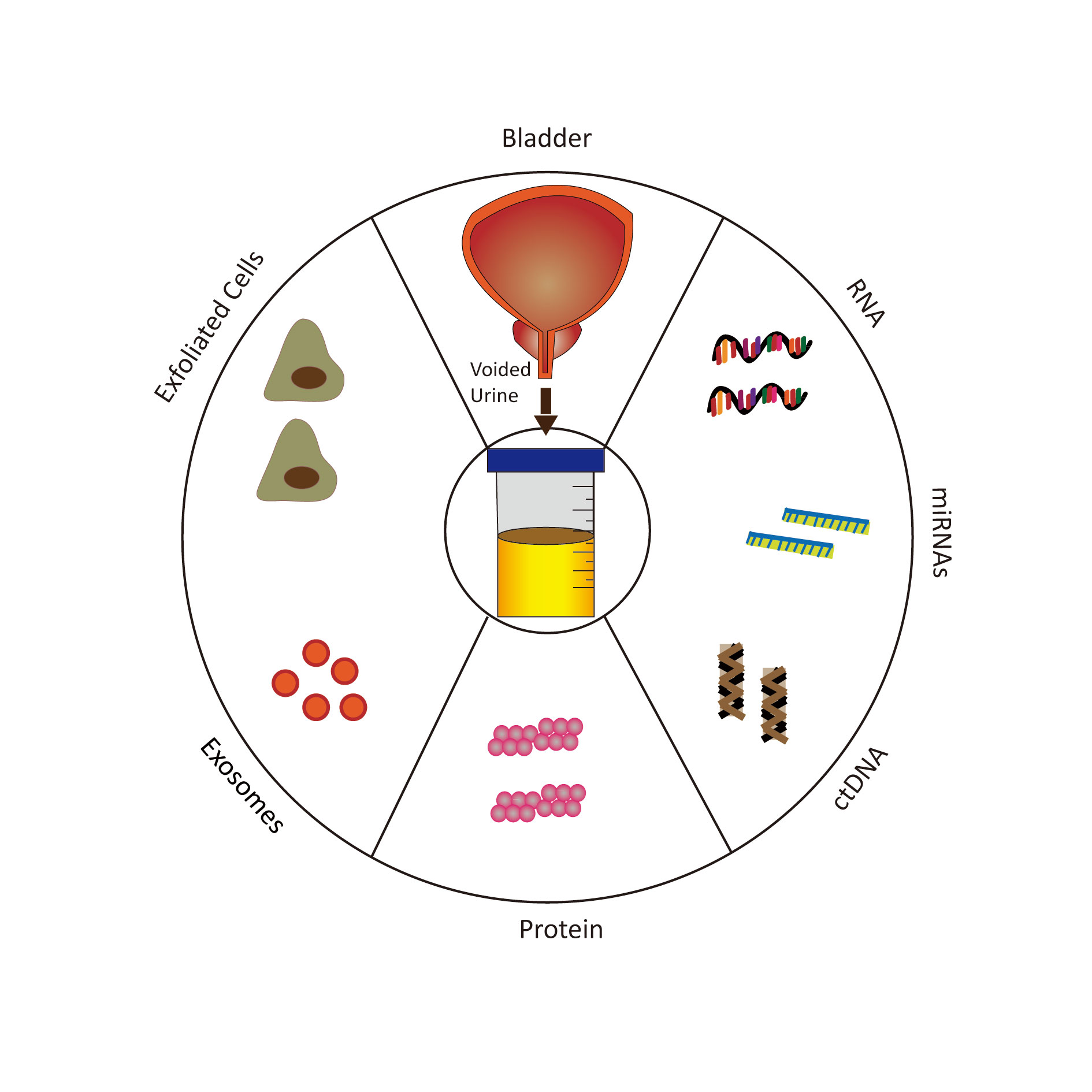 Figure 1. Schematic diagram for various urine-based biomarkers.
Figure 1. Schematic diagram for various urine-based biomarkers.
A meta-analysis of 27 studies showed an overall sensitivity of 79% and an overall specificity of 90% for urine CK20 detection of BCa. Urinary CK20 was more sensitive to the diagnosis of UBC than all other types of BCa (83% vs. 75%). In addition, the diagnostic accuracy of urinary CK20 improves with the progression of tumor stage and grade [16]. Therefore, urine CK20 may be a potential non-invasive biomarker for detecting BCa, specifically UBC. However, larger clinical studies are required to further validate urinary CK20 testing. The actual clinical value of BCa and the diagnostic significance of urinary CK20 testing for BCa remain controversial. This is an important reason restricting its application in the diagnosis of bladder cancer. Table 1 is a summary on urine markers in bladder cancer.
|
Table1. Summary on urine markers in bladder cancer. |
||||
|
Urine markers |
Definition |
Principle |
Current detection method |
References |
|
Microchromosome maintenance protein 5
|
A marker of DNA replication |
Only expressed in actively proliferating basal cell layer cells and in urothelial bladder cancer (UBC), cell proliferation is unlimited and spread throughout all layers of the urothelium, causing cells to shed from the bladder surface into the urine with MCM5 expression |
ADXBLADDER (Arquer Diagnostics, Sunderland) test |
[2], [10], [11] |
|
Cytokeratin 20
|
A low molecular weight cytokeratin encoded by the KRT20 gene |
The expression of CK20 in urothelial cells is limited to bladder surface umbrella cell and malignancies resulting in abnormally elevated CK20 expression; The diagnostic accuracy of urinary CK20 improves with the progression of tumor stage and grade, especially for UBC |
PCR |
[12-15] |
|
Abnormal glycosylated integrin α3β1 |
A high-affinity receptor for collagen, laminin and fifientin |
Abnormal glycosylation is associated with the occurrence and development of certain tumor types and the expression level of AG31 in tumor tissues is positively correlated with the severity and prognosis of BCa patients |
- |
[17-20] |
|
Tumor M2-PK |
A dimer of pyruvate kinase muscle isozyme M2 (PKM2) |
PKM2 is highly expressed in a variety of cancer types, and the poor prognosis of tumor patients is also related to the high expression of PKM2 and PKM2 expression measured by immunohistochemistry was associated with increased tumor grade in BCa compared to normal urothelium |
- |
[20-26] |
|
MicroRNAs |
Small, 18-22 nucleotides long non-coding rna transcripts |
The expression levels of let-7b-5p, miR-149-5p, miR-146a-5p and miR-423-5p in the urine of BCa patients are significantly increased and high expression of miR-149-5p and miR-193a-5p was significantly associated with lower overall survival in BCa patients |
The combined detection of BCa by miR-125b, miR-145, miR-183 and miR-221 |
[33-35] |
|
DNA methylation |
The covalent binding of methyl groups to cytosine residues and pyrimidine rings |
It affects the genomic stability and gene expression of bladder cancer |
utMeMA, the combination of GHSR/MAL genes |
[44-47] |
|
Exosomal CA9 mRNA and lncRNAs
|
A class of nanoscale extracellular vesicles (EVs) that carry cell-specific proteins, lipids, and nucleic acids |
The urine of BCa patients is rich in urinary exosomes, and CA9 mRNA can be detected in urinary exosomes |
Urinary exosome CA9mR NA, exosomal long non-coding Rnas (LNcrnas) |
[52-54] |
|
Telomerase reverse transcriptase promoter mutation |
One of the most frequently mutated genomic regions in urothelial carcinoma (UC) tissues |
TERT promoter mutations can be detected in urine 10 years before clinical diagnosis of BCa |
- |
[60-64] |
|
Urine free DNA |
Urothelial cells shed and undergo apoptosis or necrosis after releasing DNA from the cell |
Unlike normal cells, tumor cells release longer stretches of DNA with higher DNA integrity and the urine cfDNA integrity of BCa patients is much higher than that of normal individuals |
PCR, gene combinations |
[69-73] |
A recent study showed that urine AG31 detection of BCa has a sensitivity of about 90.8% and a specificity of approximately 91.5%, and urine AG31 detection can also distinguish patients with BCa from patients with other tumors of the urinary system and benign inflammatory diseases. The study also found that urine AG31 level was positively correlated with tumor stage and grade. In addition, the sensitivity and specificity of urine AG31 for the diagnosis of BCa were higher than those of NMP22 (90.6% vs. 47.2% vs. 98.2% vs. 87%). More importantly, the diagnostic accuracy of urine AG31 testing in BCa patients is not affected by hematuria, age, and gender [19]. Therefore, the detection of urine AG31 may become a promising new urine marker in the diagnosis of BCa, and it is worth further improving its clinical value in the diagnosis of BCa. However, a large number of studies are still needed to prove it in the future.
More and more studies proved that BCa increased the expression of PKM2. The content of tumor M2-PK in the urine of BCa patients was increased, and statistical score analysis showed that the sensitivity of this method was 82% [32]. Therefore, PKM2, especially tumor M2-PK, has shown potential as a urine biomarker, but its specificity in the diagnosis of BCa still needs more clinical evaluation.
Currently, relevant studies have shown that the sensitivity, specificity, positive predictive value, and negative predictive value of the combined detection of BCa by miR-125b, miR-145, miR-183 and miR-221 are 73.1%, 95.7%, 97.4% and 61.1%, respectively. The sensitivity can be increased by nearly 8% [34, 35]. Cytological examination of excess urine exfoliation alone confirms this finding. This shows a very important significance compared with other detection methods, and urine mirna has great potential as a novel biomarker for detecting BCa. However, prospective studies with more samples are needed to further validate the value of urine mirnas in the diagnosis of BCa. There are many mirnas closely related to BCa, and which miRNA combinations have the greatest diagnostic value for BCa still need to be further explored.
At present, there are many studies on DNA methylation in the field of urinary tumor detection, among which Chen et al. [40] developed an effective DNA methylation detection method utMeMA for urinary tumors. A comprehensive analysis of BCa sequencing data from 3 cohorts identified 26 BCa specific methylation sites with sensitivity and specificity of 90% and 83.1%, respectively. In addition, compared with urine flow cytology and FISH, UTMEMa-based assays significantly increased susceptibility to early BCa (Ta stage and low-grade BCa), small residual tumors, and recurrent tumors. In addition, another bladder methylation assay can detect urine tumor DNA methylation, with a sensitivity and specificity of 74% and 84%, respectively, for the diagnosis of noninvasive BCa [41-43]. Notably, the combination of GHSR/MAL genes in urine DNA methylation markers was the best for the diagnosis of BCa, outperforming single DNA methylation markers and other combinations, achieving 92% sensitivity and 85% specificity [44-47]. Therefore, urine tumor DNA methylation assessment is a rapid, high-throughput, non-invasive and promising method for early diagnosis, small residual tumor detection and BCa monitoring. In particular, the urine GHSR/MAL gene combination has shown greater accuracy in detecting BCa, which can greatly benefit patients by reducing the burden of cystoscopy and blind secondary surgery.
Carbonic anhydrase 9 (CA9) is a transmembrane member of the carbonic anhydrase family. It catalyzes reversible hydration of carbon dioxide with bicarbonate and protons, thereby maintaining a neutral pH of tumor cells in an acidic microenvironment, which plays an important role in the development of tumors. A large number of studies have found that the urine of BCa patients is rich in urinary exosomes, and CA9 mRNA can be detected in urinary exosomes, and the sensitivity and specificity of urinary exosome CA9 mRNA in the diagnosis of BCa are 85.2% and 83.2%, respectively [52]. Therefore, urinary exosome CA9mRNA may be a reliable non-invasive diagnostic biomarker for BCa. However, the separation and characterization methods of exosomes are complex and varied, and a unified and simple technique has not yet been formed. With the continuous development of smart technology, it is possible to develop more precise technology in the future that can significantly improve the detection rate of urine exosome CA9 mRNA.
More and more studies have proved that exosomal long non-coding Rnas (LncRNAs) play an important clinical role in the early diagnosis and prognosis of some cancers. Abbastabar et al. [53] found that urinary exosomal lncrnas carrying prostate cancer-associated transcript 1 (prostate cancer-associated transcript 1), antisense RNA at INK4 site (ANRIL) and PCAT-1 expression levels in BCa patients were significantly higher than those in normal subjects. The diagnostic accuracy of urinary exosome lncRNA PCAT-1 and ANRIL for BCa were 0.73 (sensitivity 43.33%, specificity 87.5%) and 0.72 (sensitivity 46.67%, specificity 87.5%), respectively. Another meta-analysis subgroup discussed the studies on exosome derived lncRNAs in urine and blood, and finally concluded that exosome derived lncRNAs have high accuracy in the diagnosis of BCa [54]. This means that exosome lncrnas in urine and blood have great potential as biomarkers for BCa diagnosis. However, due to the large heterogeneity of this study, further multicenter prospective studies are needed to verify its clinical value. However, urinary exosomal lncRNAs are promising as reliable non-invasive diagnostic biomarkers for BCa.
Current studies have shown that TERT promoter mutations can be detected in urine 10 years before clinical diagnosis of BCa, with a specificity of 100% and a sensitivity of 46.7% [64]. This means that urine TERT promoter mutations have great potential as a non-invasive biomarker for the early detection of BCa, although further studies are needed to confirm this finding and evaluate its clinical value in other longitudinal cohorts. Pakmanesh et al. [65] showed that the overall specificity and sensitivity of urinary TERT promoter mutation to detect BCa were 88.0% and 67.7%, respectively. Urinary cytology showed similar sensitivity (67.7%) but lower specificity (62.0%) for TERT promoter detection of BCa. The combination of urinary TERT promoter mutation with urinary cytology increased the sensitivity to 83.8% and reduced the specificity to 52.0%. This suggests that urine TERT promoter mutations have good diagnostic accuracy for BCa as a non-invasive urine biomarker. Another study found that BCa patients with TERT promoter mutations had a higher risk of recurrence [66, 67]. This suggests that TERT promoter mutations may also be potential predictors of BCa recurrence.
A large number of studies have proved that the specificity and sensitivity of detecting urine cfDNA for the diagnosis of BCa are 72%-84% and 57%-86% [74]. Urine cfDNA sequencing identified valuable genetic mutations that could be used to detect BCa. For example, mutations that are frequently detected in BCa, such as TERT, TP53, PIK3CA, KRAS, and FGFR3 genes are significantly altered in urine cfDNA, and the diagnostic accuracy of BCa using these five gene combinations is high (AUC of 0.94) [70]. In another study, researchers analyzed thermal gene mutations in urine cfDNA (TERT promoter and FGFR3) by drop digital PCR and showed a specificity of 100% and a sensitivity of 68.9% for urine cfDNA detection of UBC. When combined with urine cytology, the sensitivity was increased to 85.9% [75]. Therefore, the detection of urine cfDNA, especially the detection of heat gene mutations in urine cfDNA, has great potential for the diagnosis and prognosis prediction of BCa, and should be focused on in the future. Another structural feature of urine cfDNA, called "zigzag ends," can inform the diagnosis of BCa. Zhou et al. [76] evaluated single-strand terminus with 5' nucleosome prominence and noted that patients with BCa had a lower zigzag terminus index than healthy controls (AUC of 0.83). Therefore, the jagged ends of urinary cfDNA are also highly likely to serve as new urine diagnostic markers for BCa.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
RL: Conception, design of study, literature search and review, manuscript writting, approval for the final version of the manuscript and funding supports.
Competing interests
The authors have no competing interest.
Funding
None.
- Sung H, Ferlay J, Siegel RL: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Stoeber K, Swinn R, Prevost AT, de Clive-Lowe P, Halsall I, Dilworth SM, Marr J, Turner WH, Bullock N, Doble A et al: Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst 2002, 94(14): 1071-1079.
- Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2020, 70(4): 313.
- Lotan Y, Baky FJ: Urine-Based Markers for Detection of Urothelial Cancer and for the Management of Non-muscle-Invasive Bladder Cancer. Urol Clin North Am 2023, 50(1): 53-67.
- Henning GM, Barashi NS, Smith ZL: Advances in Biomarkers for Detection, Surveillance, and Prognosis of Bladder Cancer. Clin Genitourin Cancer 2021, 19(3): 194-198.
- Smolensky D, Rathore K, Cekanova M: Molecular targets in urothelial cancer: detection, treatment, and animal models of bladder cancer. Drug Des Devel Ther 2016, 10: 3305-3322.
- Lotan Y, Roehrborn CG: Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology 2003, 61(1): 109-118; discussion 118.
- Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, Kassouf W: Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol 2015, 33(2): 66.e25-31.
- El-Shal AS, Shalaby SM: Urinary exosomal microRNA-96-5p and microRNA-183-5p expression as potential biomarkers of bladder cancer. Mol Biol Rep 2021, 48(5): 4361-4371.
- Dudderidge T, Stockley J, Nabi G, Mom J, Umez-Eronini N, Hrouda D, Cresswell J, McCracken SRC: A Novel, non-invasive Test Enabling Bladder Cancer Detection in Urine Sediment of Patients Presenting with Haematuria-A Prospective Multicentre Performance Evaluation of ADXBLADDER. Eur Urol Oncol 2020, 3(1): 42-46.
- Sharma G, Sharma A, Krishna M, Ahluwalia P, Gautam G: Diagnostic performance of minichromosome maintenance 5 (MCM5) in bladder cancer: A systematic review and meta-analysis. Urol Oncol 2022, 40(6): 235-242.
- Gandhi MJ, Ferriola D, Huang Y, Duke JL, Monos D: Targeted Next-Generation Sequencing for Human Leukocyte Antigen Typing in a Clinical Laboratory: Metrics of Relevance and Considerations for Its Successful Implementation. Arch Pathol Lab Med 2017, 141(6): 806-812.
- Jiang J, Ulbright TM, Younger C, Sanchez K, Bostwick DG, Koch MO, Eble JN, Cheng L: Cytokeratin 7 and cytokeratin 20 in primary urinary bladder carcinoma and matched lymph node metastasis. Arch Pathol Lab Med 2001, 125(7): 921-923.
- Raspollini MR, Nesi G, Baroni G, Girardi LR, Taddei GL: Immunohistochemistry in the differential diagnosis between primary and secondary intestinal adenocarcinoma of the urinary bladder. Appl Immunohistochem Mol Morphol 2005, 13(4): 358-362.
- Guo B, Luo C, Xun C, Xie J, Wu X, Pu J: Quantitative detection of cytokeratin 20 mRNA in urine samples as diagnostic tools for bladder cancer by real-time PCR. Exp Oncol 2009, 31(1): 43-47.
- Mi Y, Zhao Y, Shi F, Zhang M, Wang C, Liu X: Diagnostic accuracy of urine cytokeratin 20 for bladder cancer: A meta-analysis. Asia Pac J Clin Oncol 2019, 15(2): e11-e19.
- Winograd-Katz SE, Fässler R, Geiger B, Legate KR: The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol 2014, 15(4): 273-288.
- Sachs N, Sonnenberg A: Cell-matrix adhesion of podocytes in physiology and disease. Nat Rev Nephrol 2013, 9(4): 200-210.
- Jin D, Zhang R, Chen H, Li C: Aberrantly glycosylated integrin α3β1 is a unique urinary biomarker for the diagnosis of bladder cancer. Aging (Albany NY) 2020, 12(11): 10844-10862.
- Li C, Yang Z, Du Y, Tang H, Chen J, Hu D, Fan Z: BCMab1, a monoclonal antibody against aberrantly glycosylated integrin α3β1, has potent antitumor activity of bladder cancer in vivo. Clin Cancer Res 2014, 20(15): 4001-4013.
- Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L, Jiang F: PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncol Lett 2016, 11(3): 1980-1986.
- Li C, Du Y, Yang Z, He L, Wang Y, Hao L, Ding M, Yan R, Wang J, Fan Z: GALNT1-Mediated Glycosylation and Activation of Sonic Hedgehog Signaling Maintains the Self-Renewal and Tumor-Initiating Capacity of Bladder Cancer Stem Cells. Cancer Res 2016, 76(5): 1273-1283.
- Yang Z, Zhang R, Ge Y, Qin X, Kang X, Wang Y, Zhang X, Song C, Quan X, Wang H et al: Somatic FGFR3 Mutations Distinguish a Subgroup of Muscle-Invasive Bladder Cancers with Response to Neoadjuvant Chemotherapy. EBioMedicine 2018, 35: 198-203.
- Hu H, Tu W, Chen Y, Zhu M, Jin H, Huang T, Zou Z, Xia Q: The combination of PKM2 overexpression and M2 macrophages infiltration confers a poor prognosis for PDAC patients. J Cancer 2020, 11(8): 2022-2031.
- Li TE, Wang S, Shen XT, Zhang Z, Chen M, Wang H, Zhu Y, Xu D, Hu BY, Wei R et al: PKM2 Drives Hepatocellular Carcinoma Progression by Inducing Immunosuppressive Microenvironment. Front Immunol 2020, 11: 589997.
- Papadaki C, Manolakou S, Lagoudaki E, Pontikakis S, Ierodiakonou D: Correlation of PKM2 and CD44 Protein Expression with Poor Prognosis in Platinum-Treated Epithelial Ovarian Cancer: A Retrospective Study. Cancers (Basel) 2020, 12(4): 1013.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507(7492): 315-322.
- Tang Q, Zuo W, Wan C, Xiong S, Xu C, Yuan C, Sun Q, Zhou L, Li X: Comprehensive genomic profiling of upper tract urothelial carcinoma and urothelial carcinoma of the bladder identifies distinct molecular characterizations with potential implications for targeted therapy & immunotherapy. Front Immunol 2022, 13: 1097730.
- Zhong B, Wang Y, Liao Y, Liang J, Wang K, Zhou D, Zhao Y, Jiang N: MLKL and other necroptosis-related genes promote the tumor immune cell infiltration, guiding for the administration of immunotherapy in bladder urothelial carcinoma. Apoptosis 2023, 28(5-6): 892-911.
- Zhou H, Wang X, Mo L, Liu Y, He F, Zhang F, Huang KH, Wu XR: Role of isoenzyme M2 of pyruvate kinase in urothelial tumorigenesis. Oncotarget 2016, 7(17): 23947-23960.
- Mazurek S, Boschek CB, Hugo F, Eigenbrodt E: Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol 2005, 15(4): 300-308.
- Liu W, Woolbright BL, Pirani K, Didde R, Abbott E, Kaushik G, Martin P, Hamilton-Reeves J, Taylor JA, 3rd, Holzbeierlein JM et al: Tumor M2-PK: A novel urine marker of bladder cancer. PLoS One 2019, 14(6): e0218737.
- Lin JT, Tsai KW: Circulating miRNAs Act as Diagnostic Biomarkers for Bladder Cancer in Urine. Int J Mol Sci 2021, 22(8): 4278.
- Erdmann K, Salomo K, Klimova A, Heberling U, Lohse-Fischer A, Fuehrer R, Thomas C, Roeder I, Froehner M, Wirth MP et al: Urinary MicroRNAs as Potential Markers for Non-Invasive Diagnosis of Bladder Cancer. Int J Mol Sci 2020, 21(11): 3814.
- Aveta A, Cilio S: Urinary MicroRNAs as Biomarkers of Urological Cancers: A Systematic Review. Int J Mol Sci 2023, 24(13): 10846.
- Bosschieter J, Lutz C, Segerink LI, Vis AN, Zwarthoff EC, RJ AvM, van Rhijn BW, Heymans MW, Jansma EP, Steenbergen RD et al: The diagnostic accuracy of methylation markers in urine for the detection of bladder cancer: a systematic review. Epigenomics 2018, 10(5): 673-687.
- Larsen LK, Lind GE, Guldberg P, Dahl C: DNA-Methylation-Based Detection of Urological Cancer in Urine: Overview of Biomarkers and Considerations on Biomarker Design, Source of DNA, and Detection Technologies. Int J Mol Sci 2019, 20(11): 2657.
- Santoni G, Morelli MB, Amantini C, Battelli N: Urinary Markers in Bladder Cancer: An Update. Front Oncol 2018, 8: 362.
- Ilijazi D, Pulverer W, Ertl IE, Lemberger U: Discovery of Molecular DNA Methylation-Based Biomarkers through Genome-Wide Analysis of Response Patterns to BCG for Bladder Cancer. Cells 2020, 9(8): 1839.
- Chen X, Zhang J, Ruan W, Huang M, Wang C, Wang H, Jiang Z, Wang S, Liu Z, Liu C et al: Urine DNA methylation assay enables early detection and recurrence monitoring for bladder cancer. J Clin Invest 2020, 130(12): 6278-6289.
- Laukhtina E, Shim SR, Mori K, D'Andrea D, Soria F, Rajwa P, Mostafaei H, Compérat E, Cimadamore A, Moschini M et al: Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis. Eur Urol Oncol 2021, 4(6): 927-942.
- Chiang CH, Chiang CH, Chiang CH: Re: Ekaterina Laukhtina, Sung Ryul Shim, Keiichiro Mori, et al. Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis. Eur Urol Oncol 2021, 4: 927-42: Considerations for Interpreting Data Presented in Systematic Reviews and Network Meta-analyses of Urinary Biomarker Tests. Eur Urol Oncol 2022, 5(2): 263-264.
- Laukhtina E, Shim SR, Mori K, D'Andrea D, Soria F, Rajwa P, Mostafaei H, Compérat E, Cimadamore A, Moschini M et al: Corrigendum to "Diagnostic Accuracy of Novel Urinary Biomarker Tests in Non-muscle-invasive Bladder Cancer: A Systematic Review and Network Meta-analysis" [Eur Urol Oncol 2021;4:927-42]. Eur Urol Oncol 2022, 5(4): 480-481.
- Bosschieter J, Nieuwenhuijzen JA, Hentschel A, van Splunter AP, Segerink LI, Vis AN, Wilting SM, Lissenberg-Witte BI, RJ AvM, Steenbergen RD: A two-gene methylation signature for the diagnosis of bladder cancer in urine. Epigenomics 2019, 11(3): 337-347.
- Xiao Y, Ju L, Qian K, Jin W, Wang G, Zhao Y, Jiang W, Liu N, Wu K, Peng M et al: Non-invasive diagnosis and surveillance of bladder cancer with driver and passenger DNA methylation in a prospective cohort study. Clin Transl Med 2022, 12(8): e1008.
- Andrés G, Ashour N, Sánchez-Chapado M, Ropero S, Angulo JC: The study of DNA methylation in urological cancer: present and future. Actas Urol Esp 2013, 37(6): 368-375.
- Ye F, Hu Y, Gao J, Liang Y, Liu Y, Ou Y, Cheng Z, Jiang H: Radiogenomics Map Reveals the Landscape of m6A Methylation Modification Pattern in Bladder Cancer. Front Immunol 2021, 12: 722642.
- Zhang Y, Liu Y, Liu H, Tang WH: Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 2019, 9: 19.
- Gurunathan S, Kang MH: A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int J Nanomedicine 2021, 16: 1281-1312.
- Agarwal P, Anees A, Harsiddharay RK, Kumar P, Tripathi PK: A Comprehensive Review on Exosome: Recent Progress and Outlook. Pharm Nanotechnol 2023.
- Wortzel I, Dror S, Kenific CM, Lyden D: Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell 2019, 49(3): 347-360.
- Wen J, Yang T, Mallouk N, Zhang Y, Li H, Lambert C: Urinary Exosomal CA9 mRNA as a Novel Liquid Biopsy for Molecular Diagnosis of Bladder Cancer. Int J Nanomedicine 2021, 16: 4805-4811.
- Abbastabar M, Sarfi M, Golestani A, Karimi A, Pourmand G, Khalili E: Tumor-derived urinary exosomal long non-coding RNAs as diagnostic biomarkers for bladder cancer. EXCLI J 2020, 19: 301-310.
- Wang J, Gao Y, Wang X, Gao Y, Li L, Zhang J, Zhang L, Che F: Circulating lncRNAs as noninvasive biomarkers in bladder cancer: A diagnostic meta-analysis based on 15 published articles. Int J Biol Markers 2020, 35(2): 40-48.
- Turner KJ, Vasu V: Telomere Biology and Human Phenotype. Cells 2019, 8(1): 73.
- Revy P, Kannengiesser C: Genetics of human telomere biology disorders. Nat Rev Genet 2023, 24(2): 86-108.
- Yuan X, Dai M, Xu D: Telomere-related Markers for Cancer. Curr Top Med Chem 2020, 20(6): 410-432.
- Heaphy CM, Meeker AK: The potential utility of telomere-related markers for cancer diagnosis. J Cell Mol Med 2011, 15(6): 1227-1238.
- Yuan X, Larsson C, Xu D: Mechanisms underlying the activation of TERT transcription and telomerase activity in human cancer: old actors and new players. Oncogene 2019, 38(34): 6172-6183.
- Roake CM, Artandi SE: Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol 2020, 21(7): 384-397.
- Springer SU, Chen CH, Rodriguez Pena MDC: Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. Elife 2018, 7: e43237.
- Springer SU: Correction: Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. Elife 2018, 7: e43237.
- Hayashi Y, Fujita K, Netto GJ, Nonomura N: Clinical Application of TERT Promoter Mutations in Urothelial Carcinoma. Front Oncol 2021, 11: 705440.
- Zvereva M, Pisarev E: Activating Telomerase TERT Promoter Mutations and Their Application for the Detection of Bladder Cancer. Int J Mol Sci 2020, 21(17): 6034.
- Pakmanesh H, Anvari O: TERT Promoter Mutations as Simple and Non-Invasive Urinary Biomarkers for the Detection of Urothelial Bladder Cancer in a High-Risk Region. Int J Mol Sci 2022, 23(22): 14319.
- Wan S, Liu X, Hua W, Xi M, Zhou Y, Wan Y: The role of telomerase reverse transcriptase (TERT) promoter mutations in prognosis in bladder cancer. Bioengineered 2021, 12(1): 1495-1504.
- Marchese PV, Mollica V, Tassinari E, De Biase D, Giunchi F, Marchetti A, Rosellini M, Fiorentino M, Massari F: Implications of TERT promoter mutations and telomerase activity in solid tumors with a focus on genitourinary cancers. Expert Rev Mol Diagn 2022, 22(11): 997-1008.
- Teoh JY, Kamat AM: Recurrence mechanisms of non-muscle-invasive bladder cancer - a clinical perspective. Nat Rev Urol 2022, 19(5): 280-294.
- Casadio V, Calistri D, Tebaldi M, Bravaccini S, Gunelli R, Martorana G, Bertaccini A, Serra L, Scarpi E, Amadori D et al: Urine cell-free DNA integrity as a marker for early bladder cancer diagnosis: preliminary data. Urol Oncol 2013, 31(8): 1744-1750.
- Ou Z, Li K, Yang T, Dai Y, Chandra M, Ning J, Wang Y, Xu R, Gao T, Xie Y et al: Detection of bladder cancer using urinary cell-free DNA and cellular DNA. Clin Transl Med 2020, 9(1): 4.
- Brisuda A, Pazourkova E, Soukup V, Horinek A, Hrbáček J, Capoun O, Svobodova I, Pospisilova S, Korabecna M, Mares J et al: Urinary Cell-Free DNA Quantification as Non-Invasive Biomarker in Patients with Bladder Cancer. Urol Int 2016, 96(1): 25-31.
- Koguchi D, Matsumoto K, Shiba I, Harano T, Okuda S, Mori K, Hirano S, Kitajima K, Ikeda M, Iwamura M: Diagnostic Potential of Circulating Tumor Cells, Urinary MicroRNA, and Urinary Cell-Free DNA for Bladder Cancer: A Review. Int J Mol Sci 2022, 23(16): 9148.
- Christensen E, Birkenkamp-Demtröder K, Sethi H, Shchegrova S, Salari R, Nordentoft I, Wu HT, Knudsen M, Lamy P, Lindskrog SV et al: Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J Clin Oncol 2019, 37(18): 1547-1557.
- Salvi S, Gurioli G, De Giorgi U, Conteduca V, Tedaldi G, Calistri D, Casadio V: Cell-free DNA as a diagnostic marker for cancer: current insights. Onco Targets Ther 2016, 9: 6549-6559.
- Hayashi Y, Fujita K, Matsuzaki K, Eich ML, Tomiyama E, Matsushita M, Koh Y, Nakano K, Wang C, Ishizuya Y et al: Clinical Significance of Hotspot Mutation Analysis of Urinary Cell-Free DNA in Urothelial Bladder Cancer. Front Oncol 2020, 10: 755.
- Zhou Z, Cheng SH, Ding SC, Heung MMS, Xie T, Cheng THT, Lam WKJ, Peng W, Teoh JYC, Chiu PKF et al: Jagged Ends of Urinary Cell-Free DNA: Characterization and Feasibility Assessment in Bladder Cancer Detection. Clin Chem 2021, 67(4): 621-630.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript