Case Report | Open Access
Primary Extra Renal Papillary Renal Cell Carcinoma Masquerading as A Metastatic Carcinoma: A Unique Case with Dual Malignancies
Sivaranjani Selvaraj1, Akkamahadevi Patil1, Champaka. G1, Usha Amirtham1
1Department of Pathology, Kidwai memorial institute of oncology, Bengaluru, India.
Correspondence: Sivaranjani Selvaraj (Department of Pathology, Kidwai memorial institute of oncology, No. 25, Aranga nagar, Vilankuruchi road, Peelamedu post, Coimbatore, Tamilnadu, 641004, India; E-mail: sivaranjani0892@gmail.com).
Annals of Urologic Oncology 2023, 6(3): 121-126. https://doi.org/10.32948/auo.2023.09.10
Received: 05 Aug 2023 | Accepted: 10 Sep 2023 | Published online: 16 Sep 2023
Key words renal cell carcinoma, extra-renal renal cell carcinoma, papillary, metastasis, heterotopic kidney, synchronous tumors, dual malignancies
Synchronous primary malignancies are defined by Warren and Gates’s criteria, i.e they must be histologically confirmed, must be geographically separated and distinct lesions and the probability of metastasis of either has to be excluded.
Hereby, we present the first case of an extrarenal papillary RCC arising in the peri-renal adipose tissue along with synchronous primary adenocarcinoma of rectum. Literature review studies show that conventional clear cell RCC is the most common type of extra-renal RCC. However, our case was a papillary RCC; the second reported case at an extra–renal site.
A 50-year-old male presented with abdominal pain, pain during defaecation, constipation, bleeding per rectum for 2 months. Colonoscopy showed an ulcero-proliferative growth that is 2.5 cm from anal verge.
Radiological findings
Further PET-CT was done for metastatic workup which confirmed the growth in lower rectum with mesorectal fat stranding. There was also an irregular, lobulated, encapsulated solid-cystic mass measuring 7 x 6 x 4 cm in right perinephric fat space separated from the renal capsule. Bilateral kidneys and adrenals were unremarkable. This perinephric mass was suggestive of metastasis radiologically with an SUV of 8.5 (Figure 1).
Initial histopathological and IHC findings
Biopsy of the rectum revealed a moderately differentiated adenocarcinoma. Biopsy from the right perinephric mass revealed a papillary neoplasm morphologically. The histopathologic findings favoured a papillary renal cell carcinoma. Further IHC was performed, which showed positivity for vimentin, CK7, AMACR, focally positive for CD10 and SATB2 and negative for PAX-8, RCC, CA IX CK20, CDX2 (Figure 2). On correlating with clinical, radiological findings; which had strong suspicion of metastasis, the SATB2 positivity, made us err towards a metastatic adenocarcinoma of rectum in spite of inconclusive immunomorphology.
As the lesion was encapsulated, excision of the mass was performed along with abdominoperineal resection (APR). Grossly, the APR had a 6 x 4 x 1.5 cm tumour infiltrating into the muscularis propria. The perinephric mass was completely encapsulated grossly measuring 7 x 5 x 4 cm with a solid-cystic grey-brown cut surface.
Final histopathologic evaluation
Histopathological evaluation of APR revealed a moderately differentiated adenocarcinoma of rectum, and pathologic stage of pT3 N1. Histopathological evaluation of right perinephric mass revealed a tumor arranged in papillary pattern composed of tumour cells exhibiting moderate nuclear pleomorphism, vesicular nuclei, prominent nucleoli and moderate eosinophilic cytoplasm. There were numerous psammoma bodies also identified. The above features were morphologically suggestive of a papillary renal cell carcinoma. However, the intact kidneys radiologically gave us a set back and could not explain it. The periphery of the mass showed dilated tubules lines by bland cuboidal epithelium, which were suspected to be renal tubules. Further sampling of the mass revealed a glomerulus and renal tubules exhibiting thyroidisation. This confirmed the mass to be a heterotopic kidney with papillary renal cell carcinoma arising from it. We ruled out the possibility of supernumerary kidney as radiologically, the mass did not have a separate collecting system.
IHC findings
Immunohistochemistry (IHC) performed on tumor from perinephric mass showed positivity in tumor cells for vimentin, AMACR and focally positive for CD10. PAX-8 was positive in the adjacent cystically dilated tubules. The tumor cells were negative for CK7, CK20, CAIX, NapsinA, CDX2 (Figure 3). The presence of psammoma bodies raised a suspicion of translocation associated RCC. However, TFE-3 was negative. IHC done on the rectal tumor revealed positivity for CDX2, CK20 and negative for PAX-8, CK7, AMACR and CD10 (Figure 4). This ruled out metastasis of either tumors. Summarised in Table 1.
The final diagnosis was given as dual malignancies with synchronous primary extra-renal papillary RCC and adenocarcinoma of rectum.
|
Table 1. Summary of IHC markers. |
||
|
IHC Marker |
Perinephric Mass |
Abdominoperineal Resection |
|
CK7 |
Negative |
Negative |
|
CK20 |
Negative |
Positive |
|
CDX2 |
Negative |
Positive |
|
Vimentin |
Positive |
Negative |
|
AMACR |
Positive |
Negative |
|
CD10 |
Positive(focally) |
Negative |
|
PAX-8 |
Positive (dilated tubules) |
Negative |
|
CA-IX |
Negative |
Negative |
|
Napsin-A |
Negative |
N/A |
|
TFE-3 |
Negative |
N/A |
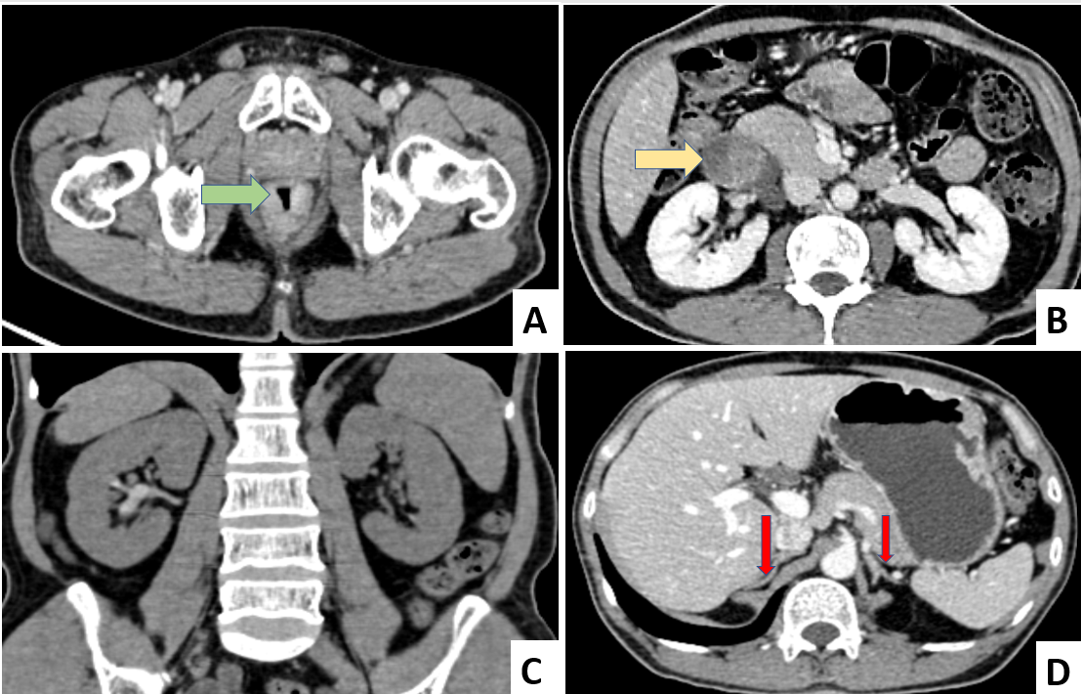 Figure 1. PET-CT. (A) Growth in the lower rectum with mesorectal fat stranding (marked by green arrow); (B) An irregular, lobulated, encapsulated solid-cystic mass identified in the right perinephric fat space which has no communication with the right kidney (marked by yellow arrow)- Suggestive of metastasis radiologically; (C) Bilateral normal Kidneys and (D) Bilateral normal Adrenals (marked by red arrow).
Figure 1. PET-CT. (A) Growth in the lower rectum with mesorectal fat stranding (marked by green arrow); (B) An irregular, lobulated, encapsulated solid-cystic mass identified in the right perinephric fat space which has no communication with the right kidney (marked by yellow arrow)- Suggestive of metastasis radiologically; (C) Bilateral normal Kidneys and (D) Bilateral normal Adrenals (marked by red arrow).
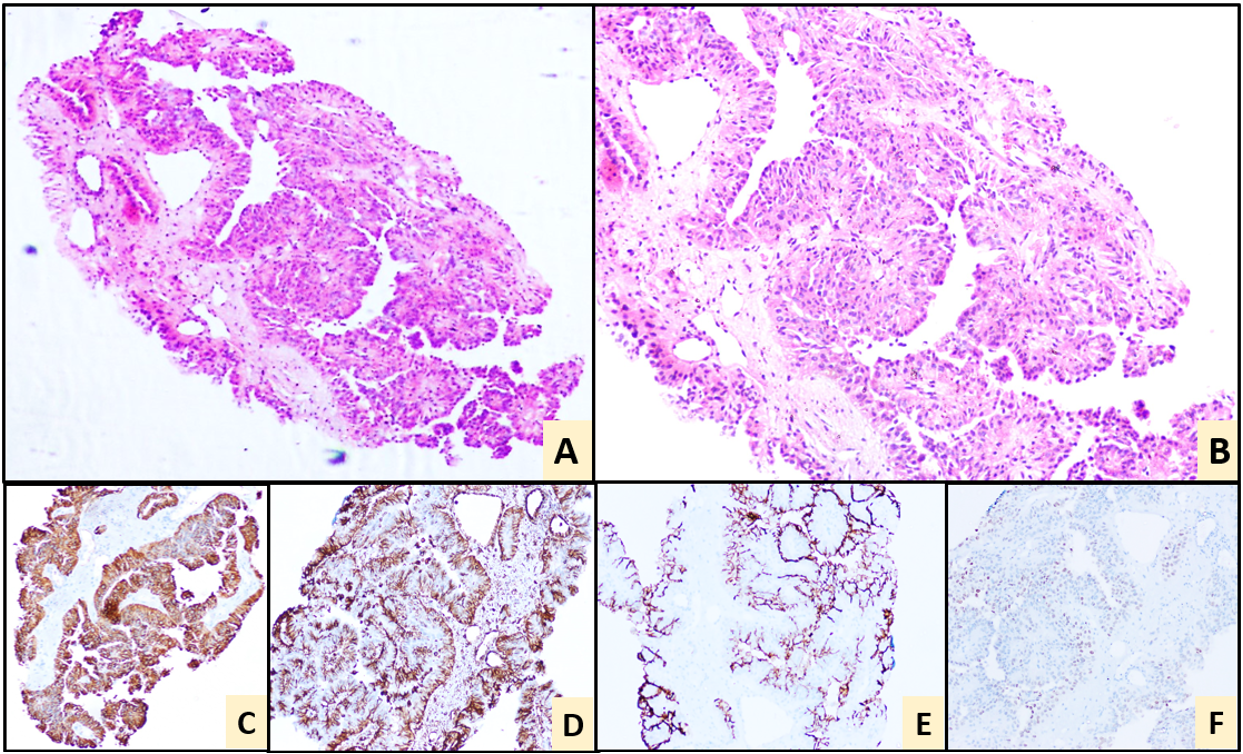 Figure 2. Perinephric mass biopsy. Haematoxylin and Eosin stain showing a papillary neoplasm (A, 10x) and (B, 20x). IHC: Positive for vimentin (C), AMACR (D) and focally positive for CD10 (E) & SATB2 (F). Negative IHC markers: PAX-8, RCC, CA IX, CK20, CDX2.
Figure 2. Perinephric mass biopsy. Haematoxylin and Eosin stain showing a papillary neoplasm (A, 10x) and (B, 20x). IHC: Positive for vimentin (C), AMACR (D) and focally positive for CD10 (E) & SATB2 (F). Negative IHC markers: PAX-8, RCC, CA IX, CK20, CDX2.
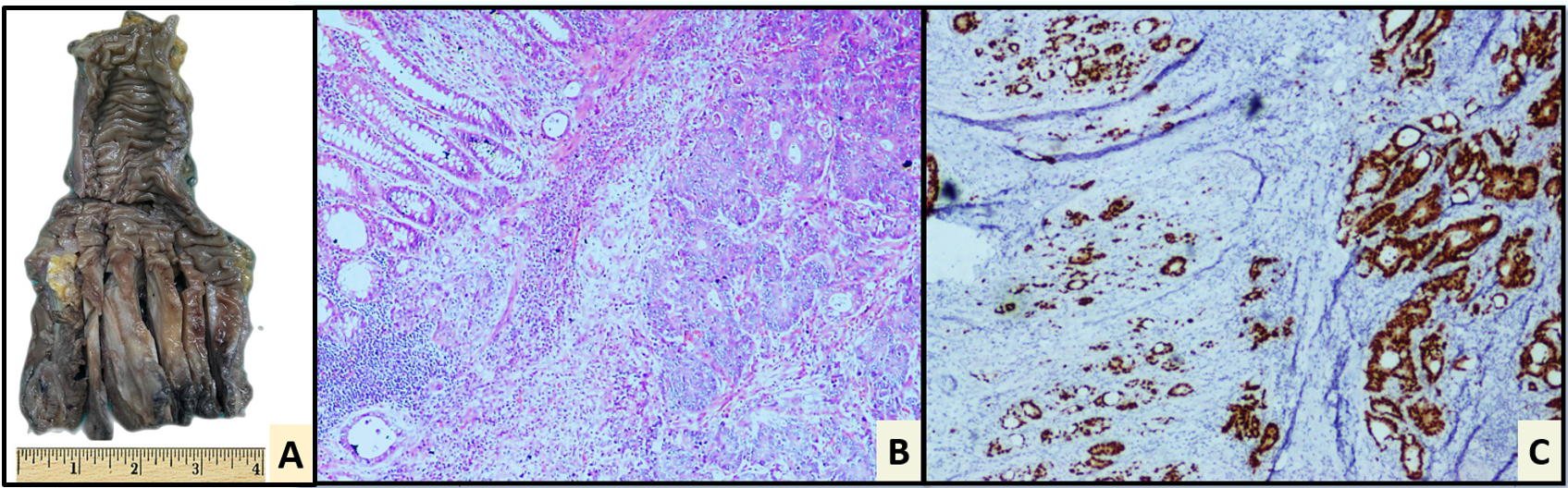 Figure 3. Abdominoperineal resection(apr). (A) Gross photograph of APR showing an ulcero-infiltrative tumour in the lower third of rectum; Haematoxylin and Eosin stain showing features of adenocarcinoma, Grade 2 (B, 20x). IHC: Positive for CDX2 (C). The tumor was MMR proficient on IHC. Negative IHC markers: PAX-8, CK7, AMACR CA-IX and CD10.
Figure 3. Abdominoperineal resection(apr). (A) Gross photograph of APR showing an ulcero-infiltrative tumour in the lower third of rectum; Haematoxylin and Eosin stain showing features of adenocarcinoma, Grade 2 (B, 20x). IHC: Positive for CDX2 (C). The tumor was MMR proficient on IHC. Negative IHC markers: PAX-8, CK7, AMACR CA-IX and CD10.
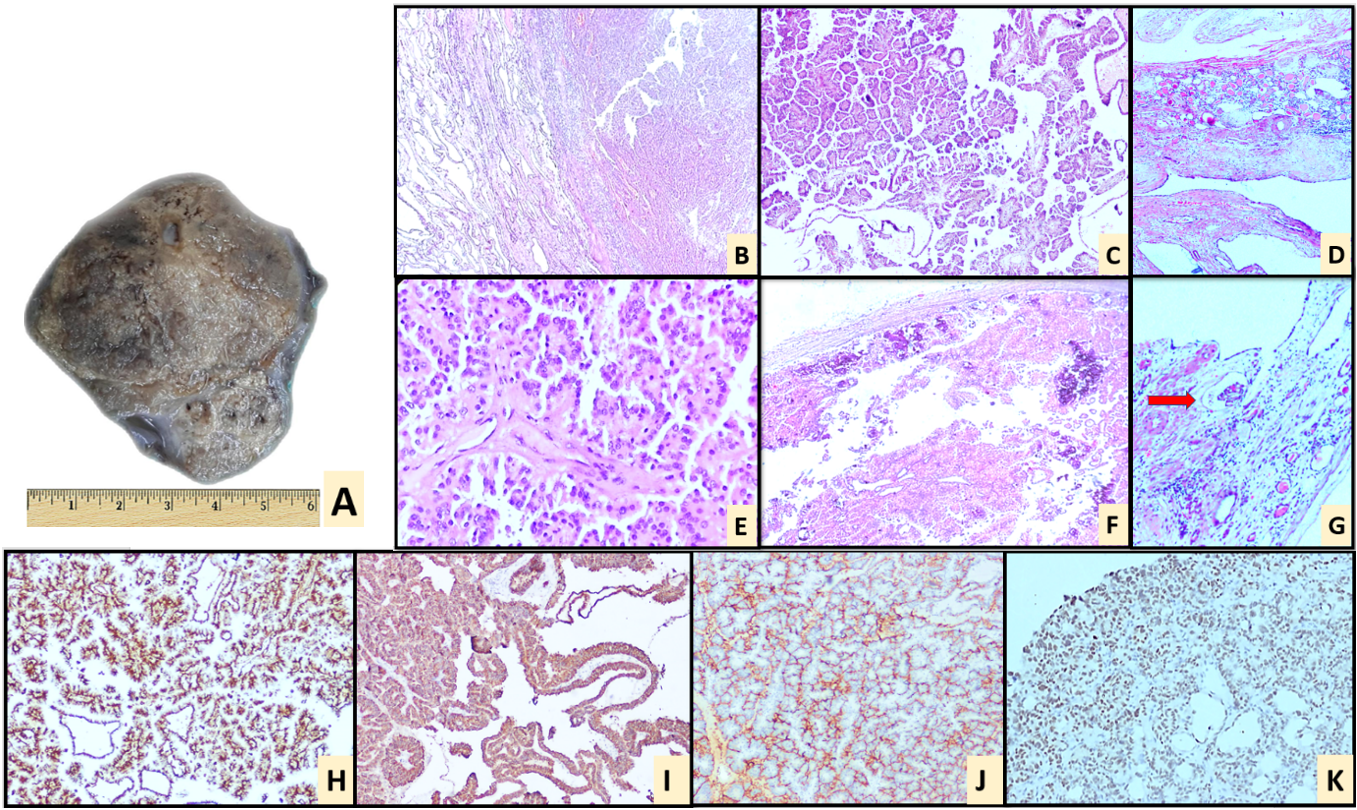 Figure 4. Perinephric mass excision. (A) Gross photograph of right perinephric space mass showing an encapsulated mass with a solid, grey-brown cut surface; Haematoxylin and Eosin stain showing an encapsulated neoplasm exhibiting a papillary architecture with adjacent tissue showing cystically dilated tubules (B, 10x), (C, 20x), (E, 40x); There are numerous psammoma bodies seen (F); On further sampling, renal tubules were identified with evidence of thyroidisation (D) along with an occasional glomerulus (marked with red arrow) (G); IHC: Excised specimen of Perinephric mass showing diffuse positivity for vimentin (H) and AMACR (I); CD10 (J) shows focal positivity, PAX-8 (K) is positive in the adjacent cystically dilated tubules. Negative IHC markers: CK7, CK20, CAIX, NapsinA, CDX2, TFE3.
Figure 4. Perinephric mass excision. (A) Gross photograph of right perinephric space mass showing an encapsulated mass with a solid, grey-brown cut surface; Haematoxylin and Eosin stain showing an encapsulated neoplasm exhibiting a papillary architecture with adjacent tissue showing cystically dilated tubules (B, 10x), (C, 20x), (E, 40x); There are numerous psammoma bodies seen (F); On further sampling, renal tubules were identified with evidence of thyroidisation (D) along with an occasional glomerulus (marked with red arrow) (G); IHC: Excised specimen of Perinephric mass showing diffuse positivity for vimentin (H) and AMACR (I); CD10 (J) shows focal positivity, PAX-8 (K) is positive in the adjacent cystically dilated tubules. Negative IHC markers: CK7, CK20, CAIX, NapsinA, CDX2, TFE3.
There are twelve reported cases of extra-renal RCC [9] of them arising from prostate, 1 from adrenal gland, peri-nephric adipose tissue, retroperitoneum, adjacent to inferior vena cava each respectively. Summary of the reported cases in medical literature are represented in the Table 2. Eleven of twelve cases were clear cell RCC and 1 case was a type II papillary RCC. Our case is a type II papillary RCC. There were areas of psommomatous calcifications identified which was further worked up for Microphthalmia translocation associated RCC and turned out to be negative. The second synchronous primary in our case was adenocarcinoma of the rectum.
The differential diagnoses while working up these cases of extra-renal RCC is very intricate and unique for each case. In our case, the first possibility considered was a metastasis as we had an established diagnosis of adenocarcinoma arising from rectum. The morphology and immunohistochemistry did not complement this possibility. The positivity of SATB2 and negativity of PAX-8 added on to the clinico-radiological suspicion, complicating the scenario. SATB2 shows variable positivity in RCCs and is shown to be a poor prognostic factor in clear cell RCC [5]. PAX-8 is negative in 10% of renal cell acrcinomas [7]. After the resection of the perinephric mass with APR, we worked up both the tumors extensively and arrived at the final diagnosis. This is first reported case of extra-renal RCC presenting as a dual malignancy and the second reported case of papillary extra-renal RCC in English literature.
The second synchronous primary in our case was adenocarcinoma of the rectum with tumour budding and deposits in serosa. The patient was in Stage III - pT3N2a. This case fulfilled the Warren and Gate’s criteria for multiple primary malignancies and was given this final diagnosis of Synchronous primaries being extrarenal Papillary RCC and carcinoma rectum. The patient is now planned for adjuvant chemotherapy for carcinoma rectum as priority following the resection.
|
Table 2. Literature review of relative case. |
|||||
|
Author |
Year |
Location of Tumour |
Pathologic Subtype |
TFE3/TFEB Positivity
|
Second primary |
|
Singh et al. [2] |
2003 |
Prostate |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Pal and Chowdhury [6]
|
2007 |
Prostate |
Renal-type Clear Cell Carcinoma |
N/A |
No |
|
Permi et al. [7] |
2011 |
Prostate |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Terada et al. [8] |
2011 |
Peri-renal adipose tissue
|
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Hasan et al. [9] |
2015 |
Adrenal gland |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Patne et al. [10] |
2015 |
Prostate |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Wang and Xue [11] |
2015 |
Prostate |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Sato et al. [12] |
2016 |
Prostate |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Liao et al. [13] |
2018 |
Prostate |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Li et al. [3] |
2019 |
Adjacent to inferior Vena Cava |
Type II Papillary RCC
|
TFE3 and TFEB positive |
No |
|
Han and Lim [14] |
2020 |
Prostate |
Renal-type Clear Cell Carcinoma
|
N/A |
No |
|
Petrinec et al. [15] |
2021 |
Retroperitoneum |
Renal-type Clear Cell Carcinoma
|
TFE3 positive |
No |
|
Current case |
2022 |
Right Peri-renal adipose tissue
|
Type II Papillary RCC |
TFE3 negative |
Yes-Adenocarcinoma, Rectum |
None.
Ethical policy
There were no human participants involved. The data was collected from archival resources.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
SS: archival retrieval and collection of data, writing the report, review of literature; AP: manuscript correction and review of literature; CG: manuscript correction and review of literature; UA: manuscript correction and review of literature.
Competing interests
No conflicts of interest.
Funding
No funding required.
Consent
Institutional consent was obtained. However, no patient details are disclosed in the case report. IRB approval was not obtained as it was only a case report.
- Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V: Renal cell carcinoma. Nat Rev Dis Primers 2017, 3: 17009.
- Singh H, Flores-Sandoval N, Abrams J: Renal-type clear cell carcinoma occurring in the prostate. Am J Surg Pathol 2003, 27(3): 407-410.
- Li Y, Qiu X, Li W, Yang Y, Yang R, Zhao X, Guo H, Li X: Primary Extrarenal Type 2 Papillary Renal Cell Carcinoma: A Case Report. Urology 2019, 123: e1-e3.
- AlShalabi O, Alsaid B, AlShalabi A: A huge extrarenal cell carcinoma developing from a heterotopic renal anlage with distant metastases: Case report and literature review. J Surg Case Rep 2020, 2020(8): rjaa227.
- Mansoor M, Young-Speirs M, Ren B, Gotto G, Merten L, Sawhney S, Siadat F, Acosta AM, Agaimy A, Trpkov K: Extrarenal renal cell carcinoma arising in the kidney proximity but without an identifiable renal primary - an intriguing dilemma: report of three cases and review of the literature. Histopathology 2022, 81(5): 635-643.
- Pal D, Chowdhury M: Renal type clear cell carcinoma of prostate. Indian J Surg 2007, 69(2): 81-84.
- Permi HS, Laxminarayana KPH, Yeshvanth SK, Shetty JK: Renal Type Clear Cell Carcinoma of the Prostate: A Diagnostic Dilemma. J Lab Physicians 2011, 3(2): 132-133.
- Terada T: Extra-renal clear cell renal cell carcinoma probably arising from mesodermal embryonic remnants. Pathol Int 2012, 62(4): 291-293.
- Hasan R, Kumar S, Monappa V, Ayachit A: Primary extra-renal clear cell renal cell carcinoma masquerading as an adrenal mass: A diagnostic challenge. Urol Ann 2015, 7(4): 513-515.
- Patne SCU, Johri N, Katiyar R, Trivedi S, Dwivedi US: Renal-type clear cell carcinoma of the prostate: a diagnostic challenge. Diagn Pathol 2015, 10(1): 193.
- Wang Q, Xue Y: Renal-type clear cell carcinoma of the prostate: A case report. Oncol Lett 2015, 9(5): 2149-2152.
- Sato Y, Kataoka M, Hata J, Akaihata H, Ogawa S, Kojima Y: Renal-type Clear Cell Carcinoma Occurring in the Prostate With Zinner Syndrome. Urol Case Rep 2016, 5: 9-12.
- Liao G, Zhang X, Li Z, Lan S, Huang M, Huang W: Renal-type clear cell carcinoma of prostate: A case report and review of literature. Indian J Pathol Microbiol 2018, 61(3): 431-433.
- Han SI, Lim S-C: Rare Case of Renal-type Clear Cell Carcinoma of the Prostate and Review of the Literature. In Vivo 2020, 34(5): 2751-2756.
- Petrinec B, Vargas BM, Harik LR, Master VA: Renal cancer without primary cancer in the kidney: Extra-renal TFE3 translocation associated renal cell carcinoma. Kidney Cancer 2021, 5(2): 107-112.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript