Review Article | Open Access
Novel Therapeutic Strategies for BCG-unresponsive Non-muscle Invasive Bladder Cancer
Peng Zhang1, Yi Ding2
1Faculty of Medicine, Wuhan Polytechnic University, Wuhan City, Hubei Province, 430000, PR. China.
2Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi'an City, Shaanxi Province, China.
Correspondence: Peng Zhang (Faculty of Medicine, Wuhan Polytechnic University, Wuhan City, Hubei province, PR China, 430000; Email: monkeyzhangpeng@163.com).
Annals of Urologic Oncology 2022; 5(2): 59-67. https://doi.org/10.32948/auo.2022.11.27
Received: 10 Nov 2022 | Accepted: 25 Nov 2022 | Published online: 29 Nov 2022
Key words BCG-unresponsive NMIBC, Intravesical chemotherapy, immunity inhibitors, PD-1 inhibitory
Next, we review recent therapeutic options that immunotherapy, intravesical chemotherapy, gene therapy, and targeted individualized therapy for BCG-unresponsive NMIBC.
Gemcitabine is effective in inhibiting DNA synthesis and is commonly administrated in systemic chemotherapy for NMIBC [14]. Its single postoperative dose setting and combined regimens as a current clinical management strategy has been used for NMIBC patients [13, 15]. As early as in 2006, Dalbagni et al. found that two courses of intravesical gemcitabine is effective in the control of tumor progression in patients with superficial bladder cancer refractory or intolerant to intravesical BCG therapy and unwilling to undergo cystectomy [16]. The study comprises of 30 patients with the encouraging results [16]. Another randomized controlled trial conducted with 40 patients in each group of gemcitabine versus second BCG induction for the treatment of recurrent NMIBC after initial BCG induction shows gemcitabine to have a significantly improved recurrence-free survival (RFS) compared with a second induction course of BCG [17].
Docetaxel
In 2006, the first clinical trial reported 18 cases with at least one prior induction course of BCG in patients that experienced recurrent Ta, T1, or carcinoma in situ, with the majority receiving two or more courses of BCG. 10 of 18 patients demonstrated a complete response 12 weeks after initiating therapy of six weekly intravesical instillations of docetaxel after TURBT [18]. Recently, Shantharam et al. performed a case series of study with 13 patients with high-risk NMIBC treated with ≥1 course of intravesical BCG who received salvage intravesical docetaxel and concluded that salvage intravesical docetaxel was well tolerated, and associated with an encouraging initial response rate of 69% and 24-month recurrence-free survival (RFS) of 25% [19]. In a large sample cohort with 54 patients suggests that thirty-two patients (59 %) had a complete initial response 12 week after initiating therapy [20]. One-year and 3-year RFS rate were 40% and 25%, respectively. The long-term 5 years disease-specific survival was 86%, and 5-year overall survival was 69% [20]. In addition, paclitaxel-hyaluronic acid was applied on bladder carcinoma in situ refractory to BCG and satisfactory response rates with minimal toxicity was observed in this clinical trial [21].
IFN-α is a pleiotropic immune modulator that has been demonstrated for its anti-proliferative activity in several preclinical studies. Combination therapy of intravesical IFN-αand BCG for NMIBC has been demonstrated to be more effective than either single agent in animal studies and suggested greater efficacy in clinical studies [22]. A Cochrane systematic review of assessment on the effects of intravesically administered BCG plus IFN-αcompared with BCG alone for treating NMIBC found that there were very low-quality evidence suggesting no clear differences in the recurrence or progression with BCG plus IFN-αcompared with BCG alone for NMIBC patients [23]. Steinberg et al. conducted a study comparing the effects of gemcitabine and docetaxel versus BCG plus interferon in patients with recurrent non-muscle invasive bladder cancer following a single induction course of BCG and their results suggested that patients with recurrent NMIBC after induction BCG had similar oncologic outcomes with induction gemcitabine/docetaxel as repeat induction with reduced dose BCG/IFN [24]. On these reports, it is anticipated that the effectiveness of BCG plus IFN-α on BCG-unresponsive NMIBC is need to be identified further.
Device-assisted chemotherapy instillation
Device-assisted chemotherapy instillation as a therapy for BCG-unresponsive NMIBC was reported in some clinical studies. Such as radiofrequency-induced thermos-chemotherapeutic effect (RITE), conductive hyperthermic chemotherapy (HCT), and electromotive drug administration (EMDA) were demonstrated as a promising attractive alternative to BCG therapy [25]. The theoretical basis is that high temperatures may enhance drug function by encouraging tumor cells to absorb more chemotherapeutic agents, redistributing their intracellular concentrations, altering metabolic patterns, and/or inhibiting repair of DNA damage [26]. Hyperthermia combined with intravesical chemotherapy has been applied to increase the effects of chemotherapy because of increased temperature leading to an enhanced blood perfusion and cell permeability, in which allows an increased MMC uptake [27]. A randomized controlled study by Tan et al. found that there was no significant difference in disease-free survival time (DFS) between RITE and institutional standard second-line therapy (control) in NMIBC patients with recurrence following BCG induction/maintenance [28]. Thermo-chemotherapy was reported there being an efficacy in patients refractory to intravesical therapies before considering early cystectomy [29]. Racioppi et al. conducted a clinical study on electroMotive drug administration (EMDA) of Mitomycin C (MMC) for high-risk “BCG failure” NMIBC with 3 years follow-up outcomes and the survival curves showed statistically significant differences (p value <0.05) [30]. The authors assumed that the EMDA®-MMC could be considered safe and effective in high-risk NMIBC unresponsive to BCG, as a “bladder sparing” therapy in selected patients [30]. The study on the assessment of effectiveness of Hyperthermic IntraVEsicalChemotherapy (HIVEC®) in BCG unresponsive NMIBC patients got a positive outcome that HIVEC® seems a valid treatment option for BCG unresponsive NMIBC patients [31]. A previous systematic review in 2011 on the combination treatment of intravesical chemotherapy and hyperthermia suggest that a 59% relative reduction in NMIBC recurrence when chemohyperthermia (C-HT), compared with MMC alone [32].
Photodynamic therapy
Photodynamic therapy (PDT) interacting between absorbed light and a retained photosensitizing agent to destroy tissue has been applied to treat NMIBC. PDT with Radachlorin was applied in a prospective, single-arm study with 34 patients and the study result show that recurrence free rates were 90.9% at 12-months, 64.4% at 24 months, and 60.1% at 30 months which suggest that PDT therapy is safe and effective treatment for NMIBC refractory or intolerant to BCG therapy in selected patients [33]. In 2010, a pilot study suggest that PDT using chlorin e6-polyvinylpyrrolidone (Ce6-PVP) was a feasible technique as a bladder sparing therapy for NMIBC refractory to intravesical BCG therapy [34].
Tumor-associated macrophages (TAMs) classified as M1 and M3 on the different polarization play a dual role in bladder cancer [41]. For M1 macrophages and M2 macrophages, the former can be induced by tumor necrosis factor (TNF)-α, interferon- (IF-γ), interleukin (IL)-1β, IL-6, IL-23, lipopolysaccharide (LPS) and suggest an inhibitory action in the initiation and/or progression of bladder cancer [42]. IL-4, IL-10, IL-13, or transforming growth factor-β (TGF-β) can activate the latter M2 macrophages which is associated with the promotion of cancer cell proliferation, migration, invasion, metastasis, and suppression of anti-tumor immune responses. Myeloid-derived suppressor cells (MDSCs) can prevent bladder cancer from attacking by CD4 T-cells, CD8 T-cells, and natural killer cells because of MDSCs from bladder cancer itself suppresses CD4 T-cells, CD8 T-cells, and NK cells. MDSCs can attract CXCL8 (IL-8) and CCL22 which is related with poor prognosis of bladder cancer [42]. Regulatory T-cells (Treg) cells were found higher levels in the peripheral blood of patients with bladder carcinoma, while Th17 cells were enriched in bladder carcinoma tissue. Polyfunctional effector cytokines such as TNF-α and IFN-γ were highly expressed in these Th17 cells in bladder tumor tissue. These evidence suggests that tumor-infiltrating Th17 cells might be functional effector T cells which directly work on the tumor cells. Some cytokines such as IL-17A, IL-17F, IL-21, IL-22, and CCL20 secreted from Th17 cells can promote the proliferation of malignant cells and induce angiogenic constituents via stimulating fibroblasts to upregulate vascular endothelial growth factor (VEGF), resulting in tumor neovascularization [43, 44].
Natural Killer (NK) cells are regarded as cytotoxic lymphocytes which are members of the innate immune system. In these cells, the antigen specificity of T and B cells are lacking but can recognize cells with downregulated human leukocyte antigen (HLA) class I and upregulated markers of cellular stress, such as MICA, MICB, ULBP-1, and the polio virus receptor. Due to such molecular properties on the cell surface suggests that NK cells are important components of both antiviral defense and tumor immunosurveillance [41]. A study by Concepcio?et al. demonstrate that CD226 expression on peripheral blood NK cells improved immunological stratification in intermediate-risk T1-T4 bladder cancer patients [45]. Another molecular biomarker Siglec-7 in blood, urine, and tumors from patients with bladder cancer are found related with poor clinical outcomes and maybe involved in the regulation of antitumor immunity mediated by NK cells in bladder cancer [46].
PD-1 and PD-L1 mediate the anti-tumor effect of the CD4+ T cells and CD8+ T cells [47]. PD-1 expressed in antigen-presenting cells (APC) can combine PD-L1 expressed in T cells, which can have an effect to the control of T cells [48]. CD4+ T cells (especial Th1 ) was regarded as a helper role in the progress of anti-tumor by CD8+ T cells after recognition of tumor antigens presented by MHC class I. CD8+ T cells and CD4+ T cells commonly suggest there were good prognosis in most human cancers while the role of CD4+, CD8+, double positive (DP) T-cells is lacking [49]. There was high expression of PD-1 of the receptor for PD-L1 in the bladder tumor tissue with a lot of CD8 T-cells infiltrating, which suggest an exhausted phenotype feature under antigenic stimulation. CD8+ T cell response is suppressed by immunosuppressive cytokines in multiple immune population and there is a lack of IFN-γand IL-12 in the immunosuppressive environment of the tumor [50]. In addition, PGE2 play an important role in cancer progression, cancer-related immune inflammation and immune evasion, which is secreted from bladder carcinoma tissues due to the high expression of the inducible inflammatory enzyme COX2 [48].
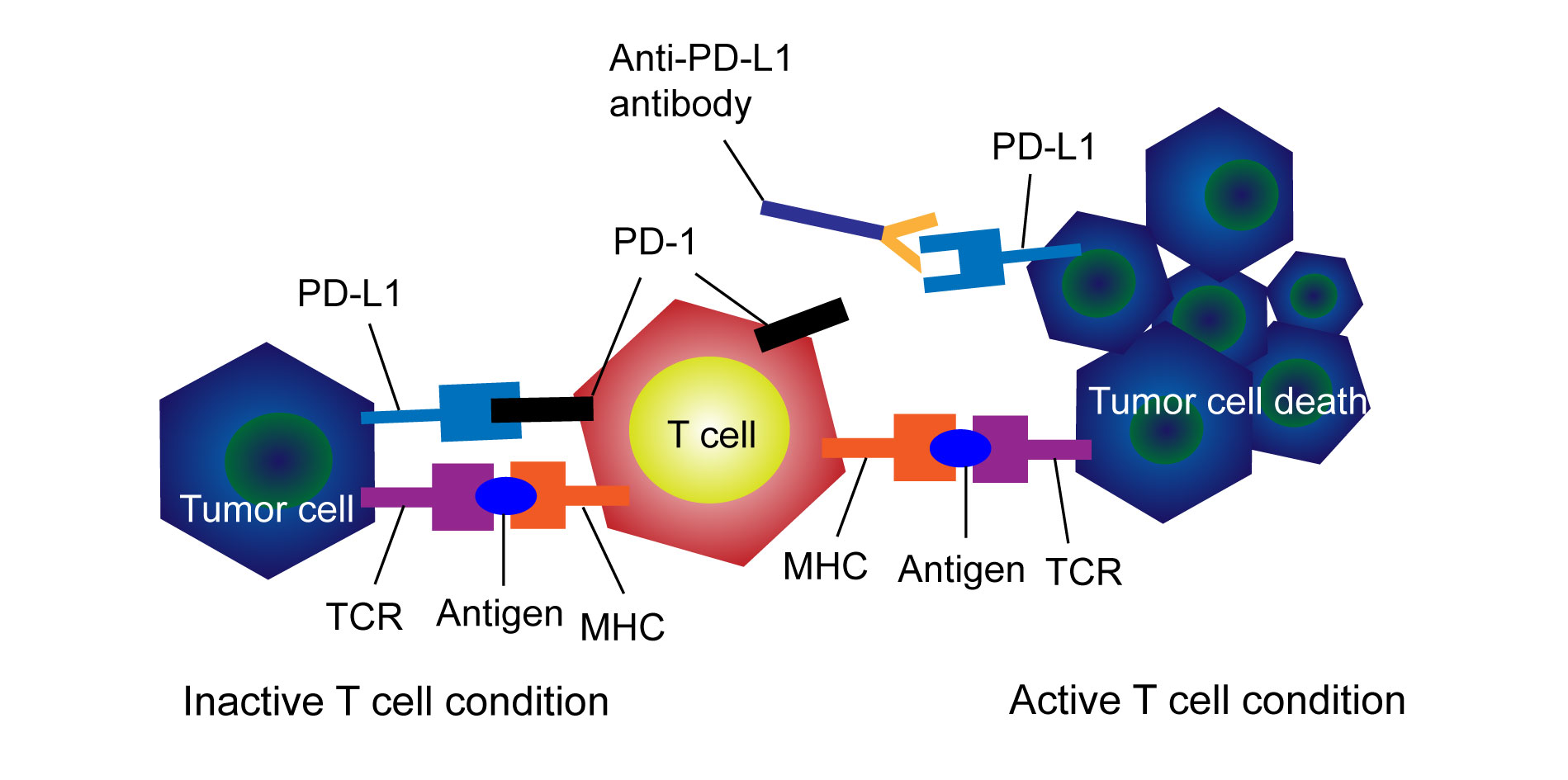 Figure 1. Schematic diagram on the mechanism of programmed death receptor and programmed death receptor ligand (PD-1/PD-L1) signals and Immune checkpoint inhibitors (ICIs). TCR: T cell receptor; MHC: major histocompatibility complex.
Figure 1. Schematic diagram on the mechanism of programmed death receptor and programmed death receptor ligand (PD-1/PD-L1) signals and Immune checkpoint inhibitors (ICIs). TCR: T cell receptor; MHC: major histocompatibility complex.
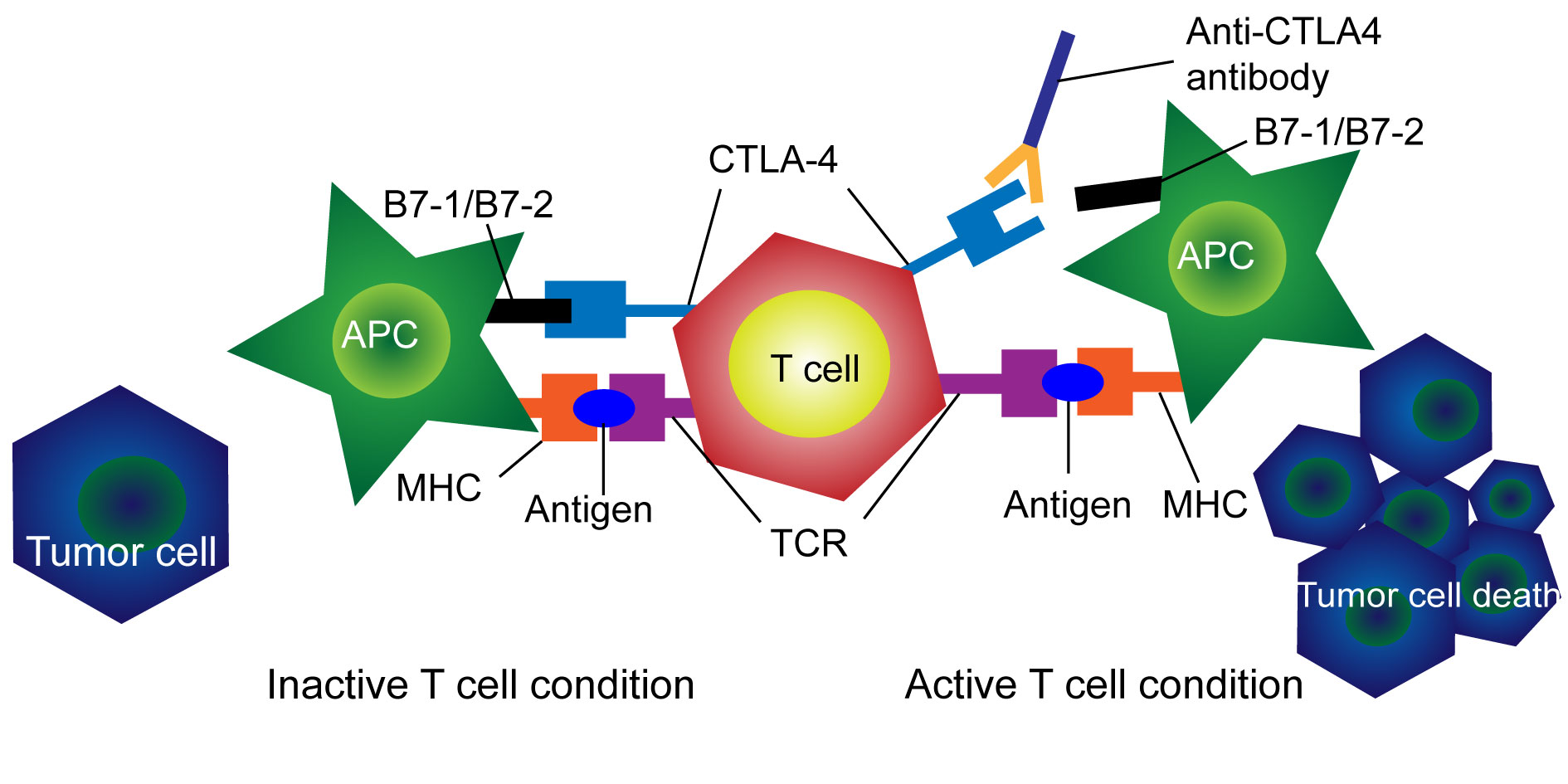 Figure 2. Schematic diagram on the mechanism of CTLA-4 signals and Immune checkpoint inhibitors (ICIs). CTLA-4: cytotoxic T lymphocyte-associated antigen-4; TCR: T cell receptor; MHC: major histocompatibility complex.
Figure 2. Schematic diagram on the mechanism of CTLA-4 signals and Immune checkpoint inhibitors (ICIs). CTLA-4: cytotoxic T lymphocyte-associated antigen-4; TCR: T cell receptor; MHC: major histocompatibility complex.
There are few ICIs approved and are in use in the clinic for bladder cancer [58-61]. In 2016, Atezolizumab as the first PD-L1 inhibitor was approved to treat patients with locally advanced or metastatic bladder cancer who progressed on or after platinum-based chemotherapy or have progressed within 12 months of neoadjuvant or adjuvant treatment with a platinum-containing chemotherapy [58]. Next year, Atezolizumab was approved to treat bladder cancer patients with locally advanced or metastatic disease and who are not eligible for cisplatin-containing therapy [58].
Recently, Pembrolizumab (KEYNOTE-057), Atezolizumab (SWOG S1605), Nivolumab, and Durvalumab were used in the clinical studies on the patients with bladder cancer after BCG failure [62-64]. The single-arm, multicenter phase II trial of KEYNOTE-057 reported that there were three-month complete response rate of 41%, and a median response time of 16.2 months in patients treated with intravenous pembrolizumab 200 mg every three weeks for two years. Complete response case of the primary endpoint is considered as a cystoscopy without evidence of disease and negative cytology. There were 12.7% of 96 patients with Grade 3/4 adverse effects and 8.8% patients abandon treatment due to toxicity. Therefore, pembrolizumab is considered to be a new alternative to cystectomy post BCG failure approved by the FDA in 2020 [65, 66].
In another single-arm multicenter phase II trial of Atezolizumab (SWOG S1605), patients with high-risk BCG-refractory bladder tumors was treated with atezolizumab 1200 mg every three weeks for one year. The primary endpoint is complete response rate at 24 weeks as defined by biopsy, which is stricter compared to trial of KEYNOTE-057. It reported that the complete response rate at three months is 42% nearly with pembrolizumab study. Only 6% of the patients dropped out of the study as a result of adverse events, with 17% of grade 3/4 treatment-related adverse events [62, 67, 68].
A current randomized-controlled trial with 700 participants, CheckMate 7G8 of Nivolumab was used in the patients with high-risk recurrences within 24 months following completion of BCG treatment, excluding BCG unresponsive tumors. Patients with BCG-refractory carcinoma in situ were treated with BCG induction and maintenance for three years plus Nivolumab 480 mg every four weeks for two years. In this trial, the primary end-point is event-free survival (defined as time to recurrence, progression or death) [62, 67]. The results are still awaited for the trial.
CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) function as activation of co-inhibitory receptors of T cells as the immune escape similar to PD-1. Ipilimumab and tremelimumab are combinations with anti-PD(L)1 and anti-CTLA-4 inhibitors which are currently ongoing in clinical trials [37]. Overall, the use of ICIs for BCG failure bladder cancer are promising alternative treatment options which require additional studies.
PANVAC is a recombinant poxviral vaccine expressing tumor associated antigens carcinoembryonic antigen and mucin-1, along with 3 costimulatory molecules. ALT-803 is a recombinant fusion protein with enhanced IL-15 activity which is important for the development and activation of natural killer and CD-8 cells [69, 70]. Two phase II studies are conducted to identify the effect of PANVAC and ALT-803 on high-grade BCG-unresponsive bladder cancer and patients who refuse or are unfit for radical cystectomy (NCT02015104 and NCT01625260) [71, 72].
III-Gene therapy
rAd–IFNα/Syn3 is non-replicating adenovirus vector with the human IFN alpha2b gene. Intravesical administration of the rAd-IFN results in transduction of the virus into the epithelial cell lining in the bladder, leading to the synthesis and expression of large amount of IFN alpha2b protein allowing for high and durable IFNα in urine as a result of IFN alpha2b gene incorporating into the cellular DNA [73]. Until now, two studies (NCT01687244 and NCT02773849) were ongoing on patients with high-grade BCG-refractory or relapsed NMIBC by intravesical rAd–IFNα/Syn3 [74, 75].
CG-0070 which is an oncolytic adenovirus that expresses the immune stimulatory cytokine GM-CSF being administered intravesically as a single-arm intervention in an open-label, phase III study (NCT02365818) [13, 76, 77].
Small-molecule inhibitors of IDO1
As a result of demonstrating the role in tryptophan catabolism, iIndoleamine 2, 3-dioxygenase (IDO), an enzyme of interest in immuno-oncology has shown immunosupressive effects [78]. IDO inhibitors were developed to work on the suppression of CD8+ T effector cells and NK cells as well as increased activity of CD4+ T regulatory cells (Treg) and MDSC [79]. The first developed IDO inhibitors are indoximod, epacadostat, and navoximod [79]. Higher IDO expression levels were found in bladder cancer compared to the noncancerous tissue highlighting their important role in bladder cancer [80]. Currently, there were several IDO inhibitors undergoing a clinical evaluation which are now in phase II clinical trials [81]. IDO inhibitors in combination with other ICIs are currently applied in clinical trials of bladder cancer [82]. Figure 3 shows that currently used novel immunotherapy approaches for NMIBC in clinical trials.
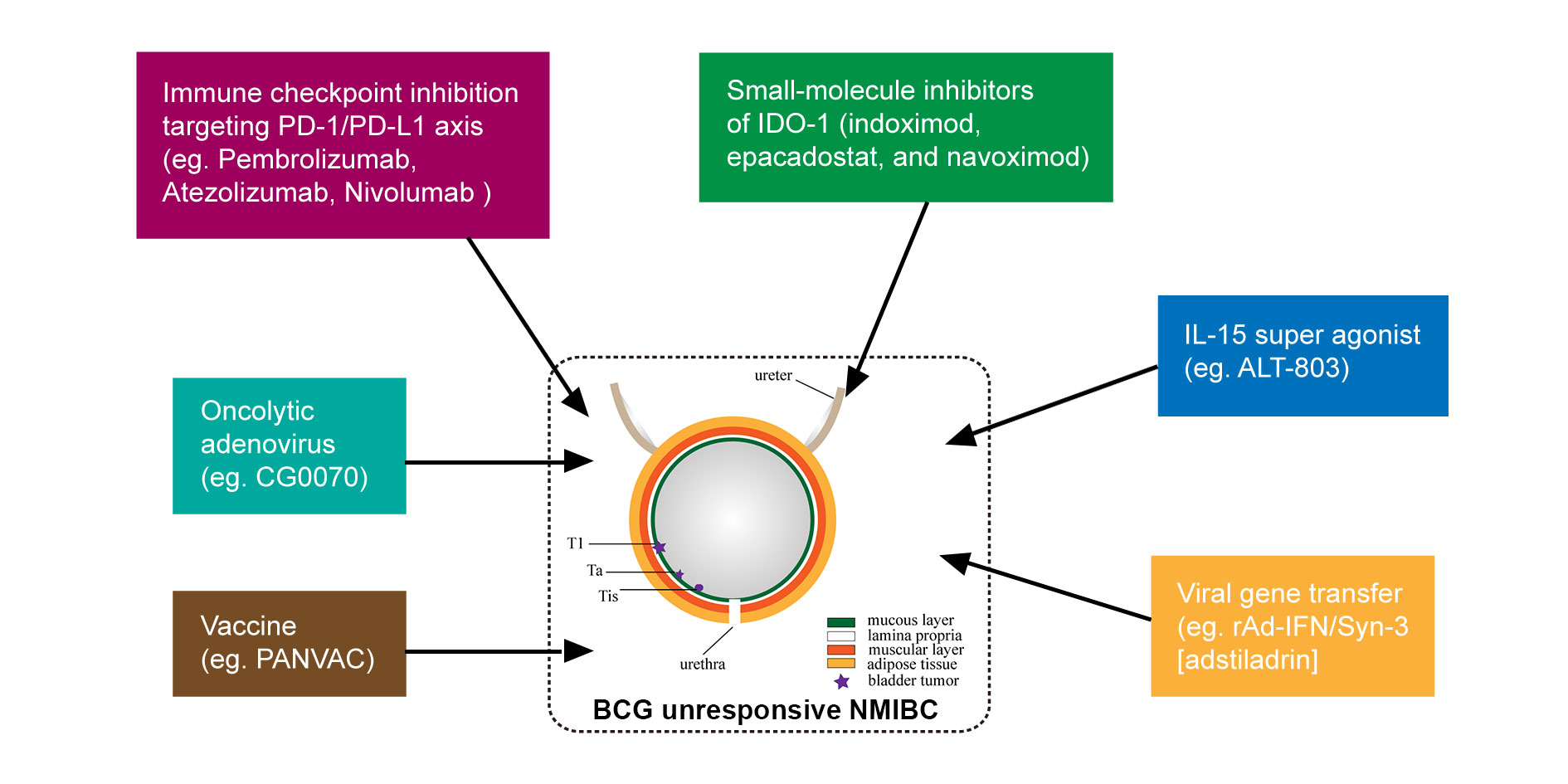 Figure 3. Current used novel immunotherapy approaches for non-muscle invasive bladder cancer (NMIBC) in clinical trials or clinic.
Figure 3. Current used novel immunotherapy approaches for non-muscle invasive bladder cancer (NMIBC) in clinical trials or clinic.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
PZ conceptualised, designed the study and was responsible for the writing of the original draft. PZ and YD reviewed, edited, and approved the final manuscript.
Competing interests
The authors declare no conflict of interest.
Funding
None.
- Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR: The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol 1997, 158(1): 62-67.
- Broughton EI, Gooden KM, Mycock KL, Rajkovic I, Taylor-Stokes G: Multi-country clinical practice patterns, including use of biomarkers, among physicians' treatment of BCG-unresponsive non-muscle invasive bladder cancer (NMIBC). BMC Urol 2022, 22(1): 27.
- Leow JJ, Reese SW, Jiang W, Lipsitz SR, Bellmunt J, Trinh QD, Chung BI, Kibel AS, Chang SL: Propensity-matched comparison of morbidity and costs of open and robot-assisted radical cystectomies: a contemporary population-based analysis in the United States. Eur Urol 2014, 66(3): 569-576.
- Stimson CJ, Chang SS, Barocas DA, Humphrey JE, Patel SG, Clark PE, Smith JA, Jr., Cookson MS: Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol 2010, 184(4): 1296-1300.
- Hounsome LS, Verne J, McGrath JS, Gillatt DA: Trends in operative caseload and mortality rates after radical cystectomy for bladder cancer in England for 1998-2010. Eur Urol 2015, 67(6): 1056-1062.
- Meng MV, Gschwend JE, Shore N, Grossfeld GD, Mostafid H, Black PC: Emerging Immunotherapy Options for bacillus Calmette-Guerin Unresponsive Nonmuscle Invasive Bladder Cancer. J Urol 2019, 202(6): 1111-1119.
- Kamat AM, Lerner SP, O'Donnell M, Georgieva MV, Yang M, Inman BA, Kassouf W, Boorjian SA, Tyson MD, Kulkarni GS et al: Evidence-based Assessment of Current and Emerging Bladder-sparing Therapies for Non-muscle-invasive Bladder Cancer After Bacillus Calmette-Guerin Therapy: A Systematic Review and Meta-analysis. Eur Urol Oncol 2020, 3(3): 318-340.
- Kamat AM, Colombel M, Sundi D, Lamm D, Boehle A, Brausi M, Buckley R, Persad R, Palou J, Soloway M et al: BCG-unresponsive non-muscle-invasive bladder cancer: recommendations from the IBCG. Nat Rev Urol 2017, 14(4): 244-255.
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS Jr, Schellhammer PF: Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007, 178(6): 2314-2330.
- Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Bohle A, Palou Redorta J et al: EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 2013, 64(4): 639-653.
- Nepple KG, Lightfoot AJ, Rosevear HM, O'Donnell MA, Lamm DL, Bladder Cancer Genitourinary Oncology Study G: Bacillus Calmette-Guerin with or without interferon alpha-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol 2010, 184(5): 1915-1919.
- Guidelines EU: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/. Accessed 15 Dec 2020Epub ahead of print.
- Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL, 3rd, Clark W, Kroeger M, Dumbadze I, Chamie K et al: An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol 2018, 36(10): 440-447.
- Han MA, Maisch P, Jung JH, Hwang JE, Narayan V, Cleves A, Hwang EC, Dahm P: Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev 2021, 6(6): CD009294.
- Klaassen Z, Kamat AM, Kassouf W, Gontero P, Villavicencio H, Bellmunt J, van Rhijn BWG, Hartmann A, Catto JWF, Kulkarni GS: Treatment Strategy for Newly Diagnosed T1 High-grade Bladder Urothelial Carcinoma: New Insights and Updated Recommendations. Eur Urol 2018, 74(5): 597-608.
- Dalbagni G, Russo P, Bochner B, Ben-Porat L, Sheinfeld J, Sogani P, Donat MS, Herr HW, Bajorin D: Phase II trial of intravesical gemcitabine in bacille Calmette-Guerin-refractory transitional cell carcinoma of the bladder. J Clin Oncol 2006, 24(18): 2729-2734.
- Di Lorenzo G, Perdona S, Damiano R, Faiella A, Cantiello F, Pignata S, Ascierto P, Simeone E, De Sio M, Autorino R: Gemcitabine versus bacille Calmette-Guerin after initial bacille Calmette-Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer 2010, 116(8): 1893-1900.
- McKiernan JM, Masson P, Murphy AM, Goetzl M, Olsson CA, Petrylak DP, Desai M, Benson MC: Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol 2006, 24(19): 3075-3080.
- Shantharam G, Amin A, Pereira J, Kott O, Mueller-Leonhard C, Mega A, Golijanin D, Gershman B: Intravesical docetaxel for high-risk non-muscle invasive bladder cancer after Bacillus Calmette-Guerin failure. Curr Urol 2021, 15(1): 33-38.
- Barlow LJ, McKiernan JM, Benson MC: Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guerin therapy. J Urol 2013, 189(3): 834-839.
- Bassi PF, Volpe A, D'Agostino D, Palermo G, Renier D, Franchini S, Rosato A, Racioppi M: Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder: results of a phase I study. J Urol 2011, 185(2): 445-449.
- Y Luo XC, T M Downs, W C DeWolf, M A O'Donnell: IFN-alpha 2B enhances Th1 cytokine responses in bladder cancer patients receiving Mycobacterium bovis bacillus Calmette-Guérin immunotherapy. J Immunol 1999, 162(4): 2399-2405.
- Shepherd AR, Shepherd E, Brook NR: Intravesical Bacillus Calmette-Guerin with interferon-alpha versus intravesical Bacillus Calmette-Guerin for treating non-muscle-invasive bladder cancer. Cochrane Database Syst Rev 2017, 3: CD012112.
- Steinberg RL, Packiam VT, Thomas LJ, Brooks N, Vitale A, Mott SL, Crump T, Wang J, DeWolf WC, Lamm DL et al: Intravesical sequential gemcitabine and docetaxel versus bacillus calmette-guerin (BCG) plus interferon in patients with recurrent non-muscle invasive bladder cancer following a single induction course of BCG. Urol Oncol 2022, 40(1): 9 e1-9 e7.
- Tan WS, Kelly JD: Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol 2018, 15(11): 667-685.
- Liu K, Zhu J, Song YX, Wang X, Zhou KC, Lu Y, Liu XQ: Thermal Intravesical Chemotherapy Reduce Recurrence Rate for Non-muscle Invasive Bladder Cancer Patients: A Meta-Analysis. Front Oncol 2020, 10: 29.
- Liem EI, Crezee H, de la Rosette JJ, de Reijke TM: Chemohyperthermia in non-muscle-invasive bladder cancer: An overview of the literature and recommendations. Int J Hyperthermia 2016, 32(4): 363-373.
- Tan WS, Panchal A, Buckley L, Devall AJ, Loubiere LS, Pope AM, Feneley MR, Cresswell J, Issa R, Mostafid H et al: Radiofrequency-induced Thermo-chemotherapy Effect Versus a Second Course of Bacillus Calmette-Guerin or Institutional Standard in Patients with Recurrence of Non-muscle-invasive Bladder Cancer Following Induction or Maintenance Bacillus Calmette-Guerin Therapy (HYMN): A Phase III, Open-label, Randomised Controlled Trial. Eur Urol 2019, 75(1): 63-71.
- Volpe A, Racioppi M, Bongiovanni L, D'Agostino D, Totaro A, D'Addessi A, Marangi F, Palermo G, Pinto F, Sacco E et al: Thermochemotherapy for non-muscle-invasive bladder cancer: is there a chance to avoid early cystectomy? Urol Int 2012, 89(3): 311-318.
- Alfred Witjes J, Hendricksen K, Gofrit O, Risi O, Nativ O: Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: experience of the European Synergo working party. World J Urol 2009, 27(3): 319-324.
- de Jong JJ, Hendricksen K, Rosier M, Mostafid H, Boormans JL: Hyperthermic Intravesical Chemotherapy for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients. Bladder Cancer 2018, 4(4): 395-401.
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, Colombo R: The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol 2011, 60(1): 81-93.
- Lee JY, Diaz RR, Cho KS, Lim MS, Chung JS, Kim WT, Ham WS, Choi YD: Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guerin immunotherapy. J Urol 2013, 190(4): 1192-1199.
- Lee LS, Thong PS, Olivo M, Chin WW, Ramaswamy B, Kho KW, Lim PL, Lau WK: Chlorin e6-polyvinylpyrrolidone mediated photodynamic therapy--A potential bladder sparing option for high risk non-muscle invasive bladder cancer. Photodiagnosis Photodyn Ther 2010, 7(4): 213-220.
- Farina MS, Lundgren KT, Bellmunt J: Immunotherapy in Urothelial Cancer: Recent Results and Future Perspectives. Drugs 2017, 77(10): 1077-1089.
- Mehta K, Patel K, Parikh RA: Immunotherapy in genitourinary malignancies. J Hematol Oncol 2017, 10(1): 95.
- Rouanne M, Roumiguie M, Houede N, Masson-Lecomte A, Colin P, Pignot G, Larre S, Xylinas E, Roupret M, Neuzillet Y: Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways. World J Urol 2018, 36(11): 1727-1740.
- Li F, Guo H, Wang Y, Liu B, Zhou H: Profiles of tumor-infiltrating immune cells and prognostic genes associated with the microenvironment of bladder cancer. Int Immunopharmacol 2020, 85: 106641.
- Fu C, Jiang A: Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front Immunol 2018, 9: 3059.
- Hurwitz AA, Watkins SK: Immune suppression in the tumor microenvironment: a role for dendritic cell-mediated tolerization of T cells. Cancer Immunol Immunother 2012, 61(2): 289-293.
- Bhardwaj N, Farkas AM, Gul Z, Sfakianos JP: Harnessing Natural Killer Cell Function for Genitourinary Cancers. Urol Clin North Am 2020, 47(4): 433-442.
- Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z: Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci 2019, 26(1): 78.
- Chi LJ, Lu HT, Li GL, Wang XM, Su Y, Xu WH, Shen BZ: Involvement of T helper type 17 and regulatory T cell activity in tumour immunology of bladder carcinoma. Clin Exp Immunol 2010, 161(3): 480-489.
- Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, Paulos CM: When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol 2018, 15(5): 458-469.
- Guillamon CF, Gimeno L, Server G, Martinez-Sanchez MV, Escudero JF, Lopez-Cubillana P, Cabezas-Herrera J, Campillo JA, Abellan DJ, Martinez-Garcia J et al: Immunological Risk Stratification of Bladder Cancer Based on Peripheral Blood Natural Killer Cell Biomarkers. Eur Urol Oncol 2021, 4(2): 246-255.
- Benmerzoug S, Chevalier MF, Villier L, Nguyen S, Cesson V, Schneider AK, Dartiguenave F, Rodrigues-Dias SC, Lucca I, Jichlinski P et al: Siglec-7 May Limit Natural Killer Cell-mediated Antitumor responses in Bladder Cancer Patients. Eur Urol Open Sci 2021, 34: 79-82.
- Littman DR: Releasing the Brakes on Cancer Immunotherapy. Cell 2015, 162(6): 1186-1190.
- Crispen PL, Kusmartsev S: Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother 2020, 69(1): 3-14.
- Bohner P, Chevalier MF, Cesson V, Rodrigues-Dias SC, Dartiguenave F, Burruni R, Tawadros T, Valerio M, Lucca I, Nardelli-Haefliger D et al: Double Positive CD4(+)CD8(+) T Cells Are Enriched in Urological Cancers and Favor T Helper-2 Polarization. Front Immunol 2019, 10: 622.
- Joseph M, Enting D: Immune Responses in Bladder Cancer-Role of Immune Cell Populations, Prognostic Factors and Therapeutic Implications. Front Oncol 2019, 9: 1270.
- Pfail JL, Katims AB, Alerasool P, Sfakianos JP: Immunotherapy in non-muscle-invasive bladder cancer: current status and future directions. World J Urol 2021, 39(5): 1319-1329.
- Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED: PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 2007, 109(8): 1499-1505.
- Siefker-Radtke A, Curti B: Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat Rev Urol 2018, 15(2): 112-124.
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL et al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515(7528): 558-562.
- Kawahara T, Ishiguro Y, Ohtake S, Kato I, Ito Y, Ito H, Makiyama K, Kondo K, Miyoshi Y, Yumura Y et al: PD-1 and PD-L1 are more highly expressed in high-grade bladder cancer than in low-grade cases: PD-L1 might function as a mediator of stage progression in bladder cancer. BMC Urol 2018, 18(1): 97.
- Nassar AH, Umeton R, Kim J, Lundgren K, Harshman L, Van Allen EM, Preston M, Dong F, Bellmunt J, Mouw KW et al: Mutational Analysis of 472 Urothelial Carcinoma Across Grades and Anatomic Sites. Clin Cancer Res 2019, 25(8): 2458-2470.
- Pierconti F, Raspollini MR, Martini M, Larocca LM, Bassi PF, Bientinesi R, Baroni G, Minervini A, Petracco G, Pini GM et al: PD-L1 expression in bladder primary in situ urothelial carcinoma: evaluation in BCG-unresponsive patients and BCG responders. Virchows Arch 2020, 477(2): 269-277.
- Inman BA, Longo TA, Ramalingam S, Harrison MR: Atezolizumab: A PD-L1-Blocking Antibody for Bladder Cancer. Clin Cancer Res 2017, 23(8): 1886-1890.
- Gupta S, Gill D, Poole A, Agarwal N: Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions. Cancers (Basel) 2017, 9(2): 15.
- Roviello G, Catalano M, Santi R, Palmieri VE, Vannini G, Galli IC, Buttitta E, Villari D, Rossi V, Nesi G: Immune Checkpoint Inhibitors in Urothelial Bladder Cancer: State of the Art and Future Perspectives. Cancers (Basel) 2021, 13(17): 4411.
- Popovic LS, Matovina-Brko G, Popovic M: Checkpoint inhibitors in the treatment of urological malignancies. ESMO Open 2017, 2(2): e000165.
- Alvarez-Maestro M, Guerrero-Ramos F, Rodriguez-Faba O, Dominguez-Escrig JL, Fernandez-Gomez JM: Current treatments for BCG failure in non-muscle invasive bladder cancer (NMIBC). Actas Urol Esp (Engl Ed) 2021, 45(2): 93-102.
- Meghani K, Cooley LF, Choy B, Kocherginsky M, Swaminathan S, Munir SS, Svatek RS, Kuzel T, Meeks JJ: First-in-human Intravesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guerin. Eur Urol 2022, 82(6): 602-610.
- Kamat AM, Shore N, Hahn N, Alanee S, Nishiyama H, Shariat S, Nam K, Kapadia E, Frenkl T, Steinberg G: KEYNOTE-676: Phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Future Oncol 2020, 16(10): 507-516.
- Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguie M, Krieger LEM, Singer EA, Bajorin DF, Grivas P et al: Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol 2021, 22(7): 919-930.
- Ahdoot M, Theodorescu D: Immunotherapy of High Risk Non-Muscle Invasive Bladder Cancer. Expert Rev Clin Pharmacol 2021, 14(11): 1345-1352.
- Gomez Del Canizo C, Rodriguez-Izquierdo Jimenez M, Pena Vallejo E, Duarte Ojeda JM, de la Rosa Kehrman F, Rodriguez Antolin A, Guerrero Ramos F: New immunotherapies for high-risk non-muscle invasive bladder cancer: Current state and future perspectives. Actas Urol Esp (Engl Ed) 2020, 44(9): 574-585.
- Garcia-Perdomo HA, Sanchez AL, Spiess PE: Immune checkpoints inhibitors in the management of high-risk non-muscle-invasive bladder cancer. A scoping review. Urol Oncol 2022, 40(9): 409 e401-409 e408.
- Huang J, Shiao SL, Furuya H, Rosser CJ: Immunogenomic Analysis of Exceptional Responder to ALT-803 (IL-15 Analogue) in BCG Unresponsive Nonmuscle Invasive Bladder Cancer: A Case Series and Review of the Literature. J Immunother 2019, 42(9): 354-358.
- Huang J, Schisler J, Wong HC, Rosser CJ, Sterbis J: Intravesical ALT-803 for BCG-unresponsive Bladder Cancer - A Case Report. Urol Case Rep 2017, 14: 15-17.
- Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, Remondo C, Cereda V, Jones JL, Pazdur MP et al: Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res 2008, 14(10): 3060-3069.
- Gomes-Giacoia E, Miyake M, Goodison S, Sriharan A, Zhang G, You L, Egan JO, Rhode PR, Parker AS, Chai KX et al: Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS One 2014, 9(6): e96705.
- Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, Bivalacqua TJ, Montgomery JS, Lerner SP, Busby JE et al: Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol 2021, 22(1): 107-117.
- Dinney CP, Fisher MB, Navai N, O'Donnell MA, Cutler D, Abraham A, Young S, Hutchins B, Caceres M, Kishnani N et al: Phase I trial of intravesical recombinant adenovirus mediated interferon-alpha2b formulated in Syn3 for Bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. J Urol 2013, 190(3): 850-856.
- Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, Gomella LG, Kamat AM, Lotan Y, Svatek RS et al: Intravesical rAd-IFNalpha/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol 2017, 35(30): 3410-3416.
- Burke JM, Lamm DL, Meng MV, Nemunaitis JJ, Stephenson JJ, Arseneau JC, Aimi J, Lerner S, Yeung AW, Kazarian T et al: A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol 2012, 188(6): 2391-2397.
- Mukherjee N, Svatek RS, Mansour AM: Role of immunotherapy in bacillus Calmette-Guerin-unresponsive non-muscle invasive bladder cancer. Urol Oncol 2018, 36(3): 103-108.
- Yentz S, Smith D: Indoleamine 2,3-Dioxygenase (IDO) Inhibition as a Strategy to Augment Cancer Immunotherapy. BioDrugs 2018, 32(4): 311-317.
- Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ: Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res 2017, 77(24): 6795-6811.
- Zhang W, Zhang J, Zhang Z, Guo Y, Wu Y, Wang R, Wang L, Mao S, Yao X: Overexpression of Indoleamine 2,3-Dioxygenase 1 Promotes Epithelial-Mesenchymal Transition by Activation of the IL-6/STAT3/PD-L1 Pathway in Bladder Cancer. Transl Oncol 2019, 12(3): 485-492.
- Hornyak L, Dobos N, Koncz G, Karanyi Z, Pall D, Szabo Z, Halmos G, Szekvolgyi L: The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front Immunol 2018, 9: 151.
- Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, Luke JJ, Balmanoukian AS, Schmidt EV, Zhao Y et al: Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J Clin Oncol 2018, 36(32): 3223-3230.
- Albisinni S, Martinez Chanza N, Aoun F, Diamand R, Mjaess G, Azzo JM, Esperto F, Bellmunt J, Roumeguere T, C DEN: Immune checkpoint inhibitors for BCG-resistant NMIBC: the dawn of a new era. Minerva Urol Nephrol 2021, 73(3): 292-298.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript