27 Dec 2024
Issue 1
Views:3013
Downloads:27
Review Article | Open Access
Advancements in Research on Non-AR- Signaling Pathways and Targeted Therapies for Castration-Resistant Prostate Cancer
Haq Dad1, Asger Hansen1
1Department of Biomedical Science, University of Bergen, Bergen 5020, Norway.
Correspondence: Asger Hansen (Department of Biomedical Science, University of Bergen, Bergen 5020, Norway; Email: Hamdiabdbmw@gmail.com).
Annals of Urologic Oncology 2025, 8(1): 12-20. https://doi.org/10.32948/auo.2024.12.25
Received: 22 Nov 2024 | Accepted: 20 Dec 2024 | Published online: 27 Dec 2024
Abstract
Prostate cancer (Pca) is a significant malignancy affecting men's health, with its incidence steadily increasing worldwide in recent years. Among its various subtypes, castration-resistant prostate cancer (CRPC) holds particular importance due to its pivotal role in the progression and management of Pca. The treatment options for Pca range from surgery to medication. However, the mechanisms underlying CRPC remain complex, with abnormal signal transduction pathways identified as a key factor in its development and progression. While the androgen receptor (AR) signaling pathway is widely recognized as central to CRPC pathogenesis, emerging research highlights the critical involvement of the non-AR signaling pathway in the disease. Prominent pathways in the disease include PI3K-Akt-mTOR, Wnt, Hippo, Hedgehog, Notch, HOXB13, and Jak2-Stat5a/b. Understanding the interplay between non-AR signaling pathways is vital for advancing therapeutic strategies for Pca. This work provides a comprehensive review of recent research progress on the relationship between non-AR signaling pathways and CRPC, aiming to offer insights and guidance for future research.
Key words prostate cancer, androgen receptor, signal transduction, castration-resistant prostate cancer
Introduction
Globally, Pca is one of the most common malignancies in men that seriously endangers men's health. According to 2018 statistics from the World Health Organization (WHO), the incidence of Pca remains high, ranking second in the incidence of all malignancies in men globally, after lung cancer [1, 2]. In the US, Pca has the highest incidence of all malignancies in men and the second highest mortality rate, after lung cancer [3]. In the past few years, the incidence of Pca in the Chinese population has increased significantly. According to the data released by the China Cancer Center in 2022, Pca ranks 6th in the incidence of malignant tumors in Chinese men and 10th in the mortality rate [4]. Some studies predict that in the next decade, the incidence of Pca in Chinese men may rise to second place [5-8]. Although the incidence of Pca in Western countries is higher than that in China, it has been on the rise in recent years, with a high proportion of middle and late stages of initial diagnosis and a large difference between urban and rural populations, which makes Pca screening, diagnosis and treatment face great challenges [9]. Treatment strategies for Pca continue to advance, including surgery, radiation therapy, hormone therapy, and the development of new drugs. In recent years, the development of new drugs such as androgen receptor (AR) signaling inhibitors, bone targeting drugs, PARP inhibitors, and immune-modulating agents has significantly improved the treatment level of prostate cancer. In addition, drugs targeting other signaling pathways, such as CDK4/6, AKT, WNT, and epigenetic markers, have also entered clinical trials. These therapeutic advances have provided more treatment options and better outcomes for Pca patients [10-15].
For patients with Pca, the commonly employed clinical treatment methods primarily include targeting of the androgen receptor (AR) signaling pathway, via androgen deprivation therapy as a significant therapeutic strategy. However, after 18 to 36 months of ADT treatment, a large number of patients will inevitably develop CRPC [4]. The mechanisms underlying CRPC generation and progression remain unclear. In addition to AR-related signaling pathways, non-AR-related signaling pathways also play an important role in CRPC progression. The exploration of these signaling pathways and their associated targeted therapies offers a promising new avenue for the treatment of CRPC. This article presents a comprehensive review of the latest advancements in research on various non-AR-related signaling pathways and their corresponding targeted drugs in the context of CRPC [5, 6].
PI3K-Akt-mTOR signaling pathway
Currently, endocrine therapy is the primary approach for treating PCa. However, a significant limitation is that prolonged treatment can lead to the transformation of PCa cells into androgen-independent cells, rendering conventional endocrine therapy ineffective [7]. mTOR (Mammalian target of rapamycin) is a serine/threonine kinase, an enzyme that plays a vital role in the transmission of multiple signaling pathways. mTOR phosphorylates eukaryotic initiation factors and regulates cell proliferation and apoptosis [8]. In Pca cells, abnormal activation of the PI3K-AKT-mTOR signaling pathway promotes the enhancement of cancer cells' resistance and promotes their survival. Studies have demonstrated that specifically targeted inhibitors of the PI3K-AKT-mTOR signaling pathway inhibit the occurrence and development of a variety of tumors. In cell biology, the PI3K-AKT-mTOR signaling pathway plays an important role, in the regulation of cell survival, proliferation, differentiation, angiogenesis and other key physiological processes [9-15]. The abnormal activation of this pathway promotes the development and progression of many diseases, including cancer. Activated Akt promotes the cell cycle through the phosphorylation of p21WAF1, which affects the inhibition of cyclin-dependent protein kinase CDK. PI3K transmits mitotic signals to p70S6K via Akt and mTOR, promoting the progression of the G1 phase and accelerating the cell cycle. mTORC1 promotes cell cycle progression by phosphorylating S6K1 and 4EBP1, thereby increasing cell proliferation. The PI3K-AKT-mTOR signaling pathway is also involved in promoting blood vessel formation, which is critical for tumor growth and metastasis. In Pca, activation of the PI3K-AKT-mTOR signaling pathway plays a key role in promoting disease progression. As a tumor suppressor protein, deletion or downregulation of PTEN can cause the activation of the PI3K-AKT-mTOR pathway, thus promoting the development of Pca [16]. Studies have also shown that during the progression of Pca to CRPC, PI3K and androgen receptor (AR) signaling pathways interact, and the loss of PTEN and activation of PI3K/AKT can prevent AR expression and activation. Loss of PTEN not only affects the PI3K-AKT-mTOR signaling pathway, but may also affect other signaling pathways, such as the MAPK signaling pathway, which also plays an important role in cancer [9, 17-20]. In Pca, the loss of PTEN occasionally promotes the proliferation of cancer cells independently of AR [21]. Current research suggest that targeted therapy of the PI3K-AKT-mTOR signaling pathway is a potential opportunity to overcome tumor complexity and genomic heterogeneity. In addition, mTORC1 and mTORC2 play critical roles in cell signaling, and their synergistic overactivation may lead to neurological abnormalities. In summary, the PI3K-AKT-mTOR signaling pathway plays a central role in the cellular physiological processes, and its abnormal activation is related to the development and progression of various diseases, making it an important target for current medical research and therapeutic strategy development.
Multidrug combination strategies are being extensively studied for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Buparlisib (BKM120), an oral panPI3K inhibitor, did not show a progression-free survival benefit in mCRPC patients either alone or in combination with the AR inhibitor enzalutamide [16]. Sonolisib (PX-866), as a pan-class I PI3K inhibitor, shows moderate activity in patients with CRPC without chemotherapy [17]. Dactolisib, a dual signal transduction inhibitor of PI3K and mTOR. The study found that the NVP-BEZ235 combined with the docetaxel treatment group was higher in terms of cytotoxicity, but was able to inhibit the growth of CRPC cell line C4-2BT6 than the monotherapy group [18-20]. However, a Phase I/II trial of the drug in combination with abiraterone in non-chemotherapy mCRPC patients was stopped at Phase I due to multiple toxic side effects [21, 22]. In the Phase II study, the efficacy of ipatasertib, an AKT inhibitor in combination with abiraterone versus abiraterone alone, was evaluated in patients with mCRPC who had been treated with metaxel chemotherapy, and there was no statistical difference in radiographic progression-free survival (rPFS) benefit between the two groups [23]. However, subgroup analysis showed that patients with PTEN deletion had rPFS benefits with different doses of ipatasertib combined with abiraterone. Results from a Phase I study with AZD5363 suggest that it may be able to overcome mCRPC resistance to enzalutamide [24]. Phase I clinical trial with 10 mg/ day of everolimus, an mTOR inhibitor, combined with 60 mg/ day of docetaxel in patients with CRPC achieved significant efficacy and safety [25]. However, in two Phase II trials of the combination of evolimus and bicalutamide in patients with CRPC, one did not show a benefit of the combination regimen, possibly due to acquired resistance to bicalutamide in these patients prior to enrollment [26]. Results from another trial showed that nearly half of the patients treated with everolimus and bicalutamide had grade 3 or 4 adverse events, but 75 percent had PSA reductions of ≥30 percent [27]. Studies have shown that the AR and PI3K-AKT-mTOR signaling pathways are not independent but interrelated. Monotherapy for patients with CRPC may not be satisfactory, and developing the best combination strategy for these drugs remains a challenge (
Figure 1).
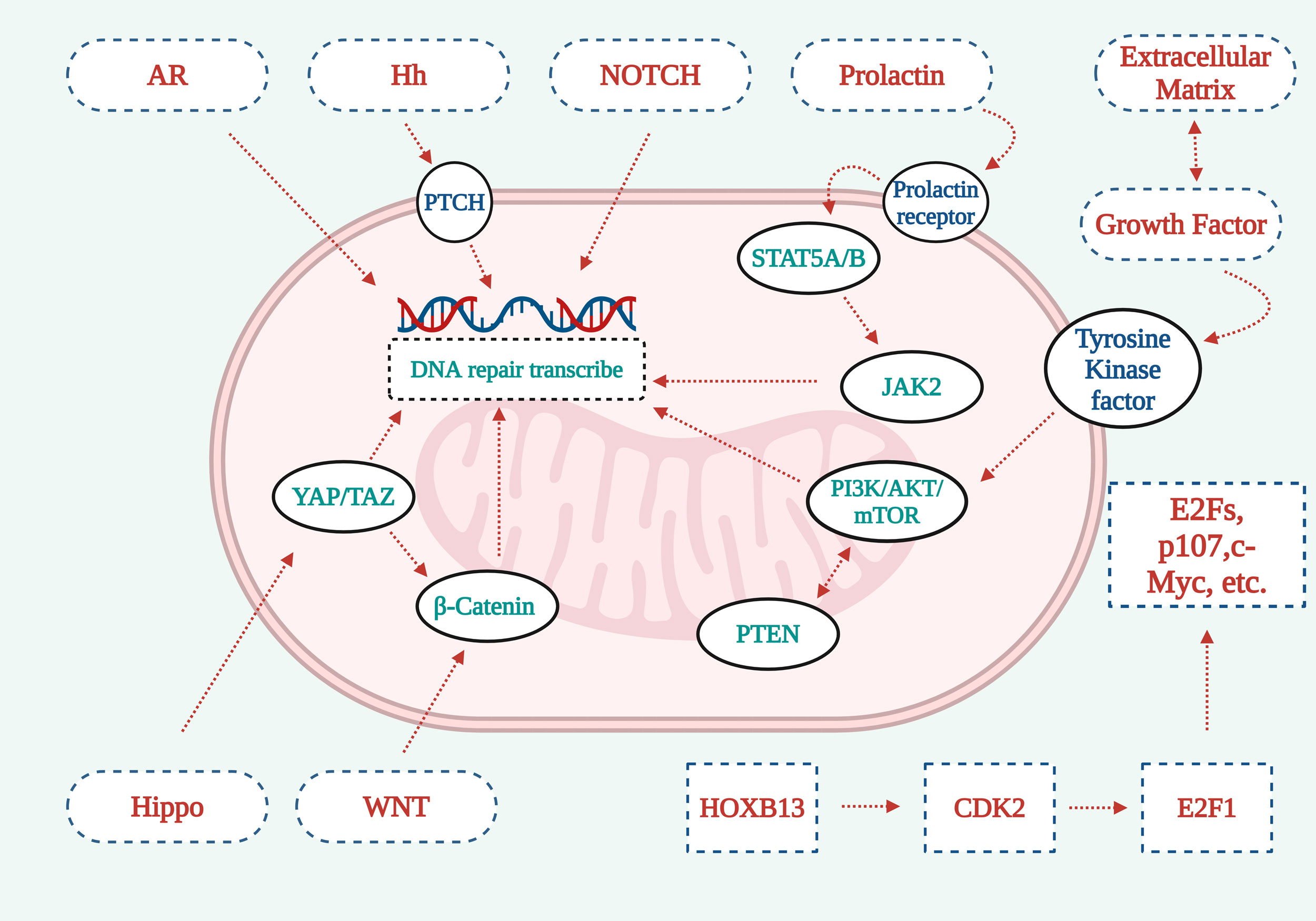 Figure 1.
Figure 1. Graphical representation explaining the operational mechanisms underlying seven vital signaling cascades. 1. The deletion or downregulation of PTEN, a tumor suppressor protein, can lead to the activation of the PI3K-AKT-mTOR pathway, subsequently promoting the development of Pca. 2. The Hippo signaling pathways YAP/TAZ components synergize with β-catenin from the WNT signaling cascade to facilitate DNA repair signal transduction. 3. AR/NOTCH signaling pathway acts directly on cells to activate signaling pathways and promote DNA repair. 4. The Hh signaling pathway promotes DNA repair through PTCH. 5. Prolactin activates Stat5a/b through the prolactin receptor, which further activates JAK2 to promote DNA repair. 6. Extracellular matrix growth factor receptors work with tyrosine kinase receptors to promote DNA repair through the PI3K-AKt-mTOR signaling pathway. 7. HOXB13 activates CDK2, which in turn activates E2F1, which recognizes cell membrane surface receptors through downstream factors such as E2Fs, p107, c-myc, and other factors, playing a signal transduction role.
Wnt signal pathway
The Wnt signaling pathway plays a central role in cell proliferation, differentiation, migration and tissue homeostasis, and is an essential intracellular signaling system [28, 29]. The Wnt signaling pathway consists of several components, mainly Wnt family ligands, curly receptors, and low-density lipoprotein receptor-associated protein 5/6 (lrr-5/6) [30]. In the absence of a Wnt ligand, β-catenin is phosphorylated, ubiquitinated, and degraded via the proteasome pathway, maintaining a low cytoplasmic concentration and inhibiting the expression of downstream target genes. When the Wnt ligand is present, it binds to frizzed and LRP5/6 receptors, preventing β-catenin degradation and allowing it to accumulate and transfer to the nucleus. Abnormally activated Wnt signaling pathway plays an essential role in the genesis, development and metastasis of tumors. Tumor stem cells (CSCs) are believed to be the origin of cancer progression, drug resistance, and distant metastasis [31]. With the help of surface markers (CD44, ALDH1, CD133), CSCs can be identified and studied. CD44 not only serves as a marker for CSCs, but also plays other key roles, such as positively regulating the Wnt/β-catenin signaling pathway through its interaction with low density lipoprotein receptor-associated protein 6 (LRP6). Moreover, silencing its expression can inhibit the accumulation of β-catenin in the nucleus and reduce the invasion and metastasis of Pca. The Wnt signaling pathway promotes epithelial-mesenchymal transition (EMT) by influencing cell proliferation and polarity, and is a key step in tumor metastasis and progression. In addition, the Wnt signaling pathway regulates the expression of metalloproteinases and extracellular matrix related factors, which are closely associated with tumor invasion and metastasis. Studies have shown that patients with metastatic castration-resistant prostate cancer (mCRPC) have abnormal Wnt pathways characterized by periodic changes in key proteins such as APC, β-catenin, and r-spondin. These findings suggest that alterations in Wnt signaling pathways may play an important role in the progression of CRPC [32]. In the absence of Wnt ligands, β-catenin is phosphorylated and degraded by APC and axin complexes. When the Wnt signaling pathway is activated, β-catenin avoids proteasome degradation, accumulates in the cytoplasm, and is transferred to the nucleus to activate transcription of Wnt target genes [33]. R-spondins can not only enhance the activity of the Wnt ligand as a co-activator, but also as an inhibitor to prevent the degradation of Wnt signaling receptors [34]. Non-classical Wnt signaling pathways can inhibit anti-androgen resistance in tumor cells [35]. At present, the research of small molecule therapy and biologics targeting the Wnt signaling pathway is still in the preliminary stage [36]. Ursolic acid, as a Wnt inhibitor, has shown antitumor activity in vitro. In addition, some polyphenols (such as quercetin, curcumin and resveratrol, etc.) can also limit the proliferation of Pca cells by inhibiting Wnt signaling pathways. Despite their potential in in vitro studies, the clinical benefit of these agents in patients with CRPC is not significant, and their potential clinical significance remains controversial [37]. Some small molecule therapeutics and biologics target the Wnt pathway, and it is necessary to further explore the potential anti-tumor mechanisms subsequent to inhibiting the Wnt pathway. In conclusion, the Wnt signaling pathway plays a crucial role in the occurrence and development of Pca, and the targeted therapy of this pathway offers a novel research direction for the treatment of CRPC. Nevertheless, converting these findings into practical clinical treatment strategies still presents numerous challenges (
Figure 2).
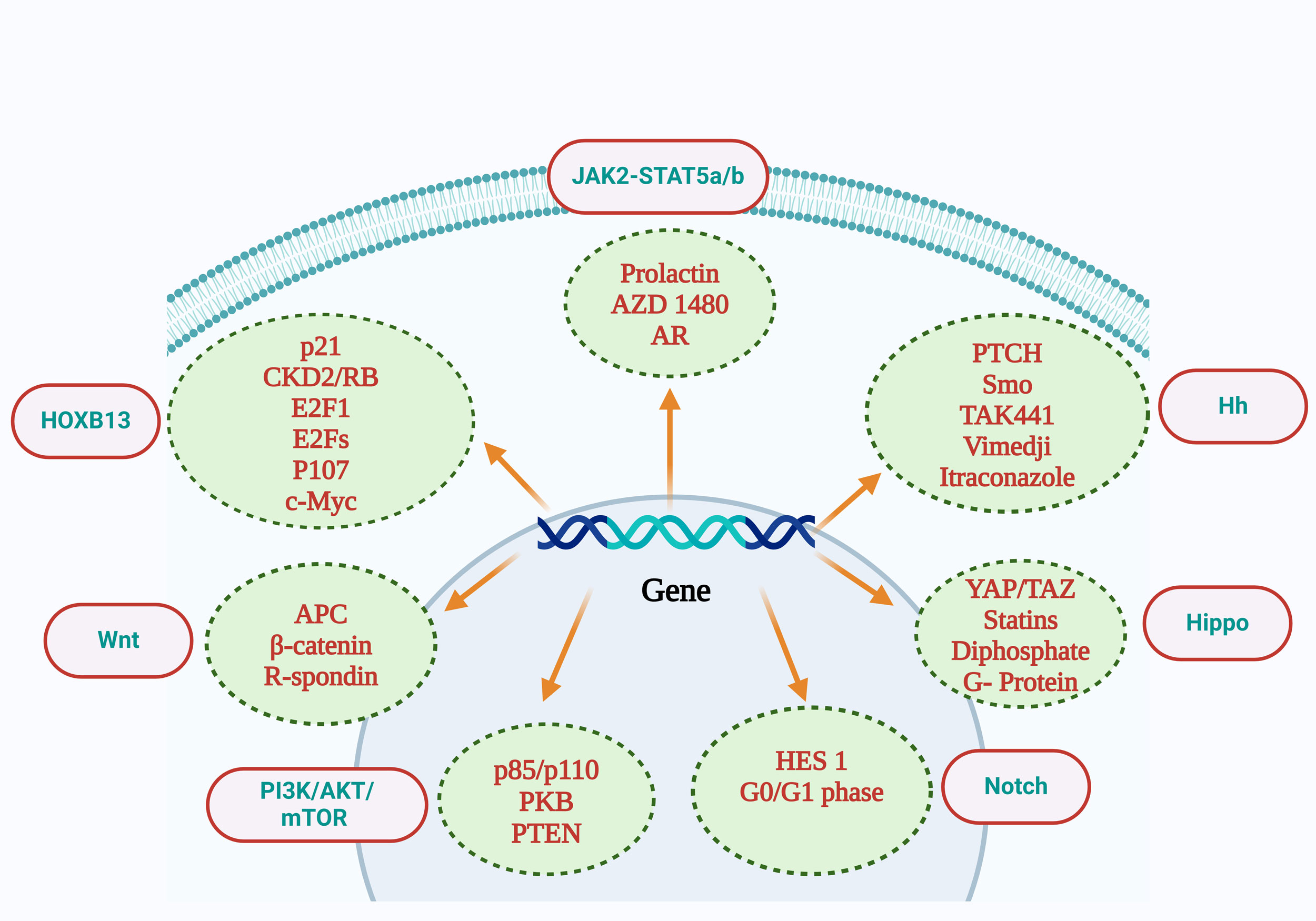 Figure 2
Figure 2. Interpretation of the relevant molecules contained in the PI3K-AKt-mTOR signaling pathway included p85/p110, PKB, and PTEN. Wnt signaling pathway includes APC, β-catenin, R-Spondin. The Notch signaling pathway includes Hes1 and the G0/G1 phase. The Jak2-Stat5a/b signaling pathway includes prolactin, AZD1480, and AR. The Hippo signaling pathway includes YAP/TAZ, statins, diphosphate, and G-protein inhibitors. The Hh signaling pathway includes PTCH,Smo,TAK441,Vimedji, Itraconazole. The HOXB13 signaling pathway includes p21, CKD2/RB, E2F1, E2Fs, P107, and c-Myc. AR: androgen receptor; PI3K: phosphatidylinositol 3-kinase; mTOR: mammalian target of rapamycin; PTEN: phosphatase and tensin homolog deleted on chromosometen; APC: adenomatous polyposis coli; PKB: Protein kinase B.
Hippo signaling pathway
The Hippo pathway was first discovered more than 20 years ago in related experimental studies in fruit flies, and has been extended to mammals, where this signaling pathway has been extensively studied. Similarly, in tumor stem cells, the Hippo signaling pathway plays an important role [38]. Studies on tumor suppressors in fruit flies have yielded sufficient and correct conclusions that this signaling pathway is present in all species, including humans [39]. It plays an important role in cell growth, proliferation, regeneration, homeostasis and organ development [40]. The Hippo pathway is influenced by factors such as cell density/polarity, mechanical conduction, nutrients, and G protein-coupled receptors [41, 42]. Independent regulation of epigenetic kinase cascades exists in YES-related proteins in the Hippo pathway (YAP) or transcriptional coactivators with PDZ-binding motifs (TAZ)[43]. Up-regulation of YAP /TAZ affects the downstream Hippo pathway and plays a central role in a variety of solid tumors [38, 40, 41]. Therefore, increased YAP /TAZ activity plays a key role in the occurrence and development of Pca. The hippo signaling pathway plays a crucial role in regulating cell proliferation, differentiation, and apoptosis, and its abnormal activation is closely related to the occurrence and development of many cancers. In Pca, YAP and TAZ act as major effectors of the Hippo signaling pathway, promoting the dryness, metastasis, and drug resistance of tumor cells [44, 45]. Studies have shown that the expression level of YAP protein is significantly increased in Pca tissues, especially in CRPC, and the increase of YAP1 is particularly significant [46]. Targeted treatment strategies for YAP and TAZ may have potential efficacy in inhibiting the occurrence and progression of Pca [47, 48]. Some drugs, such as end-anchor polymerase inhibitors, statins, bisphosphonates, geranylgeranylgeranyl converting enzyme-1, and G-protein inhibitors, are considered modulators of the YAP/TAZ pathway. Still, the exact mechanism by which these drugs inhibit the Hippo signaling pathway is not fully understood. This may be due to the difficulty in determining whether these drugs are involved in specific interactions that regulate YAP/TAZ activity, thus hindering the application of these drugs in Pca research. In addition, the study also found that the Hippo and Wnt signaling pathways interact and jointly regulate the development of Pca. For example, studies have shown that the Hippo signaling pathway and the abnormal activation of the Wnt signaling pathway can lead to the occurrence and development of CRPC. Although some drugs have been shown to inhibit the YAP/TAZ signaling pathway in vitro studies, such as ursolic acid, the clinical benefits of these drugs in Pca patients are insignificant, and their potential clinical significance is still controversial. Therefore, an in-depth study of the strong link between the Hippo signaling pathway and Pca is essential for the development of new targeted therapeutic strategies. Future studies are needed to explore further the potential anti-tumor mechanisms of inhibition of the YAP/TAZ signaling pathway to advance the development and clinical application of these drugs (Figure 1).
Hh signaling pathway
The Hedgehog (Hh) signaling pathway plays an important role in the onset and development of cancer, including Pca. Hh signaling pathway plays an important role in embryonic development, regulating wound and tissue healing, and maintaining cell drying in vertebrates and invertebrates [49]. The Hh signaling pathway usually consists of Hh ligands, two transmembrane receptors (Ptch, Smo), downstream nuclear transcription factor Gli, and some intermediate transfer molecules. In general, the Hh signaling pathway is less active [50]; Under normal circumstances, the Hh signaling pathway is involved in embryonic development and tissue regeneration, but in adults, its abnormal activation has been linked to the development of cancer. In Pca, activation of the Hh signaling pathway usually involves the following steps: Binding of Hh protein to its receptor PTCH: Hh protein (e.g., Sonic Hedgehog, SHH) binds to its receptor PTCH, resulting in the inhibition of PTCH being disengaged. In Pca, the Hh protein binds to its receptor, PTCH, resulting in the activation of the otherwise inhibited G-protein-coupled receptor, Smo, which generates a cascade of signals involved in the regulation of epithelial-mesenchymal interactions, cell survival, cell metastasis, and angiogenesis [51-54]. Itraconazole is an inhibitor of the Hh signaling pathway, and a Phase II clinical trial found that high doses of itraconazole prolonged PFS in patients with mCRPC without chemotherapy, with less toxic effects [55]. TAK-441 is a highly potent Smo inhibitor that delays the progression of Pca models in vivo trials by interfering with the Hh signaling pathway [56-58]. Vermodej is a selective inhibitor of the Hh signaling pathway. Studies have shown that Vermodej can synergistically promote docetaxel-induced apoptosis of prostate tumor cells [56]. Trials evaluating the efficacy of Vimodej in mCRPC patients are ongoing. These results suggest that the abnormal activation of the Hh signaling pathway may be related to the occurrence and development of CRPC. The mechanism of the Hh pathway inducing Pca and its clinical significance as a therapeutic target for Pca still need further study. In addition, the study showed that the BRAF protein was activated in PTEN-deficient Pca cells. In addition, Vemurafenib has been shown to inhibit PTK6, providing a new perspective for the treatment of Pca. These findings provide a scientific basis for the potential use of the Hedgehog (Hh) signaling pathway in prostate cancer treatment and carve the way for future research (Figure 1).
Notch signaling pathway
The notch signaling pathway has distinct characteristics and is highly conserved during evolution. In mammals, this complex signal transduction pathway consists of four receptors (NOTch1-4) [56], five ligands (Jagged1/2 and DLL1/4), and several downstream components [57]. Notch receptor is a single transmembrane protein that regulates a variety of cell development functions, including cell proliferation, differentiation, migration, and death [58]. Dysregulation of Notch activity can cause a variety of diseases, including cancer [59]. The role of Notch signaling pathway in Pca is multifaceted, and its role in tumor may have the dual characteristics of carcinogenesis and tumor inhibition. Studies have shown that the Notch signaling pathway is involved in the regulation of cell survival, proliferation, differentiation, apoptosis and tissue and organ formation [59]. In Pca tissues, Notch signaling pathway-related proteins such as Notch1, Notch3, and their downstream target gene Hes1 are up-regulated, especially Notch and Hh signaling are significantly enhanced in CRPC cell lines, suggesting that the activation of the Notch signaling pathway may be related to the occurrence and progression of Pca. However, some studies have found that when Notch1 is overexpressed in Pca cells, it can promote the G0/G1 phase arrest of cells, inhibit cell proliferation, and thus play a role in cancer inhibition. This indicates that the Notch signaling pathway plays a complex role in Pca, which can not only promote the dryness, metastasis, and drug resistance of tumor cells but also inhibit tumor growth by inducing cell cycle arrest and apoptosis [60-65]. Because of the complex role of the Notch signaling pathway in Pca, its use as a therapeutic target is controversial. Some drugs and natural compounds, such as sufentanil, have been found to affect the proliferation, apoptosis, and cell cycle of Pca cells through the Notch signaling pathway [66-71]. In addition, some polyphenols (such as quercetin, curcumin and resveratrol, etc.) can also limit the proliferation of Pca cells by inhibiting Notch signaling pathways [72]. These studies provide a scientific basis for the potential application of the Notch signaling pathway in the treatment of Pca, but further studies are needed to understand further the mechanism of action of a Notch signaling pathway in the occurrence and development of Pca, as well as its clinical significance as a therapeutic target (Figure 1).
HOXB13 signal path
HOXB13 is a homeobox protein specifically expressed in the prostate, which plays an important role in the development of Pca [73]. HOXB13 not only promotes the proliferation, differentiation and metastasis of Pca cells by regulating the transcription of androgen receptor (AR) target genes, but also promotes the development of CRPC independently of androgens. Studies have shown that HOXB13 can be expressed in about 51.7% of Pca tumor tissues, and tumors with high HOXB13 expression tend to have more clinicopathological factors that predict poor prognosis, including high pT stage, high Gleason score, lymph node metastasis, high PSA level, and increased risk of biochemical recurrence [74]. Studies have shown that HOXB13 may promote the activation of cyclin-dependent kinase (CDK) 2 by inhibiting the expression of p21 protein, leading to the phosphorylation of its downstream substrate RB protein, and then release E2F1 transcription factor, resulting in the expression of oncogenes such as E2Fs, p107 and c-myc, thus promoting the occurrence of CRPC [75]. Numerous studies have demonstrated that HOXB13 plays a variety of roles, such as regulating hormone-activated androgen receptor signaling pathways, DNA-linked transcription factors, and directly inhibiting AR [76]. Cells with abnormal HOXB13 expression clustered in the G1 phase, possibly because HOXB13 promoted the ubiquitination of CyclinD1. It degraded CyclinD1, thereby reducing CyclinD1 levels, while decreasing phosphorylation of pRb and slowing the entry into the S phase. Conversely, knocking out HOXB13 with siRNA increases Cyclin levels, stabilizes E2F1 and CDC25C, and thus increases pRB phosphorylation. The increase of CyclinB1 and CDC25C, through the dephosphorylation of CDK1, jointly promotes the activation of the CyclinB complex and restarts the G2/M transformation [77]. In addition, it has been found that BEZ bromine domain kinase inhibitors can inhibit the expression of HOXB13 protein and induce apoptosis, thus inhibiting the proliferation of CRPC tumor cells. This suggests that therapeutic strategies targeting HOXB13 may have potential efficacy in patients with CRPC. However, the specific pharmacological mechanisms of these drugs still require further studies. By inhibiting the expression of JNK/c-Jun, HOXB13 down-regulates the AP-1 pathway. Similarly, through the regulation of the JNK signal, HOXB13 can inhibit the expression of p21, and the change of p21 will further affect the activity of c-Jun and AP-1 [78]. Studies have shown the upregulation of ZnT transporters in Pca cells, including SLC30A10 (ZnT10) and SLC30A4 (ZnT4), which are highly HOXB13-induced zinc transporters [79]. The T allele increases HOXB13's ability to bind to transcriptional stimulators in rs339331, resulting in upregulation of RFX6, a gene associated with rs339331 [80]. By inhibiting the expression of PDEF, HOXB13 increases the expression of MMP-9 and survivin, which counteracts the inhibitory effect of PDEF on MMP-9 and survivin [81]. In summary, HOXB13 plays a vital role in the occurrence and progression of Pca, and therapeutic strategies targeting HOXB13 may provide a new direction for the treatment of CRPC [82]. Future studies are needed to explore the mechanism of action of HOXB13 in Pca more profoundly and to develop more effective targeted treatment strategies (Figure 1).
Jak2-Stat5a/b signaling pathway
The JAK2-STAT5A/B signaling pathway plays an important role in CRPC. STAT5A/B is a dual-functional protein, which is both a signaling molecule and a nuclear transcription factor and plays a crucial role in the survival of Pca cells in vitro and the growth of xenograft tumors in vivo [83, 84]. Activation of the JAK2-STAT5A /B signaling pathway is usually triggered by the binding of prolactin to its receptor. It is initiated by the autophosphorylation of JAK2 kinase, which in turn regulates target genes, including cell proliferation, apoptosis, and metastasis [85, 86]. Studies have shown functional interaction between STAT5A/B and AR (androgen receptor), and activation of STAT5A/B increases the nuclear localization and transcriptional activity of AR in Pca cells [87, 88]. In addition, blocking STAT5A/B induced apoptosis of AR-negative Pca cells. These findings suggest that STAT5A/B, in addition to directly affecting AR function, may also promote Pca growth through mechanisms independent of AR. Inhibition of the STAT5A/B cascade signaling pathway can block the expression of STAT5A/B target genes, including cytokine receptor activation, JAK2 kinase-mediated phosphorylation, STAT5A/B dimerization, DNA binding and transcriptional activity, thereby inhibiting tumor cell proliferation and inducing tumor cell apoptosis. AZD1480 is a potent JAK2 kinase inhibitor that inhibits tumor growth in CRPC xenografted mouse models by inhibiting JAK2 kinase activation and blocking STAT5A/B signaling. Studying this signaling pathway will provide new directions for therapeutic strategies for CRPC. It is currently a challenge to understand the specific mechanisms by which STAT5A/B maintains and promotes the survival of AR-independent Pca cells, and to determine whether inhibiting STAT5 can induce apoptosis of AR-negative cells in CRPC [89]. These findings suggest that inhibitors targeting the JAK2-STAT5A/B signaling pathway may be a potential therapeutic strategy for CRPC (Figure 2) [90].
Summary and prespectives
Prostate cancer (Pca) is a common malignancy in men whose treatment strategies continue to advance with a deeper understanding of the disease mechanisms. CRPC poses a significant challenge in prostate cancer treatment, as it represents a more aggressive disease state where cancer cells become resistant to conventional androgen deprivation therapy (ADT). In CRPC, the androgen receptor (AR) signaling pathway still plays an important role. Although the expression level of AR may increase, the activation mechanism of the AR signaling pathway may be more complex, including AR gene mutation, AR overexpression, changes in androgen biosynthesis, AR splicing variants, and changes in androgen cofactors. Targeted therapies focusing on the AR signaling pathway, such as abiraterone and enzalutamide, have become an important part of CRPC therapy. These drugs work by inhibiting AR activity, effectively slowing disease progression and prolonging patient survival. In addition to the AR signaling pathway, other signal transduction pathways also play an important role in the development and progression of CRPC. For example, the role of the JAK2-STAT5A/B signaling pathway in CRPC has also received attention. STAT5A/B is a dual-function protein, which is both a signaling molecule and a nuclear transcription factor and is essential for the survival and growth of Pca cells. Inhibition of the JAK2-STAT5A/B signaling pathway can inhibit the proliferation of CRPC tumor cells. In addition, HOXB13 is a homeobox protein specifically expressed in the prostate. Its mechanism of action in CRPC may be to inhibit the expression of p21 protein, promote the activation of CDK2, lead to the phosphorylation of RB protein, and then release the E2F1 transcription factor, resulting in the expression of oncogenes, resulting in CRPC progression. In terms of therapeutic strategies, traditional chemotherapy drugs such as docetaxel and cabataxel, new drugs such as PARP inhibitors (such as olaparib) and psma targeted radionuclide therapy (such as 177Lu-PSMA-617) also show potential in the treatment of CRPC. The development and application of these drugs provide more treatment options for patients with CRPC. Overall, treatment strategies for CRPC continue to advance, and the development of new targeted drugs and therapies offers new hope for improving the prognosis of patients with CRPC. However, due to the heterogeneity and complexity of CRPC, more research is still needed to gain insight into the molecular mechanisms of the disease and develop more effective and personalized treatment strategies.
Declaration
Acknowledgements
None.
Ethical policy
Non applicable.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
HD searched academic literature, wrote the draft manuscript and draw the figures; AH supervised the review writting progress and approved the final manuscript submission.
Competing interests
Authors report no conflict of interest.
Funding
None.
References
-
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71(3): 209-249.
-
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A: Global cancer statistics, 2012. CA Cancer J Clin 2015, 65(2): 87-108.
-
Torre LA, Siegel RL, Ward EM, Jemal A: Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016, 25(1): 16-27.
-
Mansinho A, Macedo D, Fernandes I, Costa L: Castration-Resistant Prostate Cancer: Mechanisms, Targets and Treatment. Adv Exp Med Biol 2018, 1096: 117-133.
-
Teo MY, Rathkopf DE, Kantoff P: Treatment of Advanced Prostate Cancer. Annu Rev Med 2019, 70: 479-499.
-
Chang AJ, Autio KA, Roach M, 3rd, Scher HI: High-risk prostate cancer-classification and therapy. Nat Rev Clin Oncol 2014, 11(6): 308-323.
-
Erdmann K, Ringel J, Hampel S, Wirth MP, Fuessel S: Carbon nanomaterials sensitize prostate cancer cells to docetaxel and mitomycin C via induction of apoptosis and inhibition of proliferation. Beilstein J Nanotechnol 2017, 8: 1307-1317.
-
Wang G, Liu M, Wang H, Yu S, Jiang Z, Sun J, Han K, Shen J, Zhu M, Lin Z, et al: Centrosomal Protein of 55 Regulates Glucose Metabolism, Proliferation and Apoptosis of Glioma Cells via the Akt/mTOR Signaling Pathway. J Cancer 2016, 7(11): 1431-1440.
-
Shorning BY, Dass MS, Smalley MJ, Pearson HB: The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int J Mol Sci 2020, 21(12): 4507.
-
Roudsari NM, Lashgari NA, Momtaz S, Abaft S, Jamali F, Safaiepour P, Narimisa K, Jackson G, Bishayee A, Rezaei N, et al: Inhibitors of the PI3K/Akt/mTOR Pathway in Prostate Cancer Chemoprevention and Intervention. Pharmaceutics 2021, 13(8): 1195.
-
Cham J, Venkateswaran AR, Bhangoo M: Targeting the PI3K-AKT-mTOR Pathway in Castration Resistant Prostate Cancer: A Review Article. Clin Genitourin Cancer 2021, 19(6): 563.e1-563.e7.
-
Edlind MP, Hsieh AC: PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl 2014, 16(3): 378-386.
-
Makwana V, Rudrawar S, Anoopkumar-Dukie S: Signalling transduction of O-GlcNAcylation and PI3K/AKT/mTOR-axis in prostate cancer. Biochim Biophys Acta Mol Basis Dis 2021, 1867(7): 166129.
-
Wylaź M, Kaczmarska A, Pajor D, Hryniewicki M, Gil D, Dulińska-Litewka J: Exploring the role of PI3K/AKT/mTOR inhibitors in hormone-related cancers: A focus on breast and prostate cancer. Biomed Pharmacother 2023, 168: 115676.
-
Xu W, Ding J, Kuang S, Li B, Sun T, Zhu C, Liu J, Zhu L, Li Y, Sheng W: Icariin-Curcumol promotes docetaxel sensitivity in prostate cancer through modulation of the PI3K-Akt signaling pathway and the Warburg effect. Cancer Cell Int 2023, 23(1): 190.
-
Armstrong AJ, Halabi S, Healy P, Alumkal JJ, Winters C, Kephart J, Bitting RL, Hobbs C, Soleau CF, Beer TM, et al: Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur J Cancer 2017, 81: 228-236.
-
Hotte SJ, Chi KN, Joshua AM, Tu D, Macfarlane RJ, Gregg RW, Ruether JD, Basappa NS, Finch D, Salim M, et al: A Phase II Study of PX-866 in Patients With Recurrent or Metastatic Castration-resistant Prostate Cancer: Canadian Cancer Trials Group Study IND205. Clin Genitourin Cancer 2019, 17(3): 201-208.e1.
-
Yasumizu Y, Miyajima A, Kosaka T, Miyazaki Y, Kikuchi E, Oya M: Dual PI3K/mTOR inhibitor NVP-BEZ235 sensitizes docetaxel in castration resistant prostate cancer. J Urol 2014, 191(1): 227-234.
-
Li J, Wang X, Ma C, Xu S, Xu M, Yang J, Wang R, Xue L: Dual PI3K/mTOR inhibitor NVP‑BEZ235 decreases the proliferation of doxorubicin‑resistant K562 cells. Mol Med Rep 2021, 23(4): 301.
-
Subtil FSB, Gröbner C, Recknagel N, Parplys AC, Kohl S, Arenz A, Eberle F, Dikomey E, Engenhart-Cabillic R, Schötz U: Dual PI3K/mTOR Inhibitor NVP-BEZ235 Leads to a Synergistic Enhancement of Cisplatin and Radiation in Both HPV-Negative and -Positive HNSCC Cell Lines. Cancers (Basel) 2022, 14(13): 3160.
-
Massard C, Chi KN, Castellano D, de Bono J, Gravis G, Dirix L, Machiels JP, Mita A, Mellado B, Turri S, et al: Phase Ib dose-finding study of abiraterone acetate plus buparlisib (BKM120) or dactolisib (BEZ235) in patients with castration-resistant prostate cancer. Eur J Cancer 2017, 76: 36-44.
-
Massard C, Chi KN, Castellano D, de Bono J, Gravis G, Dirix L, Machiels JP, Mita A, Mellado B, Turri S, et al: Corrigendum to 'Phase Ib dose-finding study of abiraterone acetate plus buparlisib (BKM120) or dactolisib (BEZ235) in patients with castration-resistant prostate cancer' [European Journal of Cancer 76 (2017) 36-44]. Eur J Cancer 2017, 81: 242.
-
de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, Arranz Arija JA, Shih KC, Radavoi GD, Xu N, et al: Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res 2019, 25(3): 928-936.
-
Kolinsky MP, Rescigno P, Bianchini D, Zafeiriou Z, Mehra N, Mateo J, Michalarea V, Riisnaes R, Crespo M, Figueiredo I, et al: A phase I dose-escalation study of enzalutamide in combination with the AKT inhibitor AZD5363 (capivasertib) in patients with metastatic castration-resistant prostate cancer. Ann Oncol 2020, 31(5): 619-625.
-
Courtney KD, Manola JB, Elfiky AA, Ross R, Oh WK, Yap JT, Van den Abbeele AD, Ryan CW, Beer TM, Loda M, et al: A phase I study of everolimus and docetaxel in patients with castration-resistant prostate cancer. Clin Genitourin Cancer 2015, 13(2): 113-123.
-
Nakabayashi M, Werner L, Courtney KD, Buckle G, Oh WK, Bubley GJ, Hayes JH, Weckstein D, Elfiky A, Sims DM, et al: Phase II trial of RAD001 and bicalutamide for castration-resistant prostate cancer. BJU Int 2012, 110(11): 1729-1735.
-
Chow H, Ghosh PM, deVere White R, Evans CP, Dall'Era MA, Yap SA, Li Y, Beckett LA, Lara PN, Jr., Pan CX: A phase 2 clinical trial of everolimus plus bicalutamide for castration-resistant prostate cancer. Cancer 2016, 122(12): 1897-1904.
-
Zhou Y, Xu J, Luo H, Meng X, Chen M, Zhu D: Wnt signaling pathway in cancer immunotherapy. Cancer Lett 2022, 525: 84-96.
-
Zhang Y, Wang X: Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 2020, 13(1): 165.
-
Hu L, Chen W, Qian A, Li YP: Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res 2024, 12(1): 39.
-
Losada-García A, Salido-Guadarrama I, Cortes-Ramirez SA, Cruz-Burgos M, Morales-Pacheco M, Vazquez-Santillan K, Rodriguez-Martinez G, González-Ramírez I, Gonzalez-Covarrubias V, Perez-Plascencia C, et al: SFRP1 induces a stem cell phenotype in prostate cancer cells. Front Cell Dev Biol 2023, 11: 1096923.
-
Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, et al: RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 2015, 349(6254): 1351-1356.
-
Park JH, Kwon HY, Sohn EJ, Kim KA, Kim B, Jeong SJ, Song JH, Koo JS, Kim SH: Inhibition of Wnt/β-catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharmacol Rep 2013, 65(5): 1366-1374.
-
Kattan J, Bachour M, Farhat F, El Rassy E, Assi T, Ghosn M: Phase II trial of weekly Docetaxel, Zoledronic acid, and Celecoxib for castration-resistant prostate cancer. Invest New Drugs 2016, 34(4): 474-480.
-
Moroishi T, Hansen CG, Guan KL: The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015, 15(2): 73-79.
-
Pan D: The hippo signaling pathway in development and cancer. Dev Cell 2010, 19(4): 491-505.
-
Park JH, Shin JE, Park HW: The Role of Hippo Pathway in Cancer Stem Cell Biology. Mol Cells 2018, 41(2): 83-92.
-
Totaro A, Panciera T, Piccolo S: YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 2018, 20(8): 888-899.
-
Yu FX, Zhao B, Guan KL: Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163(4): 811-828.
-
Kofler M, Speight P, Little D, Di Ciano-Oliveira C, Szászi K, Kapus A: Mediated nuclear import and export of TAZ and the underlying molecular requirements. Nat Commun 2018, 9(1): 4966.
-
Zanconato F, Cordenonsi M, Piccolo S: YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29(6): 783-803.
-
Zanconato F, Piccolo S: Eradicating tumor drug resistance at its YAP-biomechanical roots. Embo j 2016, 35(5): 459-461.
-
Sheng X, Li WB, Wang DL, Chen KH, Cao JJ, Luo Z, He J, Li MC, Liu WJ, Yu C: YAP is closely correlated with castration-resistant prostate cancer, and downregulation of YAP reduces proliferation and induces apoptosis of PC-3 cells. Mol Med Rep 2015, 12(4): 4867-4876.
-
Zanconato F, Battilana G, Cordenonsi M, Piccolo S: YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol 2016, 29: 26-33.
-
Cunningham R, Hansen CG: The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clin Sci (Lond) 2022, 136(3): 197-222.
-
Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K: Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A 2008, 105(8): 2883-2888.
-
Le H, Kleinerman R, Lerman OZ, Brown D, Galiano R, Gurtner GC, Warren SM, Levine JP, Saadeh PB: Hedgehog signaling is essential for normal wound healing. Wound Repair Regen 2008, 16(6): 768-773.
-
Peng YC, Joyner AL: Hedgehog signaling in prostate epithelial-mesenchymal growth regulation. Dev Biol 2015, 400(1): 94-104.
-
Nakazawa M, Kyprianou N: Epithelial-mesenchymal-transition regulators in prostate cancer: Androgens and beyond. J Steroid Biochem Mol Biol 2017, 166: 84-90.
-
Hyuga T, Alcantara M, Kajioka D, Haraguchi R, Suzuki K, Miyagawa S, Kojima Y, Hayashi Y, Yamada G: Hedgehog Signaling for Urogenital Organogenesis and Prostate Cancer: An Implication for the Epithelial-Mesenchyme Interaction (EMI). Int J Mol Sci 2019, 21(1): 58.
-
Yamamichi F, Shigemura K, Behnsawy HM, Meligy FY, Huang WC, Li X, Yamanaka K, Hanioka K, Miyake H, Tanaka K, et al: Sonic hedgehog and androgen signaling in tumor and stromal compartments drives epithelial-mesenchymal transition in prostate cancer. Scand J Urol 2014, 48(6): 523-532.
-
Antonarakis ES, Heath EI, Smith DC, Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS, Zhao M, et al: Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist 2013, 18(2): 163-173.
-
Ibuki N, Ghaffari M, Pandey M, Iu I, Fazli L, Kashiwagi M, Tojo H, Nakanishi O, Gleave ME, Cox ME: TAK-441, a novel investigational smoothened antagonist, delays castration-resistant progression in prostate cancer by disrupting paracrine hedgehog signaling. Int J Cancer 2013, 133(8): 1955-1966.
-
Sawasaki M, Tsubamoto H, Nakamoto Y, Kakuno A, Sonoda T: S-1, Oxaliplatin, Nab-paclitaxel and Itraconazole for Conversion Surgery for Advanced or Recurrent Gastric Cancer. Anticancer Res 2020, 40(2): 991-997.
-
Weng N, Zhang Z, Tan Y, Zhang X, Wei X, Zhu Q: Repurposing antifungal drugs for cancer therapy. J Adv Res 2023, 48: 259-273.
-
Ntziachristos P, Lim JS, Sage J, Aifantis I: From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell 2014, 25(3): 318-334.
-
Su Q, Xin L: Notch signaling in prostate cancer: refining a therapeutic opportunity. Histol Histopathol 2016, 31(2): 149-157.
-
Mourkioti I, Angelopoulou A, Belogiannis K, Lagopati N, Potamianos S, Kyrodimos E, Gorgoulis V, Papaspyropoulos A: Interplay of Developmental Hippo-Notch Signaling Pathways with the DNA Damage Response in Prostate Cancer. Cells 2022, 11(15): 2449.
-
Anusewicz D, Orzechowska M, Bednarek AK: Notch Signaling Pathway in Cancer-Review with Bioinformatic Analysis. Cancers (Basel) 2021, 13(4): 768.
-
Deng G, Ma L, Meng Q, Ju X, Jiang K, Jiang P, Yu Z: Notch signaling in the prostate: critical roles during development and in the hallmarks of prostate cancer biology. J Cancer Res Clin Oncol 2016, 142(3): 531-547.
-
Liu S, Hsu EC, Shen M, Aslan M, Stoyanova T: Metastasis Model to Test the Role of Notch Signaling in Prostate Cancer. Methods Mol Biol 2022, 2472: 221-233.
-
Ku SY, Wang Y, Garcia MM, Yamada Y, Mizuno K, Long MD, Rosario S, Chinnam M, Al Assaad M, Puca L, et al: Notch signaling suppresses neuroendocrine differentiation and alters the immune microenvironment in advanced prostate cancer. J Clin Invest 2024, 134(17): e175217.
-
Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, et al: Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012, 22(3): 373-388.
-
Zhao J, Shen J, Mao L, Yang T, Liu J, Hongbin S: Cancer associated fibroblast secreted miR-432-5p targets CHAC1 to inhibit ferroptosis and promote acquired chemoresistance in prostate cancer. Oncogene 2024, 43(27): 2104-2114.
-
Beier AK, Ebersbach C, Siciliano T, Scholze J, Hofmann J, Hönscheid P, Baretton GB, Woods K, Guezguez B, Dubrovska A, et al: Targeting the glutamine metabolism to suppress cell proliferation in mesenchymal docetaxel-resistant prostate cancer. Oncogene 2024, 43(26): 2038-2050.
-
Zhang J, Li S, Zhang J, Zhang W, Jiang J, Wu H, Wu E, Feng Y, Yang L, Li Z: Docetaxel resistance-derived LINC01085 contributes to the immunotherapy of hormone-independent prostate cancer by activating the STING/MAVS signaling pathway. Cancer Lett 2022, 545: 215829.
-
Lamprou I, Tsolou A, Kakouratos C, Mitrakas AG, Xanthopoulou ET, Kassela K, Karakasiliotis I, Zois CE, Giatromanolaki A, Koukourakis MI: Suppressed PLIN3 frequently occurs in prostate cancer, promoting docetaxel resistance via intensified autophagy, an event reversed by chloroquine. Med Oncol 2021, 38(10): 116.
-
Seo HK, Lee SJ, Kwon WA, Jeong KC: Docetaxel-resistant prostate cancer cells become sensitive to gemcitabine due to the upregulation of ABCB1. Prostate 2020, 80(6): 453-462.
-
Liu YQ, Wang SK, Xu QQ, Yuan HQ, Guo YX, Wang Q, Kong F, Lin ZM, Sun DQ, Wang RM, et al: Acetyl-11-keto-β-boswellic acid suppresses docetaxel-resistant prostate cancer cells in vitro and in vivo by blocking Akt and Stat3 signaling, thus suppressing chemoresistant stem cell-like properties. Acta Pharmacol Sin 2019, 40(5): 689-698.
-
Sreenath T, Orosz A, Fujita K, Bieberich CJ: Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate 1999, 41(3): 203-207.
-
Zabalza CV, Adam M, Burdelski C, Wilczak W, Wittmer C, Kraft S, Krech T, Steurer S, Koop C, Hube-Magg C, et al: HOXB13 overexpression is an independent predictor of early PSA recurrence in prostate cancer treated by radical prostatectomy. Oncotarget 2015, 6(14): 12822-12834.
-
Kim YR, Oh KJ, Park RY, Xuan NT, Kang TW, Kwon DD, Choi C, Kim MS, Nam KI, Ahn KY, et al: HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol Cancer 2010, 9: 124.
-
Kim SD, Park RY, Kim YR, Kim IJ, Kang TW, Nam KI, Ahn KY, Bae CS, Kim BY, Park SS, et al: HOXB13 is co-localized with androgen receptor to suppress androgen-stimulated prostate-specific antigen expression. Anat Cell Biol 2010, 43(4): 284-293.
-
Hamid SM, Cicek S, Karamil S, Ozturk MB, Debelec-Butuner B, Erbaykent-Tepedelen B, Varisli L, Gonen-Korkmaz C, Yorukoglu K, Korkmaz KS: HOXB13 contributes to G1/S and G2/M checkpoint controls in prostate. Mol Cell Endocrinol 2014, 383(1-2): 38-47.
-
Kim YR, Kang TW, To PK, Xuan Nguyen NT, Cho YS, Jung C, Kim MS: HOXB13-mediated suppression of p21WAF1/CIP1 regulates JNK/c-Jun signaling in prostate cancer cells. Oncol Rep 2016, 35(4): 2011-2016.
-
Kim YR, Kim IJ, Kang TW, Choi C, Kim KK, Kim MS, Nam KI, Jung C: HOXB13 downregulates intracellular zinc and increases NF-κB signaling to promote prostate cancer metastasis. Oncogene 2014, 33(37): 4558-4567.
-
Huang Q, Whitington T, Gao P, Lindberg JF, Yang Y, Sun J, Väisänen MR, Szulkin R, Annala M, Yan J, et al: A prostate cancer susceptibility allele at 6q22 increases RFX6 expression by modulating HOXB13 chromatin binding. Nat Genet 2014, 46(2): 126-135.
-
Kim IJ, Kang TW, Jeong T, Kim YR, Jung C: HOXB13 regulates the prostate-derived Ets factor: implications for prostate cancer cell invasion. Int J Oncol 2014, 45(2): 869-876.
-
Nerlakanti N, Yao J, Nguyen DT, Patel AK, Eroshkin AM, Lawrence HR, Ayaz M, Kuenzi BM, Agarwal N, Chen Y, et al: Targeting the BRD4-HOXB13 Coregulated Transcriptional Networks with Bromodomain-Kinase Inhibitors to Suppress Metastatic Castration-Resistant Prostate Cancer. Mol Cancer Ther 2018, 17(12): 2796-2810.
-
Rim EY, Clevers H, Nusse R: The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu Rev Biochem 2022, 91: 571-598.
-
Wang C, Chen Q, Xu H: Wnt/β-catenin signal transduction pathway in prostate cancer and associated drug resistance. Discov Oncol 2021, 12(1): 40.
-
Pal SK, Swami U, Agarwal N: Characterizing the Wnt Pathway in Advanced Prostate Cancer: When, Why, and How. Eur Urol 2020, 77(1): 22-23.
-
Gu L, Liao Z, Hoang DT, Dagvadorj A, Gupta S, Blackmon S, Ellsworth E, Talati P, Leiby B, Zinda M, et al: Pharmacologic inhibition of Jak2-Stat5 signaling By Jak2 inhibitor AZD1480 potently suppresses growth of both primary and castrate-resistant prostate cancer. Clin Cancer Res 2013, 19(20): 5658-5674.
-
Maranto C, Sabharwal L, Udhane V, Pitzen SP, McCluskey B, Qi S, O'Connor C, Devi S, Johnson S, Jacobsohn K, et al: Stat5 induces androgen receptor (AR) gene transcription in prostate cancer and offers a druggable pathway to target AR signaling. Sci Adv 2024, 10(9): eadi2742.
-
Lin J, Cai Y, Wang Z, Ma Y, Pan J, Liu Y, Zhao Z: Novel biomarkers predict prognosis and drug-induced neuroendocrine differentiation in patients with prostate cancer. Front Endocrinol (Lausanne) 2022, 13: 1005916.
-
Udhane V, Maranto C, Hoang DT, Gu L, Erickson A, Devi S, Talati PG, Banerjee A, Iczkowski KA, Jacobsohn K, et al: Enzalutamide-Induced Feed-Forward Signaling Loop Promotes Therapy-Resistant Prostate Cancer Growth Providing an Exploitable Molecular Target for Jak2 Inhibitors. Mol Cancer Ther 2020, 19(1): 231-246.
-
Liu X, He Z, Li CH, Huang G, Ding C, Liu H: Correlation analysis of JAK-STAT pathway components on prognosis of patients with prostate cancer. Pathol Oncol Res 2012, 18(1): 17-23.
-
Pencik J, Schlederer M, Gruber W, Unger C, Walker SM, Chalaris A, Marié IJ, Hassler MR, Javaheri T, Aksoy O, et al: STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat Commun 2015, 6: 7736.
-
Cheng B, Li L, Luo T, Wang Q, Luo Y, Bai S, Li K, Lai Y, Huang H: Single-cell deconvolution algorithms analysis unveils autocrine IL11-mediated resistance to docetaxel in prostate cancer via activation of the JAK1/STAT4 pathway. J Exp Clin Cancer Res 2024, 43(1): 67.
-
Canesin G, Krzyzanowska A, Hellsten R, Bjartell A: Cytokines and Janus kinase/signal transducer and activator of transcription signaling in prostate cancer: Overview and therapeutic opportunities. Curr Opin Endocr Metab Res 2020, 10: 36-42.
Cite this article: Dad H, Hansen A: Advancements in Research on Non-AR- Signaling Pathways and Targeted Therapies for Castration-Resistant Prostate Cancer. Ann Urol Oncol 2024, 8(1): 12-20. https://doi.org/10.32948/auo.2024.12.25
 Figure 1. Graphical representation explaining the operational mechanisms underlying seven vital signaling cascades. 1. The deletion or downregulation of PTEN, a tumor suppressor protein, can lead to the activation of the PI3K-AKT-mTOR pathway, subsequently promoting the development of Pca. 2. The Hippo signaling pathways YAP/TAZ components synergize with β-catenin from the WNT signaling cascade to facilitate DNA repair signal transduction. 3. AR/NOTCH signaling pathway acts directly on cells to activate signaling pathways and promote DNA repair. 4. The Hh signaling pathway promotes DNA repair through PTCH. 5. Prolactin activates Stat5a/b through the prolactin receptor, which further activates JAK2 to promote DNA repair. 6. Extracellular matrix growth factor receptors work with tyrosine kinase receptors to promote DNA repair through the PI3K-AKt-mTOR signaling pathway. 7. HOXB13 activates CDK2, which in turn activates E2F1, which recognizes cell membrane surface receptors through downstream factors such as E2Fs, p107, c-myc, and other factors, playing a signal transduction role.
Figure 1. Graphical representation explaining the operational mechanisms underlying seven vital signaling cascades. 1. The deletion or downregulation of PTEN, a tumor suppressor protein, can lead to the activation of the PI3K-AKT-mTOR pathway, subsequently promoting the development of Pca. 2. The Hippo signaling pathways YAP/TAZ components synergize with β-catenin from the WNT signaling cascade to facilitate DNA repair signal transduction. 3. AR/NOTCH signaling pathway acts directly on cells to activate signaling pathways and promote DNA repair. 4. The Hh signaling pathway promotes DNA repair through PTCH. 5. Prolactin activates Stat5a/b through the prolactin receptor, which further activates JAK2 to promote DNA repair. 6. Extracellular matrix growth factor receptors work with tyrosine kinase receptors to promote DNA repair through the PI3K-AKt-mTOR signaling pathway. 7. HOXB13 activates CDK2, which in turn activates E2F1, which recognizes cell membrane surface receptors through downstream factors such as E2Fs, p107, c-myc, and other factors, playing a signal transduction role.
 Figure 2. Interpretation of the relevant molecules contained in the PI3K-AKt-mTOR signaling pathway included p85/p110, PKB, and PTEN. Wnt signaling pathway includes APC, β-catenin, R-Spondin. The Notch signaling pathway includes Hes1 and the G0/G1 phase. The Jak2-Stat5a/b signaling pathway includes prolactin, AZD1480, and AR. The Hippo signaling pathway includes YAP/TAZ, statins, diphosphate, and G-protein inhibitors. The Hh signaling pathway includes PTCH,Smo,TAK441,Vimedji, Itraconazole. The HOXB13 signaling pathway includes p21, CKD2/RB, E2F1, E2Fs, P107, and c-Myc. AR: androgen receptor; PI3K: phosphatidylinositol 3-kinase; mTOR: mammalian target of rapamycin; PTEN: phosphatase and tensin homolog deleted on chromosometen; APC: adenomatous polyposis coli; PKB: Protein kinase B.
Figure 2. Interpretation of the relevant molecules contained in the PI3K-AKt-mTOR signaling pathway included p85/p110, PKB, and PTEN. Wnt signaling pathway includes APC, β-catenin, R-Spondin. The Notch signaling pathway includes Hes1 and the G0/G1 phase. The Jak2-Stat5a/b signaling pathway includes prolactin, AZD1480, and AR. The Hippo signaling pathway includes YAP/TAZ, statins, diphosphate, and G-protein inhibitors. The Hh signaling pathway includes PTCH,Smo,TAK441,Vimedji, Itraconazole. The HOXB13 signaling pathway includes p21, CKD2/RB, E2F1, E2Fs, P107, and c-Myc. AR: androgen receptor; PI3K: phosphatidylinositol 3-kinase; mTOR: mammalian target of rapamycin; PTEN: phosphatase and tensin homolog deleted on chromosometen; APC: adenomatous polyposis coli; PKB: Protein kinase B.
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
 Submit Manuscript
Submit Manuscript