Review Article | Open Access
Recent Progress on Urinary Exosomal miRNAs in Bladder Cancer
Mohan Dong1, Kaiyuan Zhou2
1Medical Department, the First Affiliated Hospital, Air Force Medical University, Xi’an, China.
2Health Service Department, Air Force Medical University, Xi’an, China.
Correspondence: Kaiyuan Zhou (Health Service Department, Air Force Medical University, 127 Changle West Street, Xi’an, 710032, China; Email: csnrzky@126.com).
Annals of Urologic Oncology 2023, 6(2): 39-46. https://doi.org/10.32948/auo.2023.05.15
Received: 18 Mar 2023 | Accepted: 15 May 2023 | Published online: 18 May 2023
Bladder cancer (Bca) is one of the most common tumors in the urinary system. Cystoscopy biopsy is a gold standard for diagnosis of Bca in the clinic. However, cystoscopy biopsy is invasive, costly and invasive process causing pain in the patients. Because the Bca cells are closely in contact with urine, and the exosomes of the Bca can pass through the base membrane and transport the microRNA (miRNAs) to the urine, so the detection of the exosome-derived miRNAs in the urine is expected to be a new non-invasive method for diagnostics of Bca. Recently, liquid biopsy for miRNAs in urine is the recent research hotspot. In this review, we mainly introduce the feasibility of the application of the miRNAs from exosome to Bca, and its feasibility to detect Bca.
Key words exosomal miRNAs, urine, bladder cancer, diagnostics
Bladder cancer (Bca) is one of the malignant tumors of the urinary system with high incidence rate and it is the ninth major cancer in the world, ranking 13th in the number of deaths worldwide [1-4]. Bca can be divided into non-muscle invasive bladder cancer (NMIBC, including pTa and pT1) and muscle invasive bladder cancer (MIBC, pT2 ~ pT4). Upon diagnoses for the first time, NMIBC constitutes about 70% of the Bca patients, and 25% of the patients were diagnosed as MIBC [5, 6]. Bca easily metastasizes to other organs outside of bladder but the mechanism(s) of tumor metastasis is currently unknown. In the clinic, cystoscopy is still a gold standard for the diagnosis of Bca and the follow-up evaluation for the patients with NMIBC [7, 8]. As an important media for intercellular communication, the exosomes may contain protein, lipids, mRNA, short-chain non-encoding RNA (miRNAs), and long-chain non-encoded RNA(LncRNAs) and other substances, which can regulate the signal pathway of receptor cells. There is a permanent contact between the bladder and urine, and the exosome in the Bca can transport the secreted miRNA to the urine [9, 10]. Multiple studies have shown that changes in the types and expression of the exosome-derived miRNAs in urine is closely related to the urinary carcinoma [11, 12]. In order to better understand the impact of the exosomal miRNAs in the urine on the occurrence and development of Bca, this review summarizes its application in the diagnosis, treatment, and prognosis of Bca.
The exosome-derived miRNAs in the urine have promoted the progress of Bca through various mechanisms, while some of them are inhibited factors of Bca.
Figure 1 shows that a diagram for exosome in urine in the patients of bladder cancer.
Exosome-derived miRNAs to promote Bca progression
Exosome-derived miRNAs to inhibit Bca progression
miRNAs can target specific genes through endogenous blocking the function of tumor suppressor gene, regulating the genes expression related with the progression of Bca, to promote down-regulation of target genes or inhibit their translation. Xu et al. [27] used immunohistochemical method to detect BCL-2 and MCL-1 expression in 28 cases of carcinoma and adjacent tissue. Further studies have found that the exosome-derived miR-29C can trigger the apoptosis of BIU-87 by reducing the expression of Bcl-2 and MCL-1, thereby restraining the progress of Bca. In addition, research by Sun et al. [28] found that Cyclin J (CCNJ) of cell cycle regulation gene is a new target for miR-205, and it proves that miR-205 can inhibit the proliferation, migration and invasion of Bca cells. Segersten et al. [29] found that there is significant difference in miR-130A-3p expression levels between Bca progressors and controls group. The study by Zhu et al. [30] found that circEHBP1 promotes lymphangiogenesis and lymphatic metastasis of bladder cancer via miR-130a-3p/TGFbetaR1/VEGF-D signaling. miR-9-3p, an exosome-derived miRNAs from osteamonal mesenchymal stem cells (BMSCS), can reduce ESM1 to inhibit the progression of Bca. Therefore, increased levels of miR-9-3p in Bca can reduce the invasion and metastasis of Bca cells and promote apoptosis. The exosome-derived miR-9-3p is expected to be a new target for Bca [31]. The above research shows that miRNAs can increase or lower its expression, directly or indirectly control gene expression to play an important role in the progress of Bca.
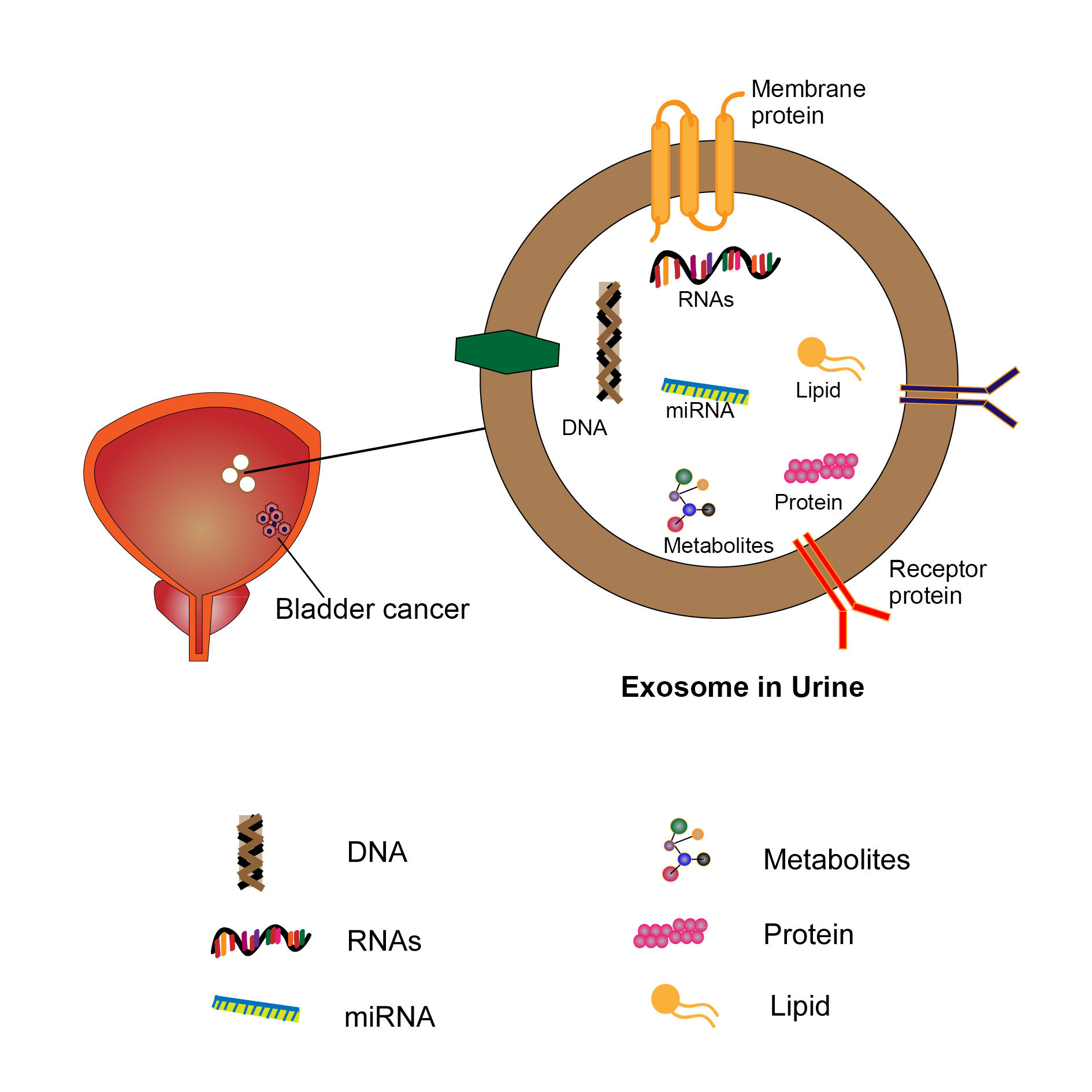 Figure 1. Exosome in urine in the patients of bladder cancer.
Figure 1. Exosome in urine in the patients of bladder cancer.
Exosome-derived miRNAs can be used as Bca biomarker
Several studies have shown that exosome-derived miRNAs from the urine can be used as biomarkersin the diagnosis and prognosis of Bca. Andreu et al. [32] used miRNA probe technology to identify 23 up-regulated miRNAs and 3 down-regulated miRNAs in the exosomes in the urine of patients with high-grade Bca, and further studies found that miR-375 was a biomarker for high-grade Bca and miR-146a was a biomarker for low-grade Bca. Hanke et al. found significant increase in the expression levels of miRNA-96 and miRNA-183 in urine in patients with Bca. However, the expression levels of these two miRNAs in the urine of the Bca patients after surgery were significantly decreased. In addition, miRNA-96 in combination with urine cytology can increase the sensitivity of urine cytology from 43.6% to 78.2%, therefore miRNA-96 can be used as a biomarker diagnosis of Bca [33-36]. Yasui et al. [37] used nanotechnology to discover a large number of urinary exosome-derived miRNAs associated with Bca: miR-558 can promote tumor growth, miR-424-3p can participate in tumor metastasis, miR-520c-3p can inhibit tumor progression, miR-378a-5p can inhibit cell proliferation and induce cell apoptosis, and miR-520b can inhibit cell invasion and migration. The above studies have proved that the exosome in urine can be used for miRNA analysis of Bca patients, and the exosome-derived miRNAs in urine can be used as a non -invasive diagnostic tool for Bca patients. It is expected to be the Bca biomarker.
Table 1 shows that a summary on exosomal miRNAs from different tissue or body fluid in bladder cancer patients.
Multi -miRNA joint test is more diagnostic significance
As mentioned earlier, the exosome-derived miRNAs in urine can be used as a non-invasive diagnosis tool for Bca patients [38, 39]. A large number of studies suggested that there is a higher diagnostic significance than a single miRNA detection for multi-miRNAs joint testing [40, 41]. For example, Chen et al. [42] combined 74 miRNAs to analyze Bca patients and healthy patients and found that 33 miRNAs were up-regulated and 41 down-regulated, among which let-7miR, mir-1268, miR-196a, miR-1, miR-100, miR-101 and miR-143 were more specific. Miah et al. [43-44] divided volunteers into Bca patients (68 cases) and age-matched control group (53 cases), and analyzed 15 types of miRNAs, and found that the sensitivity and specificity of miR-1224-3p in the diagnosis of Bca were 77% and 83%, respectively. The diagnostic sensitivity of miR-1224-3p combined with miR-15b and miR-135b could reach 94%. The above studies shows that there is a stronger application prospect in multi- miRNA combination detect analyses for the prognosis and diagnosis of Bca compared to a single miRNA detect analyses.
miRNA spectroscopy application in Bca grade
Compared with NMIBC, MIBC has a unique molecular characteristics. Some miRNAs are closely related to the invasion and migration of Bca, so their expressions in MIBC have significantly increased, so that miRNA can predict the staging of Bca by detecting miRNA. Baumgart et al. [45] analyzed the miRNA of MIBC, and found 63 specific miRNAs, of which 35 miRNAs was raised and 28 were lowered. Compared with NMIBC patients, there is altered expression in miR-141-3P, miR-146B-5P, and miR-200A-3P for MIBC patients. 6 miRNAs in the MIBC tissue and invasive cell lines are different from the expression of non-invasive cell lines. Another study by Baumgart et al. [45] compared NMIBC with MIBCwhere, 37 miRNAs show different expression through gene chip technology, among which 29 miRNAs expression levels are decreased and 8 miRNAs expression levels are increased. Further qPCR verification of exosome-derived miRNA for MIBC cells, it was found that there were 5 expression differences of miRNAs (miR-30A-3P, miR-99A-5P, miR-137-3-P, miR-141-3-P, and miR -205-5p) verified (P <0.05), while there are no difference in miR-27B-3P (P = 0.776), miR-145-5P (P = 0.864), miR-200A-3P (P = 0.456) in the urine of patients with Bca. Pardini et al. [46] found a miRNA spectrum which is capable of stratifying different Bca subtypes through next-generation sequencing analysis. Compared with the health control group, miR-205-5P among the G1/G2 patients of NMIBC is raised, and miR-21-5P, miR-106B-3P, miR-486-5P, miR-151A-3P, miR-3P, miR-3P, miR-3P, miR-3P, miR-151A-3P 200C-3P, miR-185-5P, miR-242-5P expression levels were increased, miR-30C-25P, miR-10B-5P expression levels were lowered; compared with the control group, miR-205-5P, miR-iR-5P, miR-451A, miR-25-3P, miR-71-5P expressions are higher, while miR-30A-5P expressions are lowered. Therefore, NGS technology can be used to analyze the miRNA in the urine of Bca, so as to perform staging and grading of patients with Bca. The above studies indicate that the expression levels of miRNAs in different grades are different in patients with Bca. Quantitative analysis of exosome-derived miRNAs in urine and analysis of up-regulated or down-regulated miRNAs can be used to stage and grade patients with Bca and adjust the treatment protocol.
|
Table 1. Exosomal miRNAs in Bladder Cancer Patients. |
|||
|
Tumor biomarkers |
Expression levels |
Exosome source |
Reference |
|
miRNA-23B |
Decreased |
blood |
[25] |
|
miRNA-224 |
Decreased |
blood |
[25] |
|
miR-29c |
Decreased |
tissue |
[26] |
|
miR-200c |
Decreased |
tissue |
[26] |
|
miR-146A-5P |
Increased |
urine |
[26] |
|
miR-205 |
Decreased |
blood |
[28] |
|
miR-130A-3p |
Increased |
blood |
[29] |
|
miR-9-3p |
Decreased |
tissue |
[31] |
|
miRNA-96 |
Increased |
urine |
[33] |
|
miRNA-183 |
Increased |
urine |
[33] |
|
miR-558 |
Increased |
urine |
[37] |
|
miR-424-3p |
Increased |
urine |
[37] |
|
miR-520c-3p |
Decreased |
urine |
[37] |
|
miR-378a-5p |
Decreased |
urine |
[37] |
|
miR-520b |
Decreased |
urine |
[37] |
|
mir-1268 |
Increased |
urine |
[42] |
|
miR-196a |
Increased |
urine |
[42] |
|
miR-1 |
Increased |
urine |
[42] |
|
miR-100 |
Increased |
urine |
[42] |
|
miR-101 |
Increased |
urine |
[42] |
|
miR-143 |
Increased |
urine |
[42] |
|
miR-1224-3p |
Increased |
tissue |
[43,44] |
|
miR-15b |
Increased |
tissue |
[43,44] |
|
miR-135b |
Increased |
tissue |
[43,44] |
|
miR-30A-3P |
Increased |
urine |
[45] |
|
miR-99A-5P |
Increased |
urine |
[45] |
|
miR-137-3-P |
Increased |
urine |
[45] |
|
miR-141-3-P |
Increased |
urine |
[45] |
|
miR-205-5p |
Increased |
urine |
[45] |
|
miR-21-5P |
Increased |
urine |
[46] |
|
miR-106B-3P |
Increased |
urine |
[46] |
|
miR-486-5P |
Increased |
urine |
[46] |
|
miR-151A-3P |
Increased |
urine |
[46] |
|
miR-3P |
Increased |
urine |
[46] |
|
miR-185-5P |
Increased |
urine |
[46] |
|
miR-242-5P |
Increased |
urine |
[46] |
|
miR- 200C-3P |
Increased |
urine |
[46] |
|
miR-30C-25P |
Decreased |
urine |
[46] |
|
miR-10B-5P |
Decreased |
urine |
[46] |
|
miR-451A |
Increased |
urine |
[46] |
|
miR-25-3P |
Increased |
urine |
[46] |
|
miR-71-5P |
Increased |
urine |
[46] |
|
miR-30A-5P |
Decreased |
urine |
[46] |
|
miR-23b |
Increased |
urine |
[47, 48] |
|
miR-921 |
Increased |
urine |
[47, 48] |
|
miR-29c |
Increased |
urine |
[50] |
|
miR-141 |
Increased |
blood |
[51] |
|
miR-639 |
Increased |
blood |
[51] |
|
miR-210 |
Decreased |
urine |
[58] |
|
miR-96 |
Decreased |
urine |
[58] |
In the process of tumor progression, by regulating the secretion of miRNA, the development of Bca can achieve the purpose of treatment [42]. Inhibition of miRNA secretion has been selected as a mechanism for coordinated tumor metastasis cascade activation mediated by exosomes. Ostenfeld et al. [47-48] found that silencing RAB27A or RAB27B, a member of the extracellular RAB family, can inhibit tumor secretion of miR-23b and miR-921, thus reducing tumor cell invasion. At the same time, Kosaka et al. [49] using neutral spkingomyelinase 2 (neutral sphingomyelinase 2, nSMase2) can promote the characteristics of the secretion of exosome-derived miRNAs, which is able to inhibit metastases of tumors. Xu et al. [50] found that miRP29c can be wrapped in the exosome, and Bca cell line are constructed with miRP29c is highly expressed. Transfection of miRNA-29c in Bca can increase the apoptosis of Bca and decrease the expression levels of anti-apoptotic genes such as BCLP2 and mcl1, which plays a role in directly killing Bca cells. These studies suggest the promising application prospects of exosome-derived miRNAs in the treatment of Bca.
Urine is easy to collect and contact with Bca tissue directly
Bca and urine have been exposed for a long time, and the exosomes in Bca can pass through the base membrane and transport the miRNAs to urine [8]. Urine test is a clinical routine examination and is easy to obtain. The urine collection method is simple, non-invasive, and the patients are with good compliance. Compared with blood test, urine excludes interference from other systems. The urine contains the exosome secreted by glomerular podocytes and renal tubular cells in urinary tract [50]. In 2012, Scheffer et al. [51] found that there were 22 raised miRNAs expression levels in the serum of Bca patients. Studies have found that although the levels of miR-141 and miR-639 in serum increase but it lacks specificity in the diagnosis. Therefore, miRNAs examination in urine can be an ideal liquid for analyses of miRNAs of urinary tumor biomarkers such as Bca.
A large number of undegraded miRNAs exists in urine
The exosome volume in the urine is large and it cannot across the glomerular filtration membrane in the kidneys. Most of them are directly secreted by the cells of the extrarenal urogenic tract. The protein miRNAs in the exosomes secreted by different tissues are also different and with its individual specificity. Cheng et al. [52-54] extracted and analyses these RNAs in urine, urine cell precipitation, cell-free urine, urinary supernatant without exosomes. They found that only urine has a large number of intact miRNAs in the exosome in above several specimens. Although a large number of miRNAs was detected in the precipitation of urine cells, while most of them have been degraded. The application of high-throughput depth sequencing method detected 184 exosome-derived miRNAs in 3 different subjects, while only 7 of them were shared with free-cell urine. Therefore, it can be concluded that there are a lot of RNases in the urinary system, naked miRNAs in urine are more easily degraded, and exosomes can protect miRNAs from RNase degradation. The research by Yun et al. [55] found that in urine, miRNAs has not changed significantly after several freezing, thawing cycles, and long-term preservation at room temperature, indicating that miRNAs is more stable in urine. Therefore, the study on exosome-derived miRNAs is more meaningful.
Exosome-derived miRNAs in urine is a suitable choice to detect Bca
The close correlation of miRNAs and tumor has been confirmed in the literature that it has participated in the development and metastasis of tumors and other diseases. Tumor-related exosomes miRNAs can regulate the interaction between tumor cells and tumor microenvironment by affecting the tumor-promoting process of target cells. The exosomes secreted by MIBC cells has a specific miRNA expression pattern [56]. Matsuzaki et al. [57] used qRT-PCR to find that miR-21-5p was highly expressed in exosomes of Bca cells with negative urine cytology, and the specificity and sensitivity of exosomes-derived miR-21-5p were higher than those of urine ablative cytology. These results indicate that miR-21-5p in exosomes can be used for the detection of Bca with cytological negative urine shedding. At the same time, Eissa et al. [58] identified miR-96 and miR-210 in Bca patients with a cystoscopy test negative. Sapre et al. [59-60] used 12 miRNAs as the diagnosis method of Bca. Sapre et al. [59-60] used 12 miRNAs to diagnose Bca and reduced the cystoscopy rate by 30% by increasing specificity and sensitivity, indicating that miRNA is expected to replace cystoscopy as a new noninvasive method for detecting Bca in the near future. Armstrong et al. [61] analyzed the miRNA molecular spectrum of four biological specimens: miRNA molecular spectrum of four biological specimens sources: Bca tumors, urine (free cells and exosome source), blood and plasma rich in white blood cells (circulation and exosome sources).
Studies have shown that the exosome-derived and rich white blood cell source miRNA spectrum in urine is highly related to the miRNA of Bca, and it may have the huge potential of diagnosis of Bca as a source of biomarkers for miRNA. The circulating miRNA, which has no cell plasma exosome, has no correlation with the bladder tumor miRNAs. It may not be a good choice for the biomarkers of Bca. The above studies have shown that the absence of exosome in urine is a suitable choice to detect Bca.
At the same time, there are many ways to separate and purify the exosome, the existing methods make it hard to achieve the purity required for clinical applications, and it is easy to get false negative or false results. Better technology to increase the division and purity of exosomes, and increase the yield of exosome in urine, to achieve more effective clinical applications. In the near future, through multi-disciplinary cooperation, in-depth analysis of scientists, and clinical research of large samples, the samples in urine can replace the cystoscopy test to become a new non-invasive biomarker in the diagnosis and treatment of Bca.
Acknowledgements
We thank Dr. Sanjay Gupta (Case Western Reserve University & UH Cleveland Medical Center) for his proofreading for the review.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
MHD designed the study and was responsible for the writing of the original draft. KYZ edited and approved the final manuscript.
Competing interests
All authors declare no competing interests.
Funding
None.
- Barone B, Finati M, Cinelli F, Fanelli A, Del Giudice F, De Berardinis E, Sciarra A, Russo G, Mancini V, D'Altilia N, et al: Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J Pers Med 2023, 13(3): 512.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68(6): 394-424.
- Siegel RL, Miller KD, Fuchs HE, Jemal A: Cancer statistics, 2022. CA Cancer J Clin 2022, 72(1): 7-33.
- Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F: Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017, 71(1): 96-108.
- Lenis AT, Lec PM, Chamie K, Mshs MD: Bladder Cancer: A Review. JAMA 2020, 324(19): 1980-1991.
- Ro JY, Staerkel GA, Ayala AG: Cytologic and histologic features of superficial bladder cancer. Urol Clin North Am 1992, 19(3): 435-453.
- Lee J, Heo JE, Kang SK, Lee KS, Han H, Jang WS, Choi YD: Noninvasive studies may have potential to replace cystoscopy in non-muscle invasive bladder cancer follow-up. Sci Rep 2022, 12(1): 21677.
- Malinaric R, Mantica G, Lo Monaco L, Mariano F, Leonardi R, Simonato A, Van der Merwe A, Terrone C: The Role of Novel Bladder Cancer Diagnostic and Surveillance Biomarkers-What Should a Urologist Really Know. Int J Environ Res Public Health 2022, 19(15): 9648.
- Cimmino I, Bravaccini S, Cerchione C: Urinary Biomarkers in Tumors: An Overview. Methods Mol Biol 2021, 2292: 3-15.
- Chen C, Shang A, Sun Z, Gao Y, Huang J, Ping Y, Chang W, Gu C, Sun J, Ji P, et al: Urinary Exosomal Long Noncoding RNA TERC as a Noninvasive Diagnostic and Prognostic Biomarker for Bladder Urothelial Carcinoma. J Immunol Res 2022, 2022: 9038808.
- Grimaldi AM, Lapucci C, Salvatore M, Incoronato M, Ferrari M: Urinary miRNAs as a Diagnostic Tool for Bladder Cancer: A Systematic Review. Biomedicines 2022, 10(11): 2766.
- Eissa S, Habib H, Ali E, Kotb Y: Evaluation of urinary miRNA-96 as a potential biomarker for bladder cancer diagnosis. Med Oncol 2015, 32(1): 413.
- Hashemi M, Arani HZ, Orouei S, Rostamnejad E, Ghorbani A, Khaledabadi M, Kakavand A, Tavakolpournegari A, Saebfar H, Heidari H, et al: Crosstalk of miRNAs with signaling networks in bladder cancer progression: Therapeutic, diagnostic and prognostic functions. Pharmacol Res 2022, 185: 106475.
- Catalanotto C, Cogoni C, Zardo G: MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int J Mol Sci 2016, 17(10): 1712.
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R: Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012, 1820(7): 940-948.
- Walker JM, O'Malley P, He M: Applications of Exosomes in Diagnosing Muscle Invasive Bladder Cancer. Pharmaceutics 2022, 14(10): 2027.
- Mohankumar S, Patel T: Extracellular vesicle long noncoding RNA as potential biomarkers of liver cancer. Brief Funct Genomics 2016, 15(3): 249-256.
- Zhang S, Du L, Wang L, Jiang X, Zhan Y, Li J, Yan K, Duan W, Zhao Y, Wang L, et al: Evaluation of serum exosomal LncRNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer. J Cell Mol Med 2019, 23(2): 1396-1405.
- Batrakova EV, Kim MS: Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release 2015, 219: 396-405.
- Liu Q: The emerging roles of exosomal long non-coding RNAs in bladder cancer. J Cell Mol Med 2022, 26(4): 966-976.
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT: MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 2011, 13(4): 423-433.
- Simpson RJ, Jensen SS, Lim JW: Proteomic profiling of exosomes: current perspectives. Proteomics 2008, 8(19): 4083-4099.
- Martins-Lima C, Chianese U, Benedetti R, Altucci L, Jerónimo C, Correia MP: Tumor microenvironment and epithelial-mesenchymal transition in bladder cancer: Cytokines in the game. Front Mol Biosci 2022, 9: 1070383.
- Chen X, Chen RX, Wei WS, Li YH, Feng ZH, Tan L, Chen JW, Yuan GJ, Chen SL, Guo SJ, et al: PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clin Cancer Res 2018, 24(24): 6319-6330.
- Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, et al: Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res 2014, 74(20): 5758-5771.
- Zhuang J, Shen L, Li M, Sun J, Hao J, Li J, Zhu Z, Ge S, Zhang D, Guo H, et al: Cancer-associated fibroblast-derived miR-146a-5p generates a niche that promotes bladder cancer stemness and chemoresistance. Cancer Res 2023, 83(10): 1611-1627.
- Xu XD, Wu XH, Fan YR, Tan B, Quan Z, Luo CL: Exosome-derived microRNA-29c induces apoptosis of BIU-87 cells by down regulating BCL-2 and MCL-1. Asian Pac J Cancer Prev 2014, 15(8): 3471-3476.
- Sun X, Du P, Yuan W, Du Z, Yu M, Yu X, Hu T: Long non-coding RNA HOTAIR regulates cyclin J via inhibition of microRNA-205 expression in bladder cancer. Cell Death Dis 2015, 6(10): e1907.
- Segersten U, Spector Y, Goren Y, Tabak S, Malmström PU: The role of microRNA profiling in prognosticating progression in Ta and T1 urinary bladder cancer. Urol Oncol 2014, 32(5): 613-618.
- Zhu J, Luo Y, Zhao Y, Kong Y, Zheng H, Li Y, Gao B, Ai L, Huang H, Huang J, et al: circEHBP1 promotes lymphangiogenesis and lymphatic metastasis of bladder cancer via miR-130a-3p/TGFβR1/VEGF-D signaling. Mol Ther 2021, 29(5): 1838-1852.
- Cai H, Yang X, Gao Y, Xu Z, Yu B, Xu T, Li X, Xu W, Wang X, Hua L: Exosomal MicroRNA-9-3p Secreted from BMSCs Downregulates ESM1 to Suppress the Development of Bladder Cancer. Mol Ther Nucleic Acids 2019, 18: 787-800.
- Andreu Z, Otta Oshiro R, Redruello A, López-Martín S, Gutiérrez-Vázquez C, Morato E, Marina AI, Olivier Gómez C, Yáñez-Mó M: Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur J Pharm Sci 2017, 98: 70-79.
- Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, Warnecke JM, Sczakiel G: A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol 2010, 28(6): 655-661.
- Yamada Y, Enokida H, Kojima S, Kawakami K, Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N, et al: MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: correlation with stage and grade, and comparison with urinary cytology. Cancer Sci 2011, 102(3): 522-529.
- Du L, Jiang X, Duan W, Wang R, Wang L, Zheng G, Yan K, Wang L, Li J, Zhang X, et al: Cell-free microRNA expression signatures in urine serve as novel noninvasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Oncotarget 2017, 8(25): 40832-40842.
- von Brandenstein M, Pandarakalam JJ, Kroon L, Loeser H, Herden J, Braun G, Wendland K, Dienes HP, Engelmann U, Fries JW: MicroRNA 15a, inversely correlated to PKCα, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am J Pathol 2012, 180(5): 1787-1797.
- Yasui T, Yanagida T, Ito S, Konakade Y, Takeshita D, Naganawa T, Nagashima K, Shimada T, Kaji N, Nakamura Y, et al: Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci Adv 2017, 3: e1701133.
- Berrondo C, Flax J, Kucherov V, Siebert A, Osinski T, Rosenberg A, Fucile C, Richheimer S, Beckham CJ: Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS One 2016, 11(1): e0147236.
- Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS: Urine Exosomes: An Emerging Trove of Biomarkers. Adv Clin Chem 2017, 78: 103-122.
- Pillay P, Moodley K, Moodley J, Mackraj I: Placenta-derived exosomes: potential biomarkers of preeclampsia. Int J Nanomedicine 2017, 12: 8009-8023.
- Butz H, Nofech-Mozes R, Ding Q, Khella H, Szabó PM, Jewett M, Finelli A, Lee J, Ordon M, Stewart R, et al: Exosomal MicroRNAs Are Diagnostic Biomarkers and Can Mediate Cell-Cell Communication in Renal Cell Carcinoma. Eur Urol Focus 2016, 2(2): 210-218.
- Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q, Hu L, Lou D, Li J, Dong X, et al: Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods 2021, 18(2): 212-218.
- Miah S, Dudziec E, Drayton RM, Zlotta AR, Morgan SL, Rosario DJ, Hamdy FC, Catto JW: An evaluation of urinary microRNA reveals a high sensitivity for bladder cancer. Br J Cancer 2012, 107(1): 123-128.
- Miah S, Pang K, Catto JW: MicroRNA and urothelial cell carcinoma. BJU Int 2014, 113(5): 811-812.
- Baumgart S, Meschkat P, Edelmann P, Heinzelmann J, Pryalukhin A, Bohle R, Heinzelbecker J, Stöckle M, Junker K: MicroRNAs in tumor samples and urinary extracellular vesicles as a putative diagnostic tool for muscle-invasive bladder cancer. J Cancer Res Clin Oncol 2019, 145(11): 2725-2736.
- Pardini B, Cordero F, Naccarati A, Viberti C, Birolo G, Oderda M, Di Gaetano C, Arigoni M, Martina F, Calogero RA, et al: microRNA profiles in urine by next-generation sequencing can stratify bladder cancer subtypes. Oncotarget 2018, 9(29): 20658-20669.
- Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, et al: Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res 2014, 74(20): 5758-5771.
- Ostenfeld MS, Bramsen JB, Lamy P, Villadsen SB, Fristrup N, Sørensen KD, Ulhøi B, Borre M, Kjems J, Dyrskjøt L, et al: miR-145 induces caspase-dependent and -independent cell death in urothelial cancer cell lines with targeting of an expression signature present in Ta bladder tumors. Oncogene 2010, 29(7): 1073-1084.
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T: Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010, 285(23): 17442-17452.
- Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ: Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 2016, 126(4): 1152-1162.
- Scheffer AR, Holdenrieder S, Kristiansen G, von Ruecker A, Müller SC, Ellinger J: Circulating microRNAs in serum: novel biomarkers for patients with bladder cancer. World J Urol 2014, 32(2): 353-358.
- Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF: Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney Int 2014, 86(2): 433-444.
- Cheng Y, Deng X, Yang X, Li P, Zhang X, Li P, Tao J, Lu Q, Wang Z: Urine microRNAs as biomarkers for bladder cancer: a diagnostic meta-analysis. Onco Targets Ther 2015, 8: 2089-2096.
- Cheng N, Xu Y, Luo Y, Zhu L, Zhang Y, Huang K, Xu W: Specific and relative detection of urinary microRNA signatures in bladder cancer for point-of-care diagnostics. Chem Commun (Camb) 2017, 53(30): 4222-4225.
- Yun SJ, Jeong P, Kim WT, Kim TH, Lee YS, Song PH, Choi YH, Kim IY, Moon SK, Kim WJ: Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int J Oncol 2012, 41(5): 1871-1878.
- Santoni G, Morelli MB, Amantini C, Battelli N: Urinary Markers in Bladder Cancer: An Update. Front Oncol 2018, 8: 362.
- Matsuzaki K, Fujita K, Jingushi K, Kawashima A, Ujike T, Nagahara A, Ueda Y, Tanigawa G, Yoshioka I, Ueda K, et al: MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget 2017, 8(15): 24668-24678.
- Eissa S, Matboli M, Essawy NO, Kotb YM: Integrative functional genetic-epigenetic approach for selecting genes as urine biomarkers for bladder cancer diagnosis. Tumour Biol 2015, 36(12): 9545-9552.
- Sapre N, Anderson PD, Costello AJ, Hovens CM, Corcoran NM: Gene-based urinary biomarkers for bladder cancer: an unfulfilled promise. Urol Oncol 2014, 32(1): 48.e9-48.e17.
- Sapre N, Macintyre G, Clarkson M, Naeem H, Cmero M, Kowalczyk A, Anderson PD, Costello AJ, Corcoran NM, Hovens CM: A urinary microRNA signature can predict the presence of bladder urothelial carcinoma in patients undergoing surveillance. Br J Cancer 2016, 114(4): 454-462.
- Armstrong DA, Green BB, Seigne JD, Schned AR, Marsit CJ: MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol Cancer 2015, 14: 194.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript