Research Article | Open Access
Exploration of Apoptosis in Histopathologies of Balkan Endemic Nephropathies with Both Urothelial Tumour and Atrophied Kidney
Peter Mantle1, Rohit Upadhyay2, Diana Herman3, Vecihi Batuman2
1Centre for Environmental Policy, Imperial College London, London SW7 2AY, London, UK.
2Section of Nephrology and Hypertension, John W Deming Department of Medicine, Tulane University School of medicine, New Orleans, Louisiana, USA.
3Pathology Department, County Hospital, Timisoara, Romania.
Correspondence: Peter Mantle (Centre for Environmental Policy, Imperial College London, London SW7 2AY, London, UK; Email: p.mantle@imperial.ac.uk).
Annals of Urologic Oncology 2024, 7(2): 61-69. https://doi.org/10.32948/auo.2024.06.25
Received: 18 Apr 2024 | Accepted: 20 Jun 2024 | Published online: 04 Jul 2024
Key words apoptosis, urothelial cancer, Balkan endemic nephropathy, TUNEL assay
An accidental epidemic of renal disease in about a hundred Belgian women in the 1990s was attributed to a pharmaceutical error in formulating a weight-controlling herbal medication containing aristolochic acid. As the kidney disease in these women was assumed to be at least partially involving apoptosis, we screened Balkan nephropathy cases with a urothelial tumour from Serbia using a modern TdT-mediated dUTP Nick-End Labeling (TUNEL) technique [4]. The historic renal disease foci across parts of the Balkans have become assumed to involve apoptosis, similar to sporadic renal disorders seen within parts of Asian regions historically relying on herbal medications. Although the incidence of Balkan endemic nephropathy (BEN) seems to have declined, it was still occurring in the third millennium for which the samples for assessing immunoprofiles had been obtained, and some sections of which had remained for the present study.
Detection of apoptosis has developed since its definition half a century ago, summarized more recently [5]. Its recognition in animal histology via standard haematoxylin-staining became extensive in the past 30 years, augmented by some recent methodologies. A key example was its use in Zagreb to demonstrate renal apoptosis in female rats after dosing with ochratoxin A [6], after noting that humans in endemic regions in Croatia may have been exposed to this mycotoxin [7]. For context, BEN was being more hyperendemic in women in several Balkan villages, increasingly requiring dialysis for survivalIn informal parallel exploration of BEN from the UK [8], an experimental study of a Bulgarian Penicillium isolate revealed interesting renal histopathology in male rats as the standard bioassay method [9]. Recognition of ochratoxin A in South Africa as a potent renal toxin in experimental animals since its discovery in 1965 [10] and encouraged further studies in UK with male rats focused on Bulgarian Penicillium. A more detailed fermentation product analysis [11], coupled with rat renal histopathology, focused attention to what subsequently was illustrated in publication of further studies [12], suggesting that some microbial products could cause progressive renal apoptosis [13]. That publication illustrated apoptosis in colour, but online access to The Lancet is no longer available. Subsequently, analogous apoptotic illustration [14, 15] extends the demonstration of fungal metabolites which have given further renal evidence of their apoptotic potential in rats via diet.
In 1991 a unique documentary on BEN, covering the background exclusively in Balkan contexts, was broadcast on UK television and was subsequently used in education at Imperial College in London over several years. It can now be found in the internet and uniquely illustrates some early history [16]. A visit to Karash also 1991 by PM, meeting residents illustrated in the film, was a significant personal experience.
Further staining by histology-skilled BEN researchers [18, 19], to explore apoptotic histology, have studied virtually identical sections of the Serbian samples by applying the Abcam TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) assay kit (Ab 206386). The technique detects DNA fragmentation such as in apoptosis, using a terminal deoxy-nucleotidyl-transferase to catalyse incorporation of terminal deoxy-nucleotidyl-transferase incorporation of deoxynucleotides at the 3-hydroxyl ends of fragmented DNA. Stained products then become coloured brown at sites of DNA fragmentation, but nuclear items can appear darker, almost black. Counterstain with methyl green potentially defines, by contrast, conventional tumour tissues. TUNEL assay kit (ab206386; Abcam, Cambridge, MA, USA) was used according to the manufacturer's instructions. The key steps in this protocol along with slight modifications for ease of following are given below:
Tissue Section Preparation and Rehydration: Paraffin-embedded kidney tissue sections were obtained and rehydrated according to the following protocol. Slides were immersed in xylene for two cycles of 5 minutes each at room temperature (RT), with frequent changes of xylene. Subsequently, slides were transferred to 100% ethanol for two cycles of 5 minutes each at RT, followed by immersion in 90%, 80%, and 70% ethanol for 3 minutes each at RT. Slides were briefly rinsed with 1X Tris-buffered saline (TBS) for 5 minutes, and excess liquid was carefully dried around the specimen using an absorbent wipe while ensuring the specimen was circled with a hydrophobic slide marker to contain reagent volumes and also to avoid drying of the specimen.
Permeabilization of Specimen: To permeabilize the specimen, Proteinase K was diluted 1/100 in deionized water (dH2O) (1 μL Proteinase K + 99 μL dH2O per specimen). Each specimen was covered with 100 μL of the diluted Proteinase K solution and incubated at RT for 20 minutes. Following incubation, slides were rinsed with 1X TBS for 5 minutes.
Quenching Inactivation of Endogenous Peroxidases: Endogenous peroxidases were quenched by diluting 30% hydrogen peroxide (H2O2) 1/10 in methanol (10 μL 30% H2O2 + 90 μL methanol per specimen). Specimens were covered with 100 µL of 3% H2O2 and incubated at RT for 5 minutes. Subsequently, slides were rinsed with 1X TBS for 5 minutes.
Equilibration: Specimens were covered with 100 μL of Terminal deoxynucleotidyl transferase (TdT) Equilibration Buffer and incubated at RT for 30 minutes.
Labeling Reaction: TdT Enzyme was mixed with TdT Labeling Reaction Mix in a 1: 39 ratio and applied to each specimen (40 μL per specimen). Slides were covered with a coverslip to ensure even distribution of the reaction mixture and prevent evaporation during incubation. Specimens were incubated in a humidified chamber at RT for 1.5 hours.
Termination of Labeling Reaction and Blocking: Stop Buffer was warmed to 37ºC for 5 minutes and then applied to each specimen (100 μL per specimen). Slides were incubated at RT for 5 minutes, rinsed with 1X TBS for 5 minutes, and excess liquid was carefully dried around the specimen. Specimens were covered with 100 μL of Blocking Buffer and incubated at RT for 10 minutes.
Detection: Conjugate was diluted 1/25 in Blocking Buffer (4 μL 25X Conjugate + 96 μL Blocking Buffer per specimen) and applied to each specimen. Slides were incubated in a humidified chamber at RT for 30 minutes, rinsed with 1X TBS for 5 minutes, and excess liquid was carefully dried around the specimen.
Development: DAB solution was prepared by diluting DAB Solution 1 in DAB Solution 2 (1/30 dilution). Specimens were covered with 100 μL of diluted DAB solution and incubated at RT for 15 minutes. Slides were then gently rinsed with deionized water.
Counterstain and Storage: Specimens were immediately covered with 100 μL of Methyl Green Counterstain solution and incubated at RT for 1-3 minutes. Excess counterstain was removed by pressing an edge of the slide against an absorbent towel, and slides were dipped 2-4 times in 100% ethanol. Subsequently, slides were dipped 2-4 times in 100% xylene, excess xylene was wiped off, and a glass coverslip was mounted using organic mounting media over the specimen.
An apoptosis endpoint, indicative of positive staining, was represented by a dark brown (DAB) signal. Lighter shades of brown and/or shades of blue-green to green-brown indicated a nonreactive/negative cell. After performing the assay, slides were evaluated under a fluorescent microscope (M5000, Thermo Fisher Scientific, Pittsburgh, PA, USA) with brightfield capability. We imaged slides at 4x, and 20x magnifications. In addition, we utilized Zeiss Axio ScanZ.1 Slide Scanner to digitize slides. This scanner generated slide-scanning data over an extended period. This enclosed system scanner has 25 slide holder trays, each tray has 4 slide slots, so 100 slides can be uploaded to scan. The average size of images generated through Zeiss Axio ScanZ.1 Slide Scanner was ~1GB therefore we converted them to smaller file formats like JPG for further analysis.
Application of this Abcam kit staining methodology, cited in over 150 publications, has nuclear apoptosis staining illustrated on Its website. The present imaging at x 4 and x 20 magnification has provided illustrations for a more representative description. The present staining could now indirectly link to the immune profiles of urothelial tumours in Slovakia [20], where BEN has not occurred.
A high-grade urothelial carcinoma was observed in the first case (Figure 1), with the left images and the bottom right central area exhibiting necrosis with TUNEL-positive nuclei. The right areas isolated apparent apoptotic nuclei, showing apoptosis across four urothelial tumour regions. This extensive apoptosis suggests that the tumour cells are undergoing programmed cell death, possibly due to the aggressive nature of the carcinoma or a response to therapeutic interventions.
In another instance, TUNEL-staining in the kidney cortex was noted, but the staining was not located in the nuclei (Figure 2). This implies that apoptosis might be occurring in the cytoplasmic regions or that there is a different mechanism of cell death at play, potentially related to the nephropathy itself rather than the tumour.
The urothelial tumors associated with Balkan atrophy (Figure 3) displayed slight evidence of nuclear apoptosis, with the top left image and similar items in other pictures indicating a less aggressive apoptotic process compared to other cases. This might be due to the atrophic changes in the kidney tissue, affecting the tumour's growth dynamics and apoptotic responses.
High-grade urothelial carcinoma tissue with extensive brown staining was presented in another case (Figure 4), indicating TUNEL-positive nuclei in the upper examples. Occasional inflammatory cells in the lower pictures suggest an ongoing inflammatory response, potentially contributing to the observed apoptosis. The inflammation might be a reaction to tumour necrosis or a sign of the body’s immune response to the cancer cells.
Focal intense staining of lymphocytes in the stromal compartment was evident in another urothelial carcinoma tissue (Figure 5). Brown patches implied secretion or pyknosis with elongated tumour nuclei, and there was non-specific staining of necrotic debris. This focal intense staining and presence of lymphocytes suggest an immune-mediated response, which might be causing targeted apoptosis in the tumour cells. The non-specific staining of necrotic debris indicates areas of cell death that are not solely apoptotic but could include necrosis or other forms of cell death.
A particularly notable case showed urothelial tumour tissues where the centrally-stained material was necrotic or nearly necrotic (Figure 6). This extensive necrosis indicates a high level of cell death, possibly due to severe tumour progression or a lack of sufficient blood supply to the tumour center, leading to hypoxia and subsequent cell death.
In the final case, a solid and invasive urothelial tumour was observed (Figure 7), with occasional or frequent evidence of nuclear apoptosis, similar to observations in Figures 1 and 4. The invasiveness of the tumour likely contributes to the observed apoptosis, as invasive tumours tend to have higher rates of cell turnover and death.
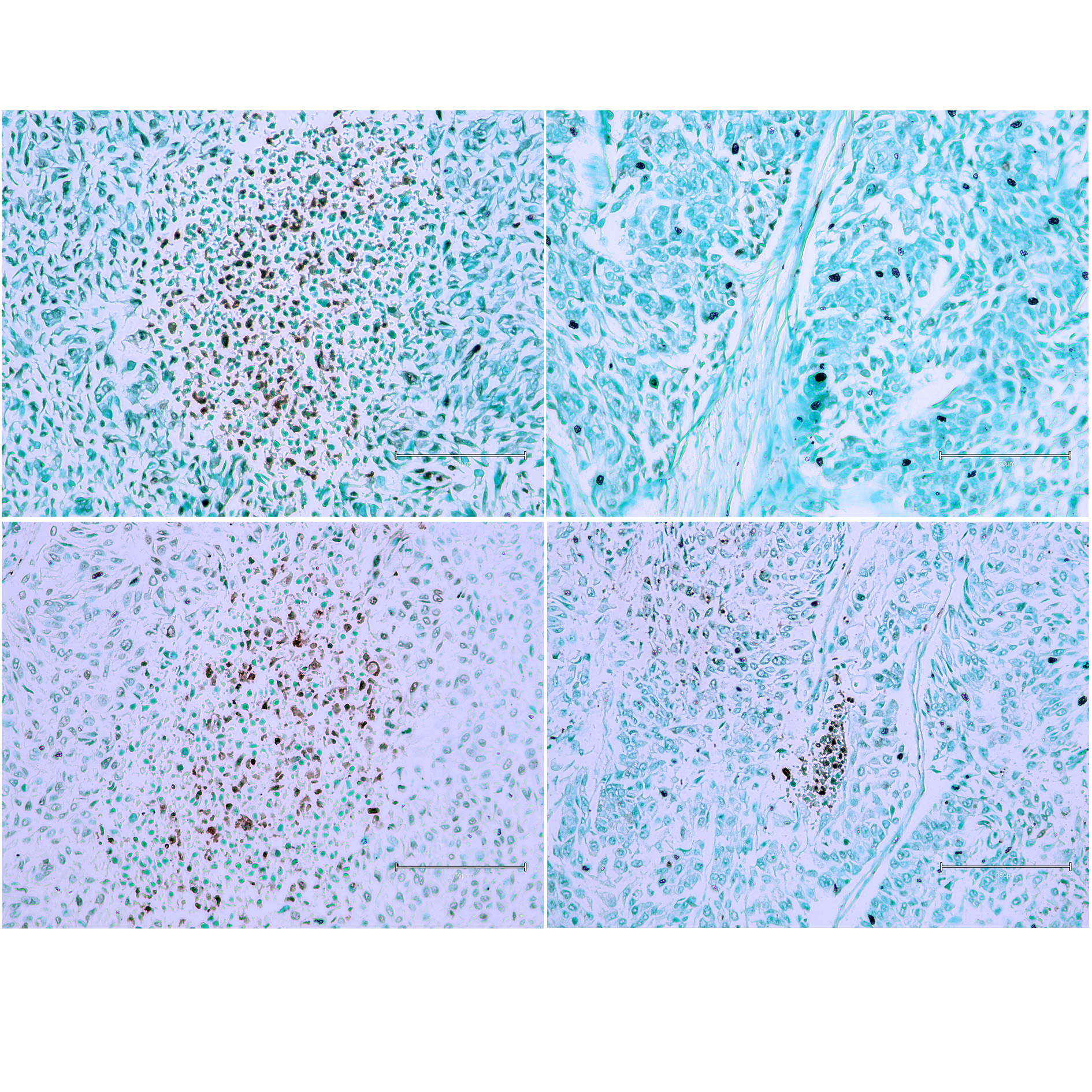 Figure 1 (Case 1 [17]). High-grade urothelial carcinoma. Panels (A) and (C) show the central area of necrosis with TUNEL-positive nuclei. Panels (B) and (D) display isolated apparent apoptotic nuclei. Apoptosis is observed across four urothelial tumour regions. Bar 150 µm.
Figure 1 (Case 1 [17]). High-grade urothelial carcinoma. Panels (A) and (C) show the central area of necrosis with TUNEL-positive nuclei. Panels (B) and (D) display isolated apparent apoptotic nuclei. Apoptosis is observed across four urothelial tumour regions. Bar 150 µm.
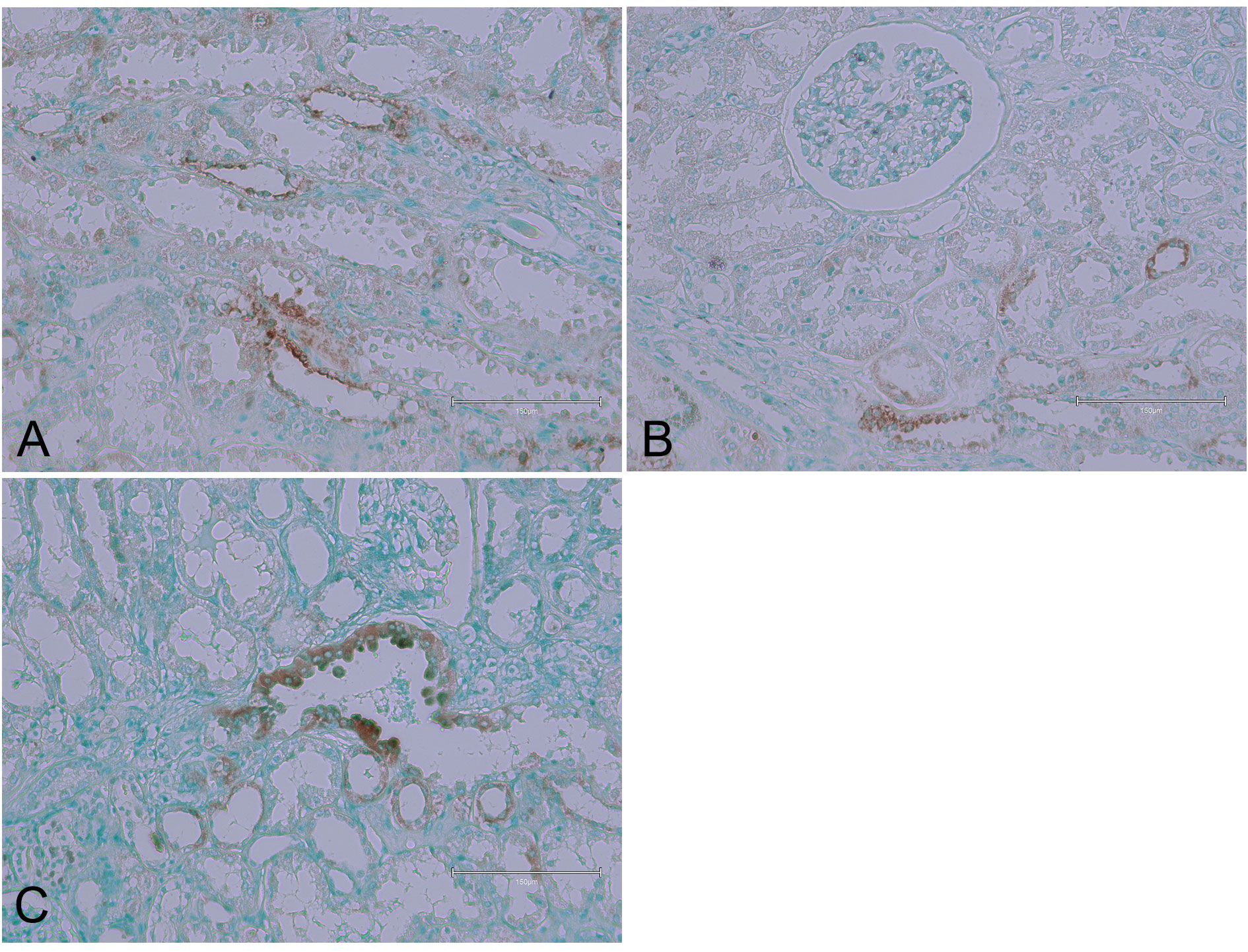 Figure 2 (Case 2 [17]). Examples of TUNEL-staining in the kidney cortex, but the staining is not located in the nuclei. Panels (A), (B), and (C) illustrate the TUNEL-positive areas. Bar 150 µm.
Figure 2 (Case 2 [17]). Examples of TUNEL-staining in the kidney cortex, but the staining is not located in the nuclei. Panels (A), (B), and (C) illustrate the TUNEL-positive areas. Bar 150 µm.
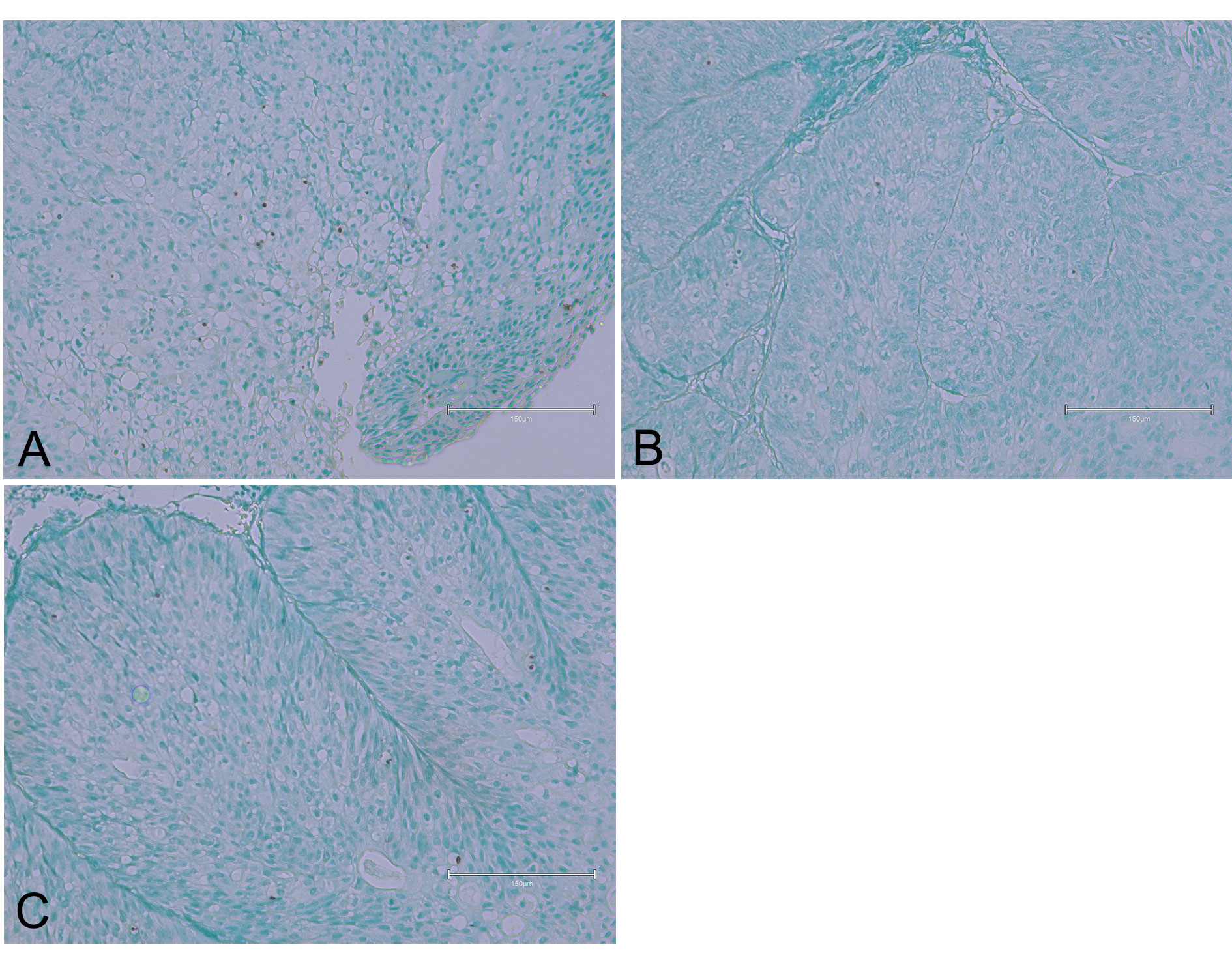 Figure 3 (Case 3 [17]). Displaying urothelial tumour tissues associated with Balkan atrophy. Panel (A) shows slight evidence of nuclear apoptosis. Panels (B) and (C) present similar findings, with two or three apoptotic nuclei observed in each image. Bar 150 µm.
Figure 3 (Case 3 [17]). Displaying urothelial tumour tissues associated with Balkan atrophy. Panel (A) shows slight evidence of nuclear apoptosis. Panels (B) and (C) present similar findings, with two or three apoptotic nuclei observed in each image. Bar 150 µm.
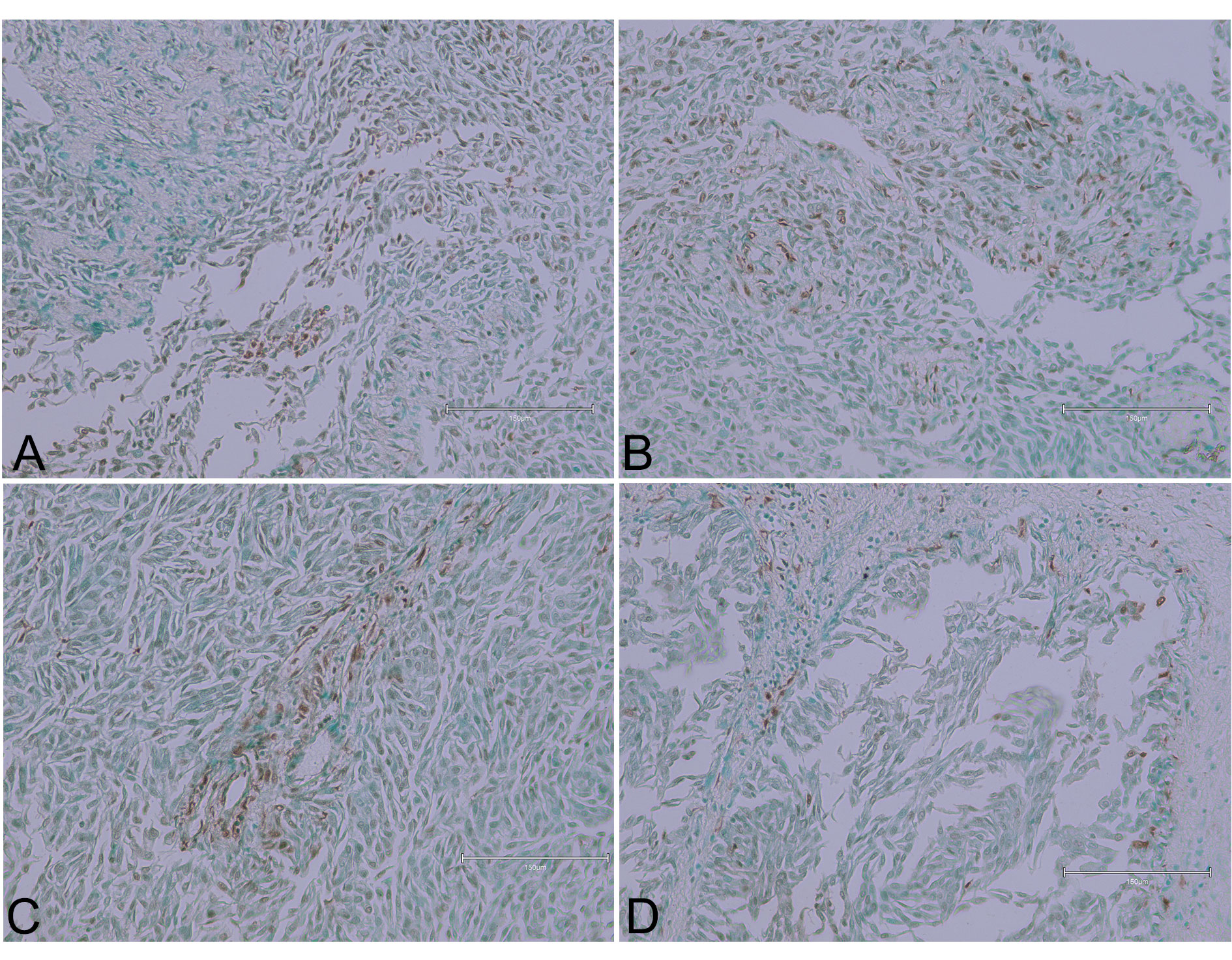 Figure 4 (Case 4 [17]). Upper examples (panels A and B) show high-grade urothelial carcinoma tissue areas with fairly extensive brown staining, indicating TUNEL-positive nuclei. Lower pictures (panels C and D) display occasional inflammatory cells. Bar 150 µm.
Figure 4 (Case 4 [17]). Upper examples (panels A and B) show high-grade urothelial carcinoma tissue areas with fairly extensive brown staining, indicating TUNEL-positive nuclei. Lower pictures (panels C and D) display occasional inflammatory cells. Bar 150 µm.
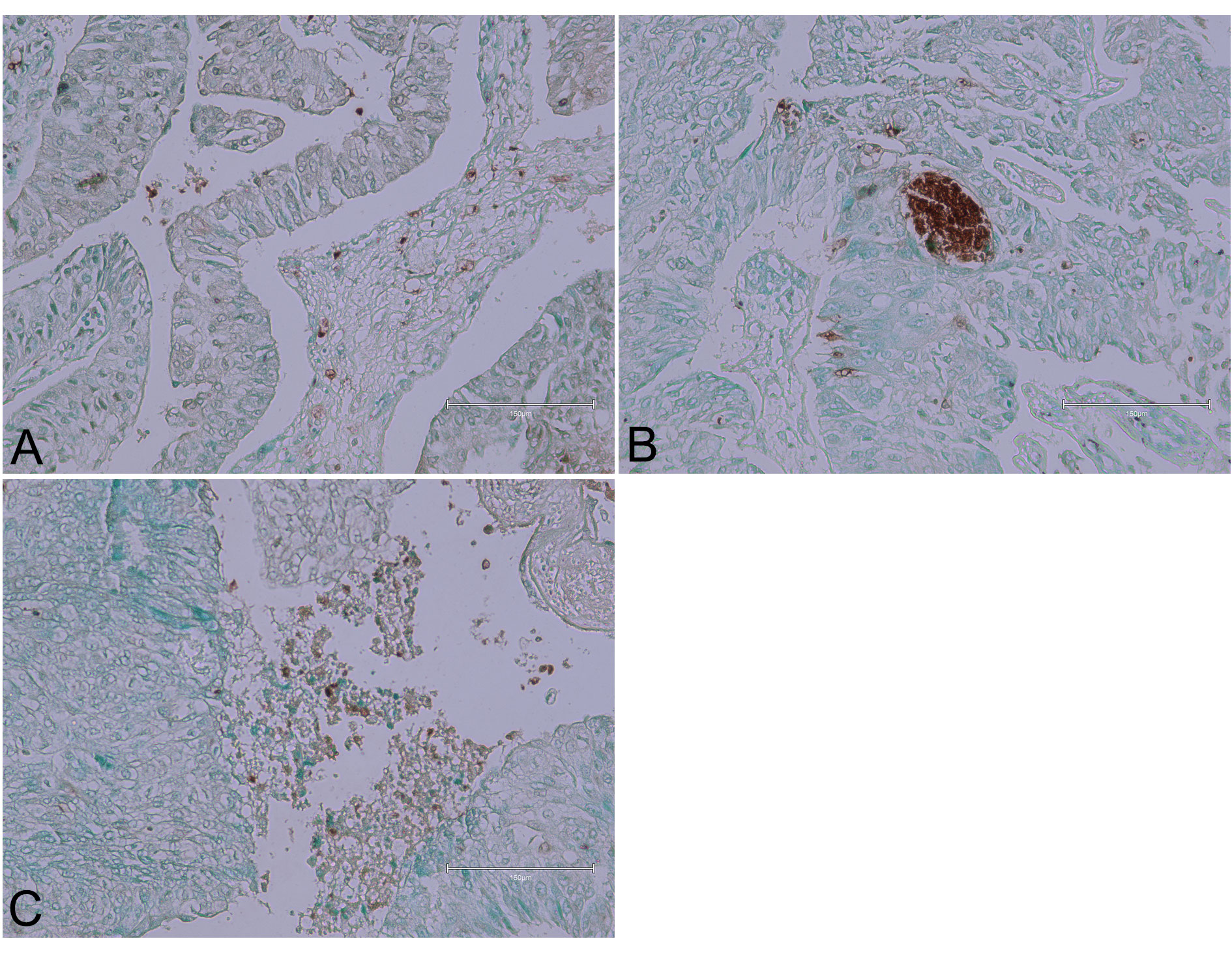 Figure 5 (Case 5 [17]). Urothelial carcinoma tissues. Panel (A) shows focal intense staining of lymphocytes in the stromal compartment. Panel (B) exhibits brown patches implying secretion or pyknosis, with a few elongated tumour nuclei. Panel (C) displays non-specific staining of necrotic debris. Bar 150 µm.
Figure 5 (Case 5 [17]). Urothelial carcinoma tissues. Panel (A) shows focal intense staining of lymphocytes in the stromal compartment. Panel (B) exhibits brown patches implying secretion or pyknosis, with a few elongated tumour nuclei. Panel (C) displays non-specific staining of necrotic debris. Bar 150 µm.
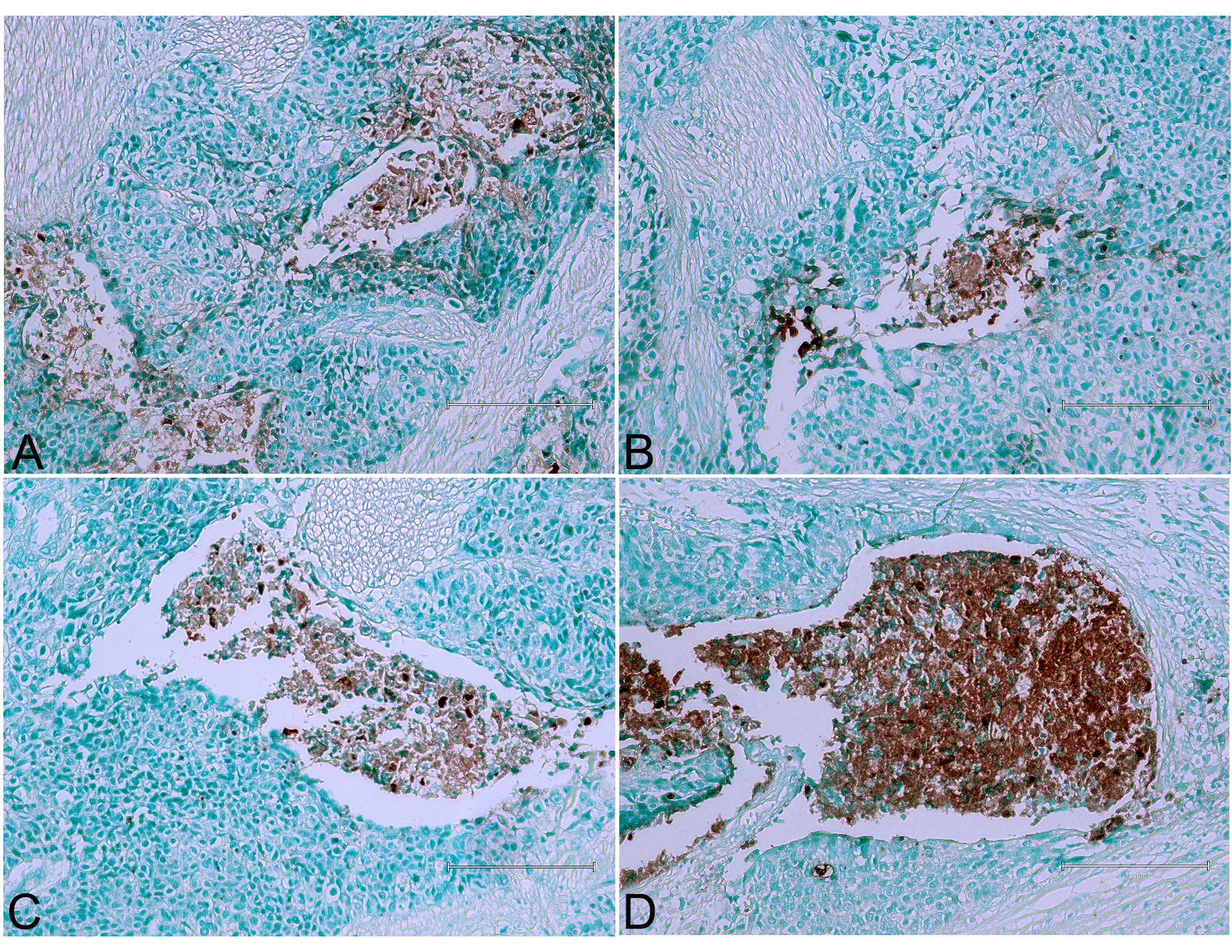 Figure 6 (Case 7 [17]). Examples of urothelial tumour in which the centrally-stained material is necrotic or almost necrotic. Panels (A), (B), (C), and (D) illustrate these findings. Bar 150 µm.
Figure 6 (Case 7 [17]). Examples of urothelial tumour in which the centrally-stained material is necrotic or almost necrotic. Panels (A), (B), (C), and (D) illustrate these findings. Bar 150 µm.
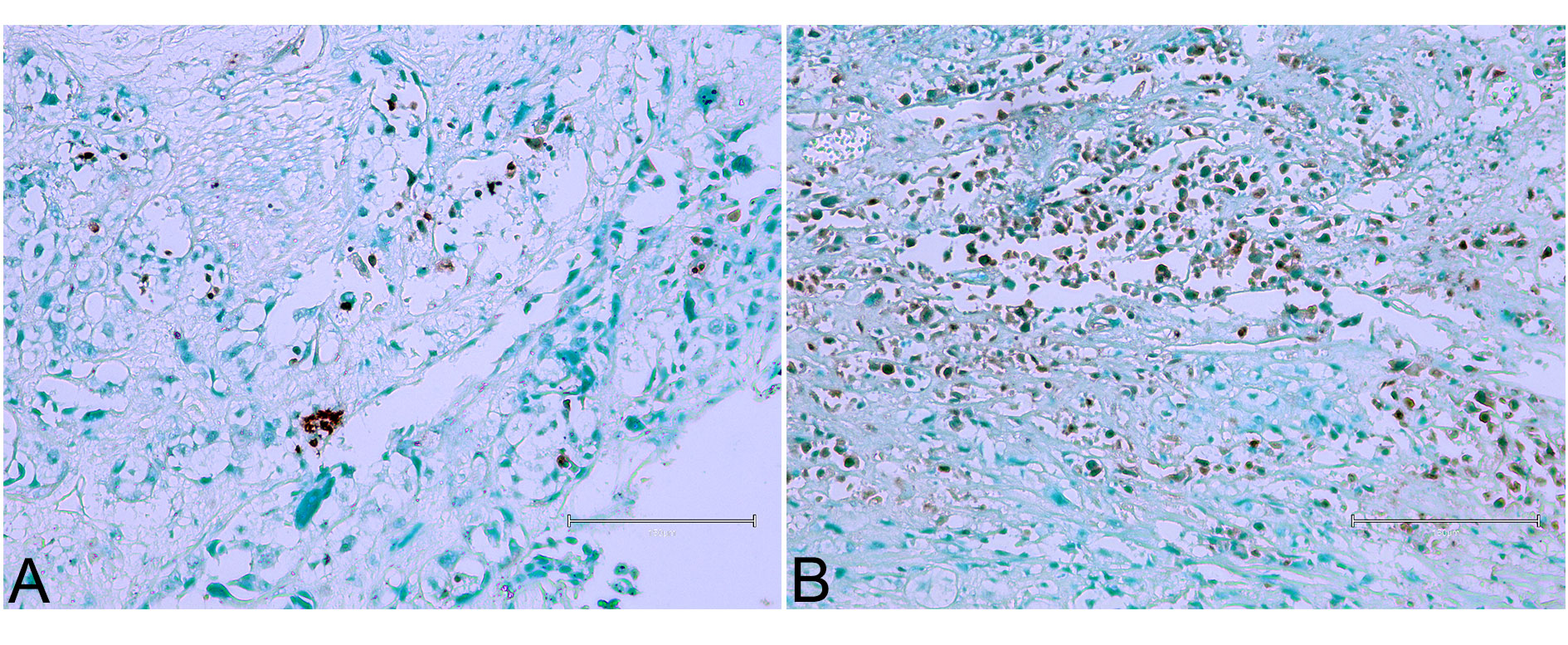 Figure 7 (Case 8 [17]). Urothelial tumour, particularly solid and invasive, with occasional or frequent evidence of nuclear apoptosis similar to observations in Figures 1 and 4. Panels (A) and (B) illustrate these findings. Bar 150 µm.
Figure 7 (Case 8 [17]). Urothelial tumour, particularly solid and invasive, with occasional or frequent evidence of nuclear apoptosis similar to observations in Figures 1 and 4. Panels (A) and (B) illustrate these findings. Bar 150 µm.
To overcome the limited supply of natural BEN tissues, we used rat tissues exposed to ochratoxin A through their diet. Ochratoxin A is known to be a potential human kidney toxin, making it important for this study. We exposed rats to ochratoxin A over a long period to see how it affected their kidney tissues. The staining with Ab 206386 showed significant changes in the rat kidney tissues, similar to those seen in BEN tissues. These results, shown in Figure 8, highlight the strong impact of ochratoxin A on kidney tissues and suggest that it could be an important factor in human kidney disease. This comparison between BEN and ochratoxin A-exposed tissues helps us better understand the potential health risks of ochratoxin A.
Demonstration was made in London nearly 25 years ago that ochratoxin A, as a candidate mycotoxin for causing BEN, could be absorbed by intact coffee roots within soil and potentially contaminate human foodstuffs [21]. This has led eventually, via analogous Balkan experimentation, to a similar conclusion for aristolochic acid; informal discussion concerning its suspected involvement in BEN occurred during a BEN conference in Belgrade. Thus, the present renal histopathology exploration of a few Serbian BEN cases has been complemented separately by adding several examples from OTA/rat experimentation. We present here (Figure 8) two examples of response to the Abcam histology staining in the kidney of a male rat after half-lifetime dietary exposure to OTA, sufficient subsequently to cause a renal carcinoma (not displayed here). This has revealed Abcam-staining exclusively in cortical proximal nephron epithelia but not in their nuclei. Also displayed, for another rat on continuous OTA, the tumour has extensive TUNEL-stained nuclei, consistent with apoptosis. The rat and human examples in the present studies display either lifetime consequences of an artificial, generally-tolerated and principally-renal toxin, or near lifetime consequence of rural life in some areas of the Balkans. For humans this is apparently the first significant evidence of apoptosis in the urothelial tumour, although tumour origin was by definition outside the kidney. The principal reason for the unilateral nephrectomy must have included evidence of reduced renal mass. Thus the origin of that must have been the principal reason(s) for its BEN diagnosis. The present BEN tissue samples focused initially on the tumours for defining their immunoprofile range [17]; being distant from renal cortex, that tissue remains as yet unexplored for any TUNEL evidence that might be involved early in BEN initiation. Perhaps the source of the present sections also still has archived renal tissues from these unilateral nephrectomies. Expanded publication of the presently-parallel Abcam-stained study of OTA/rat renal pathology, focusing on renal cortex as shown here (Figure 8), will illustrate that in further detail.
Active UK exploratory interest in the Balkans’ nephropathy commenced in the 1970s, while easy access to the region was limited, and even admission of the disease occurrence in Romania was forbidden. Study of climate across relevant countries recorded extreme rainfall and humidity patterns. Questioning of the incidence of fungal saprophytes for agricultural use yielded samples of moulded produce amongst which a fungus, currently named as Penicillium aurantiogriseum, was found to be common. Experimental biomedical study in UK [9] had followed the exploration in South Africa in the 1960s of mould toxicity to experimental rats and the discovery of ochratoxin A [22]. To focus on Balkan fungi the UK study used a natural mould from the Vratsa BEN area in NW Bulgaria and discovered rat renal toxicity somewhat different from that caused by ochratoxin A, and not obviously mimicking it. Meanwhile, a Yugoslavian report [23] described apoptosis in kidneys of female rats given ochratoxin A. This mycotoxin, found to be native of local agriculture of BEN villages in Serbia, refocused Balkan research attention on that mycotoxin. Also, renewed study of the Bulgarian Penicillium isolate [24] refined its histopathology in male rats, while exploring its cause [25].
In 1985 a preliminary autumnal survey of fungi in Yugoslavia associated with human food in the BEN-hyperendemic village of Kaniza, now just a part of Croatia, had revealed a wide range of moulds, particularly of Penicillium aurantiogriseum and P. commune, but none revealed they could produce ochratoxin A [26]. More recently, focus across Serbia [27] explored home-produced meat products confirming association with Penicillia, notably the P. aurantiogriseum (since revised to P. verrucosum var. cyclopium) and P. commune, but not exploring their mycotoxin potential. Human risk from OTA seems thus to have declined, according to literature [28].
Twenty years ago, a visit to BEN areas in the Romanian region of Dobreta Turnu Severin included informal social interaction along with a welcome response in the hyperendemic village of Erghevita to requests for urine samples as part of informal analyses comparing residential populations in the Balkans [29]. Diversion of two of the Erghevita population from normal was reported after detailed UK pharmaceutical analysis. Apparently, no fresh diagnosis of BEN in that village has since been declared, but the published study illustrated indigenous Aristolochia growing in residential property. More recently, literature focus has been on Aristolochic acid as a cause of BEN [30] and its natural latitude reflection across Europe from the Belgian accidental epidemic to the Balkans. General conformity to adverse natural herbal accidental poisoning in similar Asian latitudes allowed conclusion that BEN’s or BEN-like kidney disease might be endemic worldwide. An example of this recent Chinese involvement [30] is on detailed assessment of treatment of aristolochic acid’s kidney fibrosis in mice, while Asian interest remains in avoiding human exposure via cultural organic medicine.
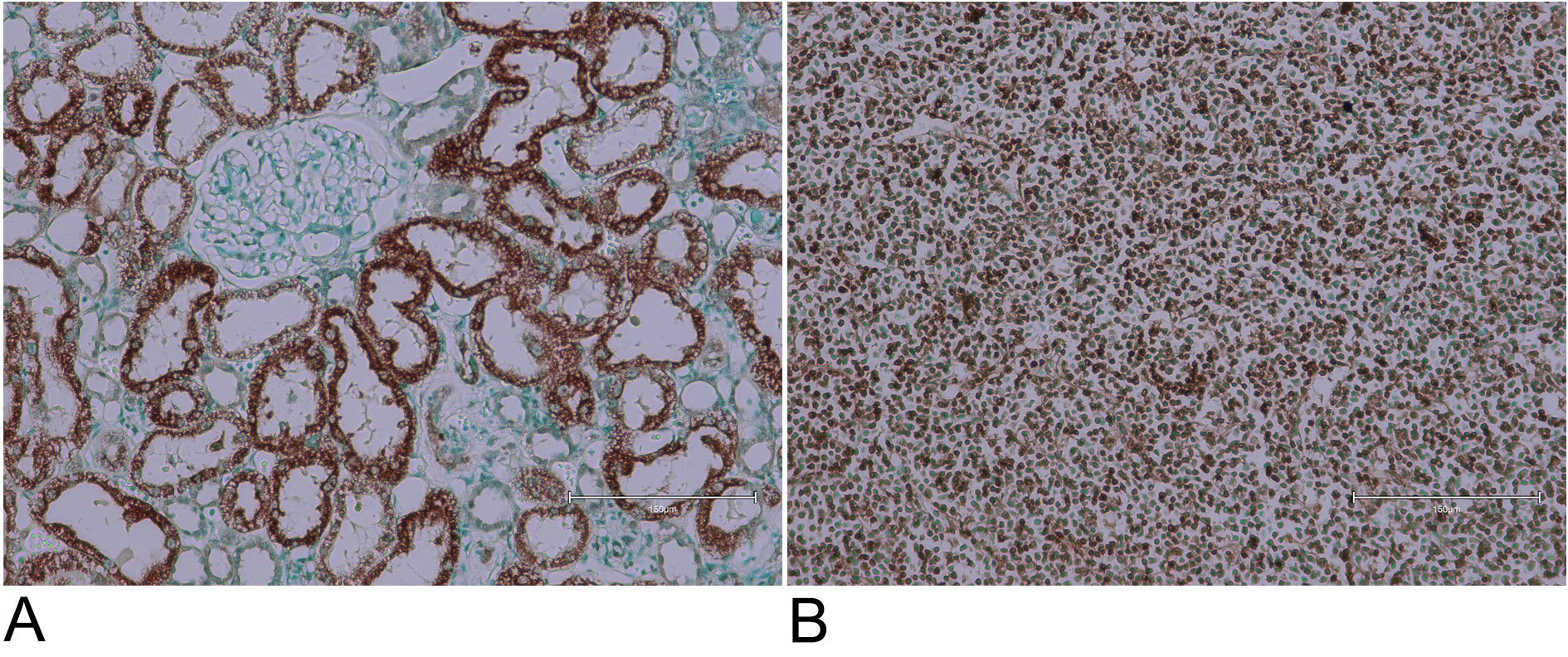 Figure 8. Examples of male rat kidney cortex and renal tumour, stained with Abcam 206386 kit concurrently with the BEN tissues. (A) Selective staining of proximal nephron epithelia, generated while on dietary ochratoxin A during the first year of adult life, and retained through a second year of life without dietary mycotoxin. (B) Renal carcinoma, after dietary ochratoxin A for the whole of adult life. Bar 150 µm.
Figure 8. Examples of male rat kidney cortex and renal tumour, stained with Abcam 206386 kit concurrently with the BEN tissues. (A) Selective staining of proximal nephron epithelia, generated while on dietary ochratoxin A during the first year of adult life, and retained through a second year of life without dietary mycotoxin. (B) Renal carcinoma, after dietary ochratoxin A for the whole of adult life. Bar 150 µm.
None.
Ethical policy
The research was approved by the Ethics Committee of the General Hospital Leskovac, Serbia on 16-11-2018. No. 11550/2.
Availability of data and materials
None additional.
Author contributions
PM, conception, data analysis, drafting, critical revision, final approval; RU, data collection, photography, critical revision, final approval; DH, histopathology scrutiny, final approval; VB, critical and nephrological medical revision, final approval.
Competing interests
The authors have no competing interest.
Funding
Personal only.
- Tanchev Y, Evstatiev Z, Dorossiev D, Pencheva J, Zvetkov G: Studies on the nephritides in the district of Vratza. Sovrem Med 1956, 7: 14-29.
- Krogh P: Causal associations of mycotoxic nephropathy. Acta Pathol Microbiol Scand 1978, Sect. A A suppl. 279, 1-28.
- Boorman GA: Toxicology and carcinogenesis studies of ochratoxin A. NIH Publication 1989, 2813.
- Herman D, Stanojevic G, Pavlovic N, Mantle P: Histopathological review of renal pelvic tumours of Balkan nephropathy cases from Southern Serbia. Ann Urol Oncol 2020, 3(1): 2617-2673.
- Kerr JF, Wyllie AH, Currie AR: Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972, 26(4): 239-257.
- Peraica M, Ferencic Z, Fuchs R, Cvoricec D: Single and multiple doses of ochratoxin A cause apoptosis in kidney tubular epithelium of rat. Rev Med Vet 1998, 149: 650: (sourced electronically via The British Library).
- Radic B, Fuchs R, Peraica M, Lucic A: Ochratoxin A in human sera in the area with endemic nephropathy on Croatia. Toxicol Lett 1997, 91(2): 105-109.
- Austwick PKC: Balkan nephropathy. The Practitioner 1981, 225(1357): 1031-1038.
- Barnes JM, Austwick PKC, Carter RL, Flynn FV, Peristianis GC, Aldridge WN: Balkan (endemic) nephropathy and a toxin-producing strain of Penicillium verrucosum var. cyclopium: an experimental model in rats. The Lancet 1977, 1(8013): 671-675.
- Van der Merwe KJ, Steyn PS, Fourie L: Ochratoxin A, a toxic metabolite produced by s Aspergillus ochraceus Wilh. Nature 1965, 205(976): 1112-1113.
- Mantle PG, McHugh KM, Adatia R, Gray T, Turner DR: Persistent karyomegaly caused by Penicillium nephrotoxins in the rat. Proc Roy Soc Lond. B Biol Sci 1991, 246(1317): 251-259.
- Mantle PG, McHugh KM, Adatia R, Heaton JM, Gray T, Turner DR: Penicillium aurantiogriseum-induced persistent renal histopathological changes in the rat. In Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours. M. Castegnaro, R Plestina, G Dirheimer, I N Chernozemsky, H. Bartsch, Eds. International Agency for Research in Cancer, Lyon, publication 115, 119-127.
- Mantle PG, Miljkovic A, Udupa A, Dobrota M: Does apoptosis cause renal atrophy in Balkan endemic nephropathy? The Lancet 1998, 352(9134): 1118-1119.
- Miljkovic A, Mantle P: Renal apoptosis in the mycotoxicology of Penicillium polonicum and ochratoxin A in rats. Life 2022, 12(3): 352.
- Miljkovic A, Mantle P: Chromatographic fractionation of penicillium polonicum fermentation metabolites in search of the nephrotoxin(s) for rats. Life 2022, 12(5): 747.
- The Curse of Karash, BBC Horizon programme 1991 (see YouTube).
- Herman D, Stanojevic G, Pavlovic N, Mantle P: Histopathological review of renal pelvic tumours of Balkan nephropathy cases from Southern Serbia. Ann Urol Oncol 2020, 3: 2617-2673.
- Batuman V: Fifty years of Balkan endemic nephropathy: daunting questions, elusive answers. Kidney Int 2006, 69(4): 644-646.
- Upadhyay R, Batuman V: Aristolochic acid I induces proximal tubule injury through ROS/HMGB1/ mt DNA mediated activation of TLRs. J Cell Mol Med 2022, 26(15): 4277-4291.
- Rajkani J, Kajo K, Adamkov M, Moravekova E, Felkanova D, Bencat M: Immunohistochemical characterization of urothelial carcinoma. Bratislava Med J 2013, 114(8): 431-438.
- Mantle PG: Uptake of radiolabelled ochratoxin A from soil by coffee plants. Phytochemistry 2000, 53(3): 377-378.
- Van der Merwe KJ, Steyn PS, Fourie L: The constitutions of ochratoxins A, B and C, metabolites of Aspergillus ochraceus Wilh. J Chem Soc 1965, 7083-7099.
- Peraica M, Ferencic Z, Fuchs R, Cvoriscec D, Domijan AM, Lucic A, Medugorac- Popovski M, Radic B: Single and multiple doses of ochratoxin A cause apoptosis in kidney tubular epithelium of rat. Rev Med Vet 1998, 149: 650. (sourced electronically via The British Library).
- Mantle PG, McHugh KM, Adatia R, Gray T, Turner DR: Persistent karyomegaly caused by Penicillium nephrotoxins in the rat. Proc Roy Soc Lond B 1991, 246(1317): 251-259.
- Mantle PG, McHugh KM, Adatia R, Heaton JM, Gray T, Turner DR: Penicillium aurantiogriseum-persistent renal histopathological changes in rats; an experimental model for Balkan endemic nephropathy competitive with ochratoxin A. IARC Sci Publ 1991, 115: 119-127.
- Macgeorge KM, Mantle PG: Nephrotoxic fungi in a Yugoslavian community in which Balkan nephropathy is hyperendemic. Mycol Res 1991, 95: 660-664.
- Chan W, Pavlović NM, Li W, Chan CK, Liu J, Deng K, Wang Y, Milosavljević B, Kostić EN: Quantitation of Aristolochic Acids in Corn, Wheat Grain, and Soil Samples Collected in Serbia: Identifying a Novel Exposure Pathway in the Etiology of Balkan Endemic Nephropathy. J Agric Food Chem 2016, 64(29): 5928-5964.
- Lešić T, Vahčić N, Kos I, Zadravec M, Sinčić Pulić B, Bogdanović T, Petričević S, Listeš E, Škrivanko M, Pleadin J: Characterisation of traditional Croatian household-produced dry- fermented sausages. Foods 2020, 9(8): 990.
- Mantle P, Modalca M, Nicholls A, Tatu C, Tatu D, Toncheva D: Comparative 1H NMR metabolic urinalysis of people diagnosed with Balkan endemic nephropathy, and healthy subjects, in Romania and Bulgaria: a pilot study. Toxins 2011, 3(7): 815-833.
- Wang F, Wang S, Wang J, Huang K, Chen G, Peng Y, Liu C, Tao Y: Pharmacological mechanisms of Fuzheng Huaya formula for aristolochic acid 1-involved kidney fibrosis through network pharmacology. Front Pharmacol 2022, 13: 156865.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript