REVIEW | Open Access
Renal Cell Carcinoma with Sarcomatoid and Rhabdoid Dedifferentiation: Clinico Pathological Significance- Review
Sunil V. Jagtap1, Shubham S. Jagtap2, Harshkumar Macchi1, Pranjal Shah1, Devika Borade1
1Department of Pathology, KVV Deemed to be University, Krishna Institute of Medical Sciences, Karad, India.
2Department of Medicine, KVV Deemed to be University, Krishna Institute of Medical Sciences, Karad, India.
Annals of Urologic Oncology 2024, 7(3): 90-96. https://doi.org/10.32948/auo.2024.08.22
Received: 07 Aug 2024 | Accepted: 24 Aug 2024 | Published online: 27 Aug 2024
Case report A 78-year-old male presented with complaints hematuria, pain abdomen, burring maturation of 2 months duration. The patient had a history of hypertension and diabetes mellitus. Computed tomography revealed a well-defined lobulated, exophytic ball-type, isodense lesion, measuring (~ 7.8 x 7.5 x 11.0 cm) arising from mid and lower poles of right kidney involving renal sinus. Features were suggestive of renal cell carcinoma. The patient underwent a right radical nephrectomy. On histopathological findings reported as clear cell RCC, sarcomatoid and rhabdoid dedifferentiation, Fuhrman grade IV. There was no evidence of any metastasis. The immunochemistry was positive for Paired box 8 (PAX8), Carbonic anhydrase 9 (CA9). The BAP1: BRCA1 Associated Protein-1 was retained.
Conclusion Herewith present an uncommon case of highly aggressive tumor clear cell RCC sarcomatoid and rhabdoid differentiation for its clinical, radioimaging, histopathological and immunohistochemitry significance with review.
Key words renal tumors, renal cell carcinoma, rhabdoid differentiation, sarcomatoid differentiation, paired box 8 (PAX8), carbonic anhydrase 9 (CA9)
The sarcomatoid renal cell carcinomas (SRCC) represents 5%–8% of RCCs and may arise from any subtypes of RCC.
Rhabdoid features is observed rarely in adult RCC. Rhabdiod features is noted in various types of high-grade sarcomas, such as synovial sarcomas, extraskeletal myxoid chondrosarcoma, and leiomyosarcoma [2].
Recent molecular and genetic evidence suggest that sarcomatoid component is transformed from a common progenitor of the associated RCC and the TP53 gene plays a pivotal role in this process. The sarcomatoid dedifferentiation in sarcomatoid component has shown increased incidence of alterations in TP53, CDKN2A, and the Hippo pathway.
In the literature clear cell RCC with rhabdoid features histopathologic shows very few reports. Herewith we report a rare case of clear cell RCC, sarcomatoid and rhabdoid dedifferentiation, Fuhrman grade IV in right nephrectomy and literature review.
The patient underwent a right radical nephrectomy. We received a specimen labelled as Right radical nephrectomy with IVC cuff excision, totally measuring 17 x 13 x 9 cm and weighs 750 grams with attached perinephric fat. External surface of the kidney is grey brown, bosselated and covered with intact renal capsule. Cut surface showed a variegated, firm, grey white to yellowish tumor measured 9 x 7 x 5 cm arising in the mid and lower pole of the kidney (Figure 2), with areas of hemorrhage, necrosis, calcification, cystic degeneration, and satellite nodules. On serial sectioning of the perenephric fat it was unremarkable. On histopathology reported as clear cell RCC with sarcomatoid and rhabdoid diiferentiation, 42% and 21% respectively, Fuhrman grade IV (Figure 3, 4, 5). Tumor grade was IV (Grading as per International Society of Urologic Pathology (ISUP). Tumor showed predominantly solid and tubular pattern with extreme nuclear pleomorphism, with prominent nucleoli, scattered multinucleated giant cells. Extensive area of hemorrhage and necrosis noted. Focal proliferation of vascular channels were seen. Tumor is reaching upto renal capsule and renal pelvis. Renal artery, renal vein, perinephric fat, ureter were free from tumor. The immunochemistry was positive for PAX8, Carbonic Anhydrase 9 (CA9). The BAP1: BRCA1 Associated Protein-1 was retained. In this case right radical nephrectomy was done, the tumor was localised and there was no metastatis. Patient was advised regular follow up.
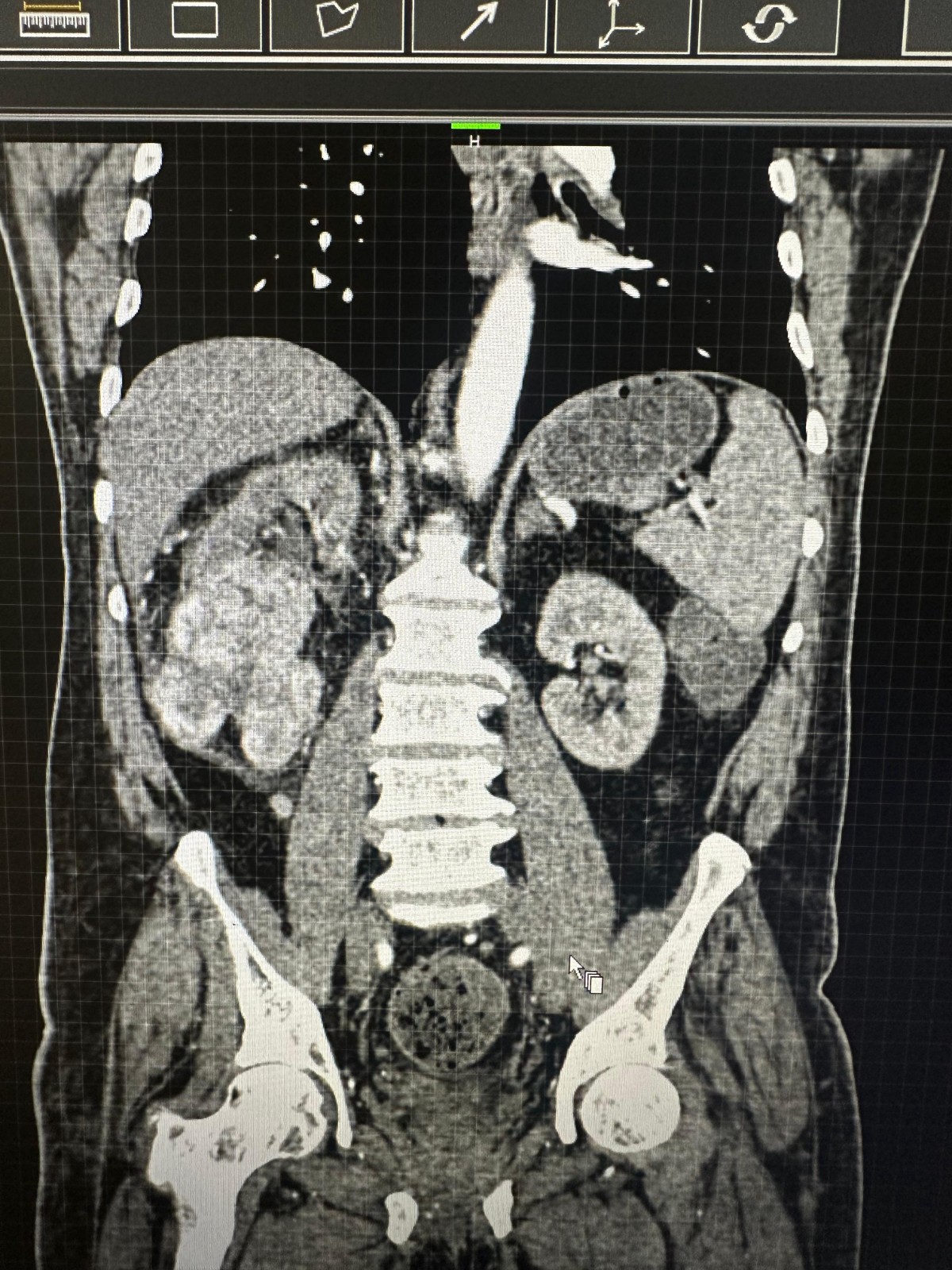 Figure 1. CT scan abdomen pelvis showing well-defined lobulated, exophytic, isodense lesion arising from mid and lower poles of right kidney.
Figure 1. CT scan abdomen pelvis showing well-defined lobulated, exophytic, isodense lesion arising from mid and lower poles of right kidney.
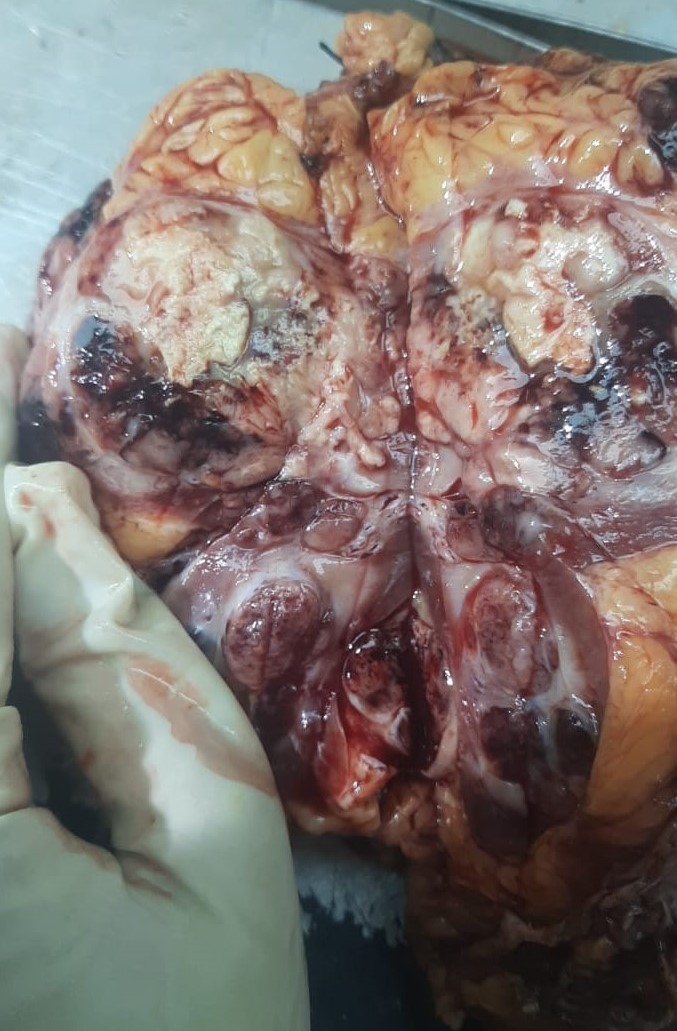 Figure 2. Right radical nephrectomy specimen showing tumor measured 9 x7 x 5 (cm). The cut surface revealed a variegated, solid, firm, greyish white mass with areas of cysts, hemorrhage and necrosis.
Figure 2. Right radical nephrectomy specimen showing tumor measured 9 x7 x 5 (cm). The cut surface revealed a variegated, solid, firm, greyish white mass with areas of cysts, hemorrhage and necrosis.
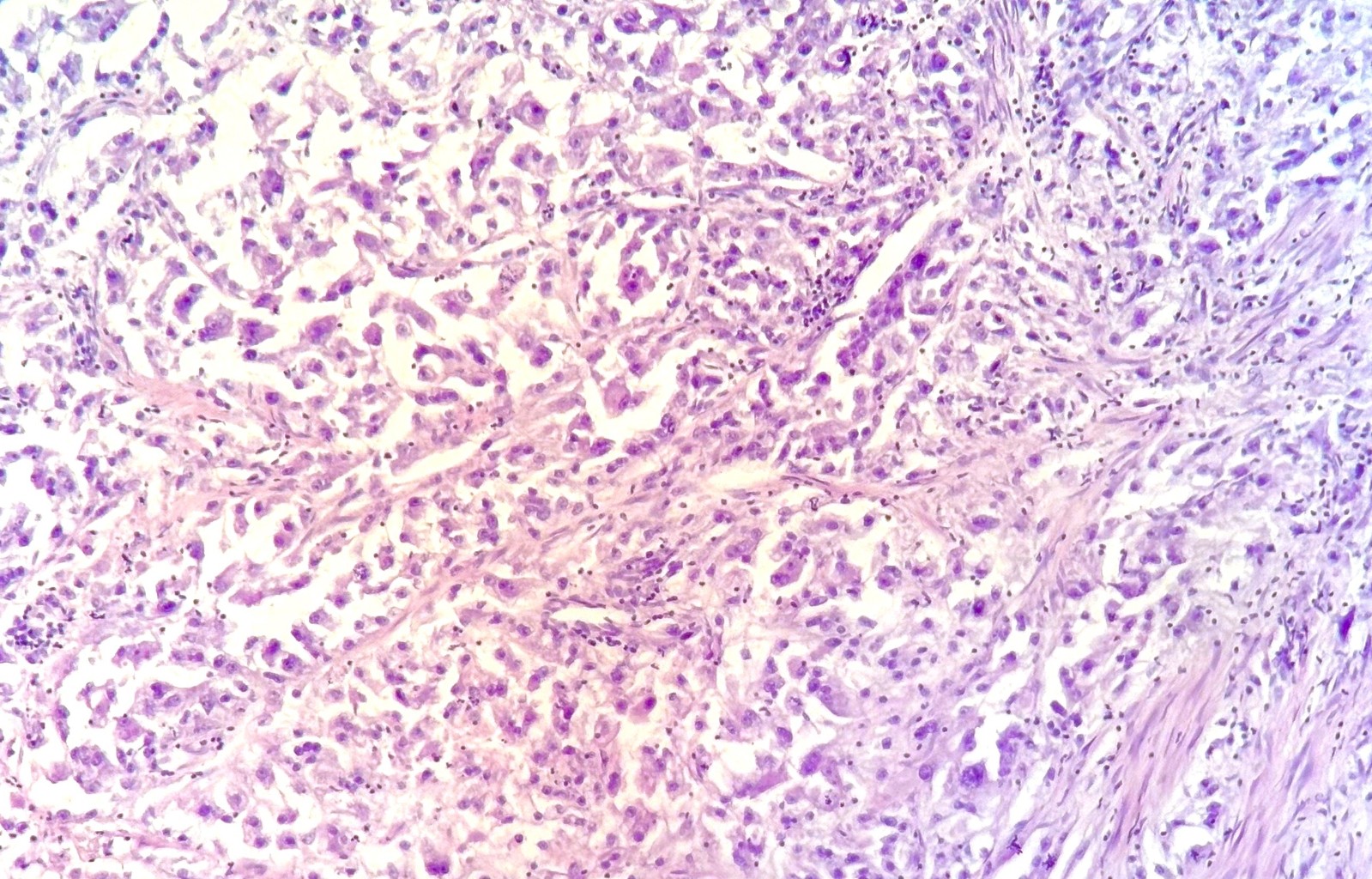 Figure 3. Photomicrograph showing clear cell renal cell carcinoma with spindle cell sarcomatoid differentiation, (Hematoxylin–Eosin stain, 40×).
Figure 3. Photomicrograph showing clear cell renal cell carcinoma with spindle cell sarcomatoid differentiation, (Hematoxylin–Eosin stain, 40×).
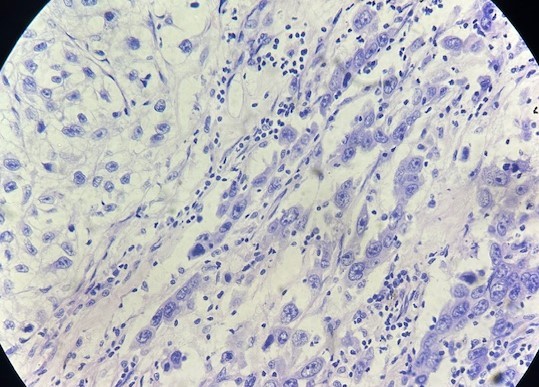 Figure 4. Photomicrograph showing clear cell renal cell carcinoma with spindle cell sarcomatoid differentiation, (Hematoxylin–Eosin stain, 100×).
Figure 4. Photomicrograph showing clear cell renal cell carcinoma with spindle cell sarcomatoid differentiation, (Hematoxylin–Eosin stain, 100×).
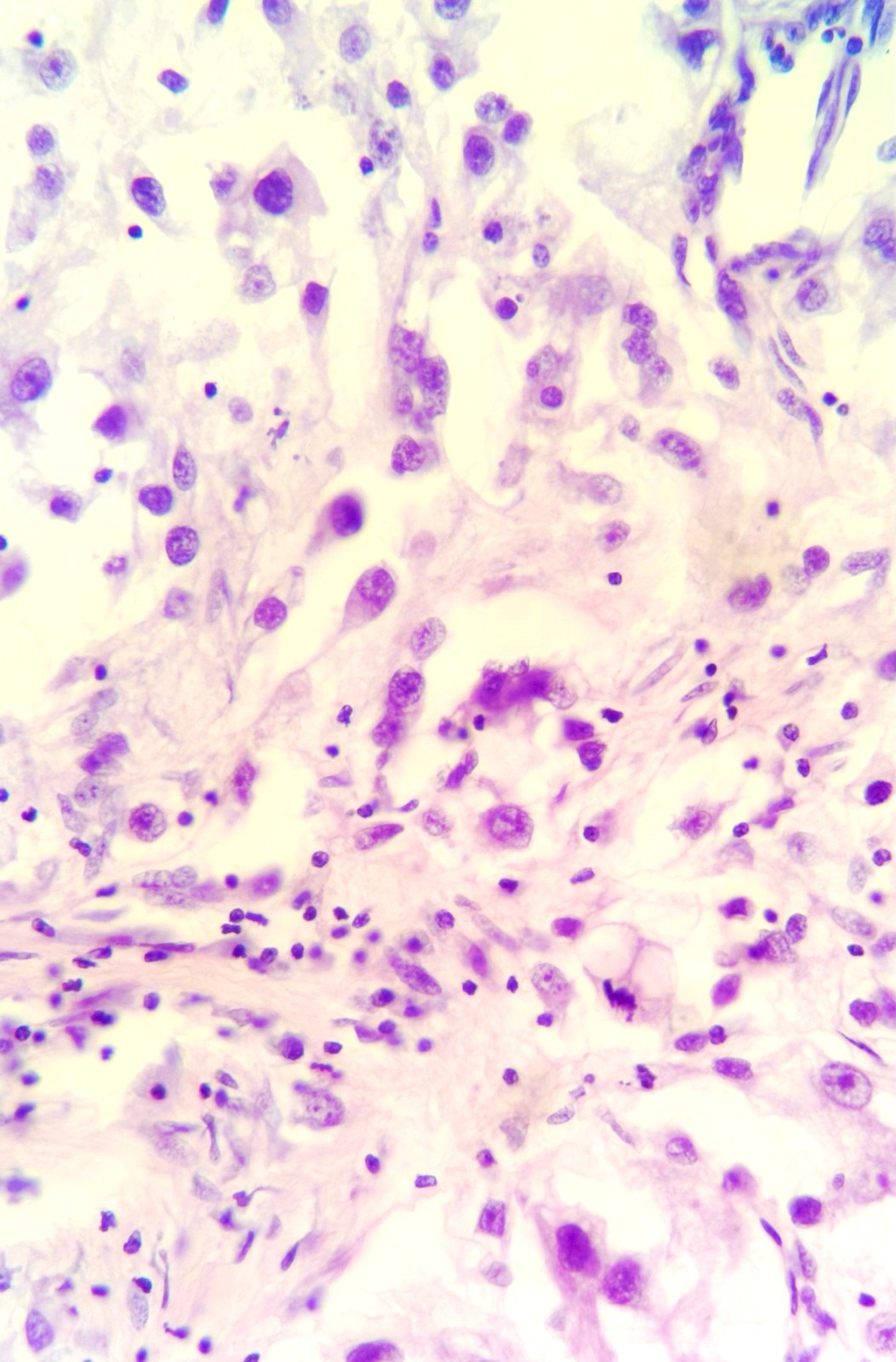 Figure 5. RCC with rhabdoid differentiation, (Hematoxylin–Eosin stain, 100×).
Figure 5. RCC with rhabdoid differentiation, (Hematoxylin–Eosin stain, 100×).
RCC with sarcomatoid differentiation
These typically has biphasic features with an epithelial component and a sarcomatoid component. The tumor shows an atypical, spindle cells with marked nuclear pleomorphism. Scattered tumor giant cells may be noted.
The areas of sarcomatoid dedifferentiation may be heterogenous or uniform and may display fibrosarcoma-like, pleomorphic undifferentiated sarcoma. RCC with sarcomatoid differentiation more frequently has larger tumor size, higher risk of necrosis and higher tumor stage and histopathological grade [5].
Cheville JC, et al. done an analysis of associations with patient outcome, observed that the presence of sarcomatoid RCC is significantly associated with poor outcome even in Fuhrman grade IV clear cell RCC [6].
de Peralta-Venturina et al. noted that the amount of sarcomatoid component varied with a mean of 40%–50% [7].
Shuch et al. found that an increased percentage of sarcomatoid component is associated with a worse prognosis [4].
Renal cell carcinoma with rhabdoid differentiation: (RCC-R)
In the literature there are 280 reported cases of RCC-R. This is second case of RCC-R reported from Indian subcontinent.
It is a recently described variant of RCC. Rhabdoid morphology is classically defined as sheets clusters or discohesive rhabdoid cells of variably cohesive, large epithelioid cells with eccentrically placed vesicular nuclei, prominent nucleoli and large paranuclear intracytoplasmic inclusions with densely eosinophilic cytoplasm. Abrupt transition between conventional clear cell carcinoma and rhabdoid morphology is noted on microscopy. Rhabdoid dedifferentiation was observed in 5% of patients with RCC in a pathologic series [8].
Rhabdoid differentiation refers to the development of cancer cells that resemble rhabdomyoblasts and may involve anywhere from 5% to 90% of the tumor. Rhabdoid dedifferentiation has many parallels to sarcomatoid differentiation, but it is a distinct entity with significantly less known about the biology and therapeutic implications.
IHC and Molecular study
In ccRCC, rhabdoid differentiation is most frequently observed. Also noted in a variety of variant histologic subtypes, including papillary, collecting duct carcinoma, and chromophobe [9]. Majority of cases positive for vimentin, EMA, pan CK, p53, CAIX, PAX2, PAX8, CD10, NSE, PAS. RCC with rhabdoid features shows an aggressive neoplasm with overexpression of p53 [10].
Recently, Przybycin et al. presented a clinicopathologic analysis of the largest series (76 cases) of RCC with rhabdoid differentiation and noted rhabdoid differentiation is associated with aggressive behavior in renal cell carcinoma [11].
Clear cell renal cell carcinoma with rhabdoid differentiation shows metastases in up to 70% of cases, and the cancer specific mortality rate is 40% to 50%.
In our case cc RCC with both sarcomatoid and rhabdoid dedifferentiation was noted 32% and 25% respectively.
Grade 4 is considered in any of the three following features: (a) presence of tumor giant cells and/or marked nuclear pleomorphism, (b) sarcomatoid carcinoma, or (c) carcinoma showing rhabdoid differentiation.
The differential diagnoses for RCC with sarcomatoid differentiation are pleomorphic undifferentiated sarcoma, primary renal leiomyosarcoma, fibrosarcoma, and malignant peripheral nerve sheath tumor [12].
The immunohistochemistry markers associated with a renal origin of the tumor includes CD10, CAIX and PAX8 are usually observed in sarcomatoid RCC. On immunohistochemistry epithelial markers in tumor cells are positive for cytokeratin AE1, AE3, and Vimentin strongly and diffusely positive in clear cell carcinoma as well as in spindle cell and rhabdoid components. It is important to note that sarcomatoid transformation can lead to loss of renal cell carcinoma markers, particularly CAIX, CD10 and PAX8.
Patients with renal cell carcinoma with sarcomatoid dedifferentiation shows genomic characterization of alterations in TP53 (42.3%), CDKN2A (26.9%), and NF2 (19.2%) more common than in ccRCC without dedifferentiation, and TP53 and NF2 alterations were mutually exclusive [13].
Treatment update and prognosis
The treatment approach to sarcomatoid and rhabdoid differentiation RCC has changed dramatically over the last decades. As sarcomatoid and rhabdoid differentiation both have a poor prognosis and are considered World Health Organization (WHO)/International Society of Urological Pathology (ISUP) as grade 4 neoplasm [14]. In patients diagnosed at an early stage, surgical intervention remains the treatment of choice. The sarcomatoid differentiation has specific therapeutic options like cytotoxic chemotherapy, targeted therapy, or immune checkpoint therapy.
M. Alevizakos' study showed that the patients with sarcomatoid dedifferentiation, the 5-year disease-specific survival was 77.7%, 67.8%, 35.4%, and 3.5% for stage I, II, III, and IV disease at diagnosis [15].
The presence of even a small component of sarcomatoid differentiation was shown to independently predict poor survival. Sarcomatoid dedifferentiation treated with surgical procedure of nephrectomy. The recurrence rates are high even with optimal surgical management in patients with localized disease. Toni K. Choueiri et al. noted that the pembrolizumab treatment led to a significant improvement in disease-free survival who were at high risk for recurrence in RCCs [16, 17]. Recent data suggest sarcomatoid or rhabdoid differentiation RCC is especially responsive to immune checkpoint inhibitor based therapies.
A study by Motzer RJ, et al. showed that immune checkpoint therapy has drastically changed the treatment landscape for metastatic RCCs. It consists of immune checkpoint therapy combinations, particularly nivolumab plus ipilimumab which has shown remarkable responses [18].
Pichler R, et al. suggested new therapeutic hope strategies include vascular endothelial growth factor (VEGF)-targeted monotherapy and combined strategies with sunitinib plus gemcitabine or gemcitabine plus doxorubicin [19]. The immunohistochemistry for BAP1 serves as an adverse clinicopathological features [20]. BAP1 loss was associated with high tumor necrosis, high Fuhrman grade of tumor, advanced pT stage, sarcomatoid dedifferentiation. In our case showed all these features.
Genomic analyses: Loss of 11p was specific for rhabdoid differentiation, with loss found in 29.4% of rhabdoid components compared with 0% of clear cell areas [21]. Molecular study shows inactivation /mutations located on chromosome 3p, tumor suppressor genes BAP1 or PBRM1 has been reported in a few cases of RCCs with rhabdoid features [22].
Sarcomatoid RCCs shown to have higher PD1 and PDL1 expression than other subtypes of RCCs. The key genomic aberrations e.g., TP53, CDKN2A, copy number changes present in sRCC which explain its aggressive clinical course and may become potential targets for therapy [23]. The nephrectomy (Table 1) and newer combinations of immune checkpoint inhibitor immunotherapies particularly nivolumab plus ipilimumab, are suggested to improved treatment responses and overall outcomes [24-34].
|
Table 1. Review cases of clear cell renal cell carcinoma with rhabdoid differentiation. |
|||||||||
|
Authors |
Cases |
Duration |
Histopathological diagnosis |
Age (yr.) |
Male /Female |
Renal Side; Right/left |
Main clinical presentation |
Surgical procedure |
ISUP grade of RCC |
|
Przybycin, Christopher G, et al. [11] |
76 |
2014 |
cRCC with rhabdoid differentiation |
- |
- |
- |
flank pain, hematuria, wt loss |
Nephrectomy |
3-4 |
|
Milena PotićFloranović, et al. [24] |
2 |
2020 |
papillary RCC rhabdoid differentiation and cRCC |
83 and 63 |
male |
right |
abdominal pain and microscopic haematuria |
Radical nephrectomy |
2 and 4 |
|
XiaoqunYang, et al. [25] |
10 |
2015 |
cRCC with rhabdoid differentiation |
51-77 |
7 / 3 |
6/4 |
flank pain |
Radical nephrectomy |
3-4 |
|
Zhang BY, et al. [26] |
111 |
2015 |
RCC with rhabdoid differentiation |
- |
- |
- |
flank pain, haematuria, abdominal pain |
Radical nephrectomy |
4 |
|
Carmen M. Perrino, et al. [27] |
60 |
Jan1989 to Aug 2013 |
RCC with rhabdoid differentiation |
42 to 73 |
30/30 |
- |
haematuria, abdominal pain |
Nephrectomy |
3-4 |
|
Krishnamoorthy V, et al. [28] |
1 |
2016 |
cRCC with rhabdoid differentiation |
52 |
male |
left |
weight loss, hypertensive |
Lower pole partial nephrectomy |
4 |
|
Arash Samiei, et al. [29] |
36/12 |
2008 to 2016 |
RCC with rhabdoid and sarcomatoid differentiation |
47-88 |
8/4 |
- |
haematuria, abdominal pain, wt loss |
Nephrectomy and ICI and TKI
|
4 |
|
Xavier Leroy, et al. [30] |
14 |
1999 -2005 |
adult RCC with rhabdoid features |
32-77 |
6/8 |
8/6 |
flank discomfort, hematuria |
Radical nephrectomy |
3/4 |
|
B. Shannon, et al. [31] |
1 |
2003 |
chromophobe RCC with rhabdoid differentiation |
76 |
male |
right |
right flank pain |
Radical nephrectomy |
3 |
|
S Fukata, et al. [32] |
1 |
2009 |
RCC with rhabdoid and sarcomatoid change |
54 |
female |
left |
left flank pain and macrohematuria |
Left radical nephrectomy |
4 |
|
Divya A, et al. [33] |
2 |
2016 |
cRCC with rhabdoid differentiation |
59 and 65 |
male |
right |
loss of appetite, pain abdomen, haematuria |
Radical Nephrectomy |
3/4 |
|
Ryuji Matsumoto, et al. [34] |
2 |
2015 |
cRCC with rhabdoid features |
76 and 76 |
male and female |
right |
hematuria |
Radical Nephrectomy |
4 |
|
Sunil V Jagtap (present case) |
1 |
2024 |
cRCC with rhabdoid and sarcomatoid differentiation |
78 |
male |
right |
hematuria, pain, and burring maturation |
Radical Nephrectomy |
4 |
|
cRCC: clear cell Renal Cell Carcinoma, ISUP: International Society of Urological Pathology, ICI: Immune checkpoint inhibitors, TKI: Tyrosine kinase inhibitors.
|
|||||||||
Not applicable.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
That data is available from the corresponding author on request.
Author contributions
SVJ, SSJ: conceptualisation, data curation, formal analysis, writing original draft supervision, review and editing; HM: conceptualisation, formal analysis, review and editing; PS, DB: resources, data curation, supervision, review and editing.
Competing interests
The authors have no conflicts of interest to declare.
Funding
None.
- Siegel RL, Giaquinto AN, Jemal A: Cancer statistics, 2024. CA Cancer J Clin 2024, 74(1): 12-49.
- Mauricio A, Paláu L, Pham Tina Thu, Barnard Nicola, Merino Maria J: Primary sarcoma of the kidney with rhabdoid features. Int J Surg Pathol 2007, 15(4): 421-28.
- Farrow GM, Harrison EG Jr., Utz DC: Sarcomas and sarcomatoid and mixed malignant tumors of the kidney in adults. Cancer 1968, 22(3): 556-563.
- Shuch B, Said J, La Rochelle JC, Zhou Y, Li G, Klatte T, Kabbinaavar FF, Pantuck AJ, Belldegrun AS: Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology – Is up-front resection indicated and, if not, is it avoidable? J Urol 2009, 182(5): 2164-2171.
- Gu L, Li H, Wang H, Ma X, Wang L, Chen L, Zhao W, Zhang Y, Zhang X: Presence of sarcomatoid differentiation as a prognostic indicator for survival in surgically treated metastatic renal cell carcinoma. J Cancer Res Clin Oncol 2017, 143(3): 499-508.
- Cheville JC, Lohse CM, Zincke H, Weaver AL, Leibovich BC, Frank I, Blute ML: Sarcomatoid renal cell carcinoma: An examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol 2004, 28(4): 435-441.
- de Peralta-Venturina M, Moch H, Amin M, Tamboli P, Hailemariam S, Mihatsch M, Javidan J, Stricker H, Ro JY, Amin MB: Sarcomatoid differentiation in renal cell carcinoma: A study of 101 cases. Am J Surg Pathol 2001, 25(3): 275-284.
- Gökden N, Nappi O, Swanson PE, Pfeifer JD, Vollmer RT, Wick MR, Humphrey PA: Renal cell carcinoma with rhabdoid features. Am J Surg Pathol 2000, 24(10): 1329-1338.
- SV Jagtap, A Beniwal, SS Jagtap, A Huddedar: Papillary Renal Cell Carcinoma Type-II: A Distinct clinicopathological Subtype of Renal Epithelial Neoplasm. Ann Pathol Lab Med 2016, 03(06): (Suppl) 279-282.
- Leroy X, Zini L, Buob D, Ballereau C, Villers A, Aubert S: Renal cell carcinoma with rhabdoid features: an aggressive neoplasm with overexpression of p53. Arch Pathol Lab Med 2007, 131(1): 102-106.
- Przybycin CG, McKenney JK, Reynolds JP, Campbell S, Zhou M, Karafa MT and Magi-Galluzzi C: Rhabdoid differentiation is associated with aggressive behavior in renal cell carcinoma: a clinicopathologic analysis of 76 cases with clinical follow-up. Am J Surg Pathol 2014, 38(9): 1260-1265.
- Jagtap SV, Jagtap S S, Agarwal G, Huddedar A: Leiomyosarcoma of the Renal Pelvis: Rare Mesenchymal Tumor. Journal of Datta Meghe Institute of Medical Sciences University 2021, 16(4): 746-748.
- Malouf GG, Ali SM, Wang K, Balasubramanian S, Ross JS, Miller VA, Stephens PJ, Khayat D, Pal SK, Su X, et al: Genomic characterization of renal cell carcinoma with sarcomatoid dedifferentiation pinpoints recurrent genomic alterations. Eur Urol 2016, 70(2): 348-357.
- Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch H, Grignon DJ, et al: The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 2013, 37(10): 1490-1504.
- Alevizakos M, Gaitanidis A, Nasioudis D, Msaouel P, Appleman LJ: Sarcomatoid renal cell carcinoma: population-based study of 879 patients. Clin Genitourin Cancer 2019,17(3): e447-e53.
- Andrew W. Hahn, Justin Lebenthal, Giannicola Genovese, Kanishka Sircar, Nizar M. Tannir, Pavlos Msaouel: The significance of sarcomatoid and rhabdoid dedifferentiation in renal cell carcinoma. Cancer Treat Res Commun 2022, 33: 100640.
- Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, Hajek J, Symeonides SN, Lee JL, Sarwar N, et al: Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021, 385(8): 683-694.
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, et al: Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018, 378(14): 1277-1290.
- Pichler R, Compérat E, Klatte T, Pichler M, Loidl W, Lusuardi L, Schmidinger M: Renal Cell Carcinoma with Sarcomatoid Features: Finally New Therapeutic Hope? Cancers (Basel) 2019, 11(3): 422.
- Kapur P, Christie A, Raman JD, Then MT, Nuhn P, Buchner A, Bastian P, Seitz C, Shariat SF, Bensalah K, et al: BAP1 immunohistochemistry predicts outcomes in a multi-institutional cohort with clear cell renal cell carcinoma. J Urol 2014, 191(3): 603-610.
- Perrino CM, Hucthagowder V, Evenson M, Kulkarni S, Humphrey PA: Genetic alterations in renal cell carcinoma with rhabdoid differentiation. Hum Pathol 2015, 46(1): 9 -16.
- Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, et al: BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 2012, 44(7): 751-759.
- Blum KA, Gupta S, Tickoo SK, Chan TA, Russo P, Motzer RJ, Karam JA, Hakimi AA: Sarcomatoid renal cell carcinoma: biology, natural history and management. Nat Rev Urol 2020, 17(12): 659-678.
- Milena Potić-Floranović, Ana Ristić-Petrovi, Filip Veličković, Milan Potić, Tanja Džopalić1, Ljubinka Janković-Veličković: Rhabdoid differentiation in different types of renal cell carcinoma: A Report of Two Cases and Literature Review. Acta facultatis medicae Naissensis 2020, 37(1): 79-86.
- Yang X, Xi C, Jin J, Zhou L, Su J, Liu L, Liu Y: Adult renal cell carcinoma with rhabdoid differentiation: incidence and clinicopathologic features in Chinese patients. Ann Diagn Pathol 2015, 19(2): 57-63.
- Zhang BY, Cheville JC, Thompson RH, Lohse CM, Boorjian SA, Leibovich BC and Costello BA: Impact of rhabdoid differentiation on prognosis for patients with grade 4 renal cell carcinoma. Eur Urol 2015, 68(1): 5-7.
- Perrino Carmen M, Vishwanathan H, Michael E, Shashikant K, Peter A: Genetic alterations in renal cell carcinoma with rhabdoid differentiation. Human Pathology 2015, 46(1): 9-16.
- Krishnamoorthy V, Gowda KK, Rao RN: Incidentally detected clear cell renal cell carcinoma with rhabdoid differentiation. Indian J Pathol Microbiol 2016, 59(4): 507-509.
- Arash S, Pritam T, Angela S, Ralph M, John L, Jeffrey C, Shifeng M: The clinical outcome of renal cell carcinoma with rhabdoid and sarcomatoid differentiation. J Clin Oncol 2019, 37: 15: suppl- e16083.
- Leroy X, Zini L, Buob D, Ballereau C, Villers A, Aubert S: Renal Cell Carcinoma With Rhabdoid Features: An Aggressive Neoplasm With Overexpression of p53. Arch Pathol Lab Med 2007, 131(1): 102-106.
- Shannon BA, Cohen RJ: Rhabdoid differentiation of chromophobe renal cell carcinoma. Pathology 2003, 35(3): 228-230.
- Fukata S, Karashima T, Toi M, Kuroda N, Shuin T: Renal cell carcinoma with rhabdoid features and sarcomatoid change: a case report. Hinyokika kiyo 2010, 56(4): 221-223.
- Divya AA, Siddhi GS, Avinash RJ, Pallavi DB: Renal cell carcinoma with rhabdoid differentiation: A novel entity with immunohistochemical study. Indian J Pathol Microbiol 2016, 59(4): 565-567.
- Matsumoto R, Shinohara N, C-Hatanaka K, Kuroda N, Tsuchiya K, Maruyama S, Abe T, Nonomura K: Concurrent occurrence of renal cell carcinoma with rhabdoid features in a married couple: a case report. BMC Res Notes 2015, 8: 3.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript