Case Report | Open Access
Isolated Large Renal Hydatid Cyst Treated by Laparoscopic Nephrectomy
Veda Murthy Reddy Pogula1, Ershad Hussain Galeti1, Bhargava Reddy Kanchiv1, Ifrah Ahmad1, Ayesha Galeti1
1Narayana medical college, Nellore, Andhra Pradesh, India.
Correspondence: Ershad hussain galeti (Department of urology, Narayana medical college, nellore, 524003, Andhra pradesh, India; Email: dr.ershadhussain@gmail.com).
Annals of Urologic Oncology 2021, 4(1): 5-9. https://doi.org/10.32948/auo.2021.08.16
Received: 19 May 2021 | Accepted: 16 Aug 2021 | Published online: 20 Aug 2021
Hydatid disease is caused by Echinococcus granulosus, which causes rare isolated presentation in the kidneys, and is estimated to be about 2-4% of all cases. We herein present a case of a 45-year-old symptomatic male patient with a large primary hydatid cyst in the left kidney that was treated successfully by laparoscopic left nephrectomy.
Key words renal hydatid disease, laparoscopic nephrectomy, hydatiduria, urogenital
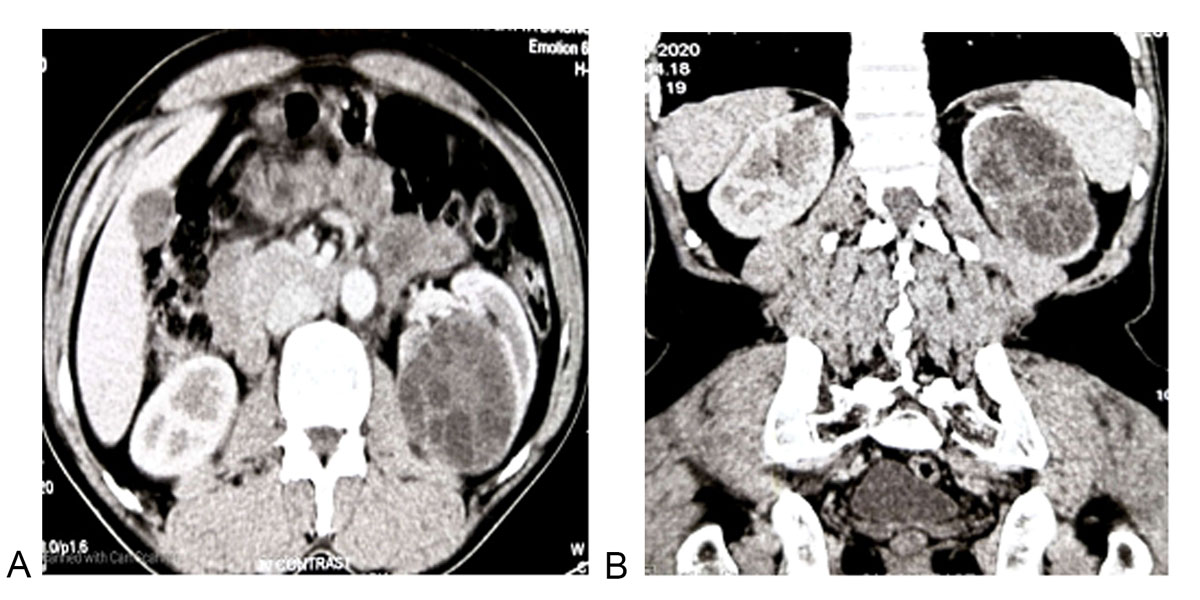
Figure 1 . Abdominal CECT. a: Axial view showing a multi-loculated hydatid cyst involving the left kidney; b: Coronal view showing a multi-loculated Hydatid cyst involving the majority of the left kidney.
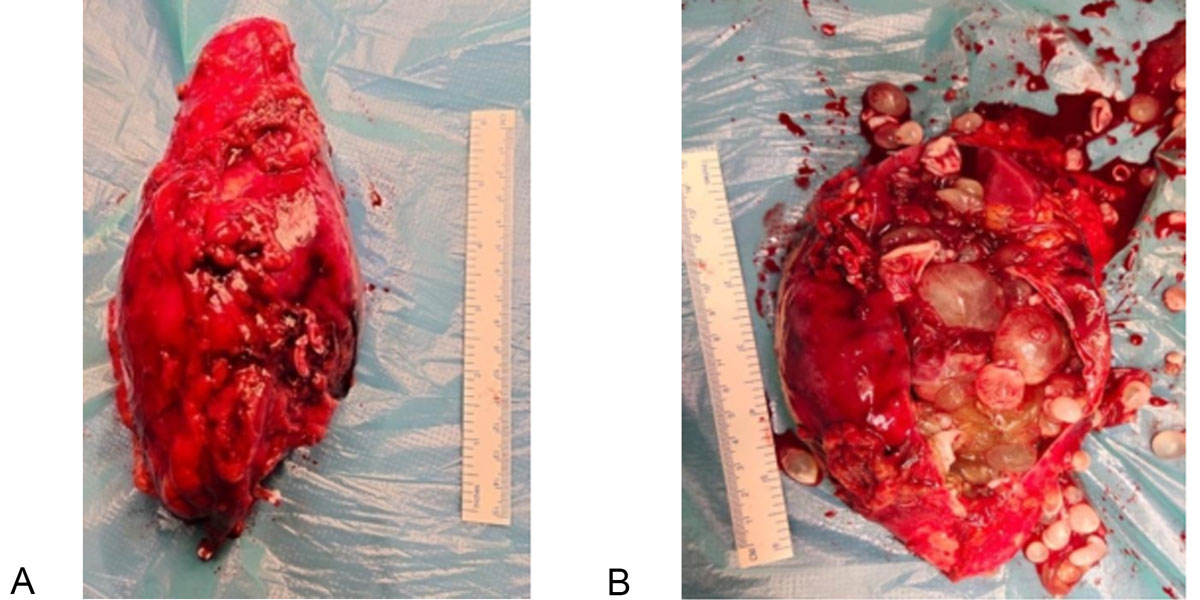
Figure 2. Nephrectomy specimen. a: Laparoscopic left nephrectomy gross specimen; b: Left Nephrectomy specimen with multiple daughter cysts.
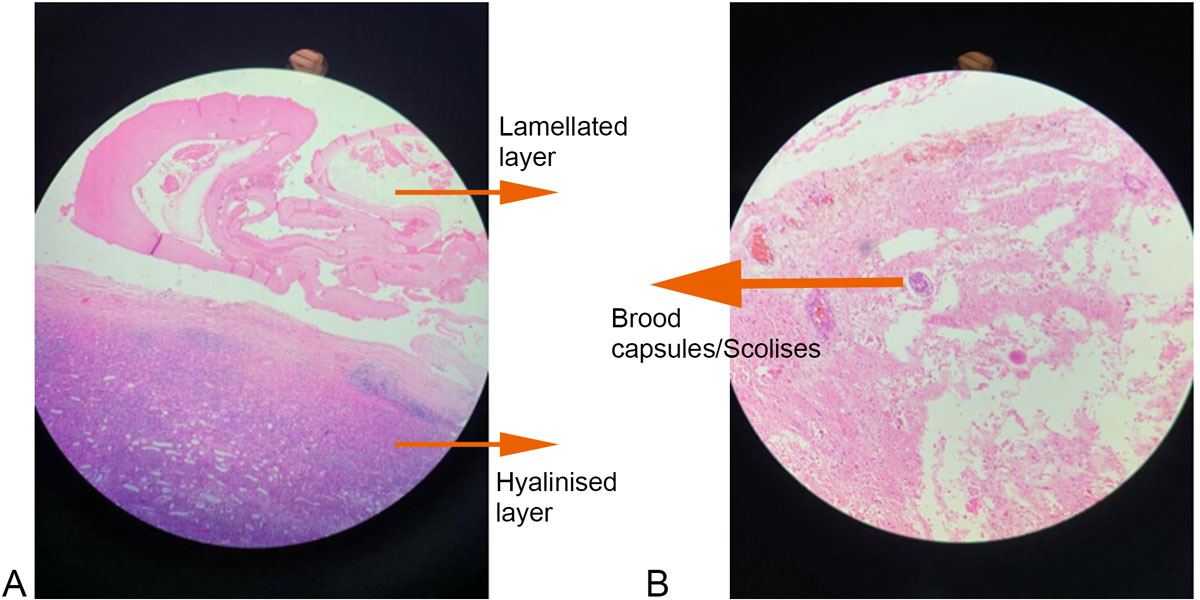
Figure 3a: Hematoxylin and Eosin stain x10 magnification of cyst wall. Figure 3b: Hematoxylin and Eosin stain x40 magnification of cyst wall.
Although it has no specific clinical presentation, the renal hydatid cyst has a characteristic appearance on imaging, surgical cut-surface and histopathological examination. The triad of relevant clinical history, imaging tools, and laboratory investigations provides a reliable preoperative diagnosis in about half of the cases. Despite serological tests and having significant imaging techniques, the diagnosis of renal cyst hydatid cannot be made in 1/3 of the patients before the operation [4]. In our case, renal hydatid cyst was diagnosed before the surgery.
On imaging studies in the current case, a well-demarcated multilocular renal cyst was seen. The detached germinative membrane had a two-layered membrane, with the cyst wall, which is referred to as ″double-contour″ appearance. This appearance has been previously described as ″water-lilysign?. Echinococcal cystic lesions have been classified by the WHO into five grades (Table 1). Based on this WHO classification, imaging findings of the current case refers to Grade 3b hydatid cyst [5,6]. Symptoms and signs of the hydatid cyst disease depend on the involved organ and the secondary spread. The most common clinical finding is a palpable mass. Patients with renal hydatid cystic disease usually present vague pain in the flank region [7]. The cyst's may rupture into the collecting system causing hydatiduria, which is a pathognomonic sign of renal hydatidosis. It is present in only 15–20% of the cases. Eosinophilia is noted in 50% of cases. Pre and post-operatively use of albendazole decreases the cyst wall tension thus reducing the risk of spillage during surgery and prevent the chance of anaphylaxis [8]. Surgery is the treatment option, either through an open or laparoscopic approach [9]. The cyst should be removed without rupture to avoid the spread of the disease, and the kidney sparing cyst removal is performed by cystectomy and pericystectomy when possible. Superficial renal hydatid cysts that do not involve the renal parenchyma can be treated by cystectomy or pericystectomy. However, in cases in which the cyst extends deep into the renal parenchyma, partial nephrectomy can be performed. However, an attempt must be made to preserve as much of the parenchyma as possible [10]. These nephron-sparing surgeries are possible in 75% of the cases, and total nephrectomy should be reserved only for cases in which the entire kidney is occupied by the cyst [2]. Nephrectomy is usually needed when the hydatid cyst invades a major renal part or in cases of hydatiduria. The other problem faced in laparoscopic treatment of hydatid cysts is the difficulty in evacuating the articulate contents of the cyst, the daughter cysts, and laminated membranes. Failure to remove all viable protoscolices in the initial operation may result in local recurrence [11]. There are few reports of laparoscopic removal of renal hydatid but chances of cyst rupture, dissemination, and incomplete removal of the hydatid cyst are quite high [12]. In our case, we considered feasible a laparoscopic left-sided nephrectomy because the hydatid cyst invaded a majority of the renal parenchyma. Ozden et al. also applied pericystectomy with a laparo- scopic retroperitoneal approach to their patient to avoid risk of intraperitoneal contamination [13]. We did not apply peri-cystectomy in our patient. The postoperative period was uncomplicated, and the patient received albendazole for four weeks. After a six-month follow-up, the patient was asymptomatic. The laparoscopic approach can either be retroperitoneal [14] or trans-peritoneal [15]. In our opinion, the laparoscopic retroperitoneal approach may be preferred in cases without giant hydatid cysts. However, the laparoscopic transperitoneal approach will be more appropriate when it is large or giant-sized, as in our case. Although there is a risk of intraperitoneal contamination, the transperitoneal approach provides a better workspace for the operating surgeon if carefully done.
We conclude by stating that laparoscopic renal hydatid cyst surgery may be performed with minimal morbidity, minimal intraoperative blood loss, without intraperitoneal spillage, improved cosmetic results, shorter hospital stay, and an early return to work, making it the gold standard in the treatment of renal hydatid disease.
|
Table 1: Classification of imaging results based on the WHO classification of cystic echinococcosis. |
|
|
WHO Classification |
Imaging characteristics |
|
CL |
Univesicular, cystic lesion with uniform echoes, clear boundary, thin visible wall. If it is a hydatid cyst, it is active. |
|
CE1 |
Univesicular anechoic cyst. Presence of hydatid sand, snow flake sign and double wall sign. The hydatid is active. |
|
CE2 |
Multivesicular, multiseptated cysts; cysts septations produce ‘‘wheel-like’’structures, and presence of daughter cysts is indicated by ‘‘rosette-like’’ or ‘‘honeycomb-like’’ structures. |
|
CE3a |
Detachment of laminated membrane from the cyst wall visible as ‘‘big snake sign’’ or as ‘‘water-lily sign’’. The hydatid status is transitional. |
|
CE3b |
Intracystic shadow of the daughter vesicles and solid septation, manifested as complex cyst shadow. The hydatid is dying. |
|
CE4 |
Heterogenous hypoechoic or hyperechoic contents ecurrence of hydatid disease. |
|
CE5 |
Intracystic solid degeneration and calcification of the cystic wall. The hydatid is inactive. |
|
*CE- Cystic Echinococcosis |
|
Acknowledgements
NIL
Ethical policy
Approval was taken from institutional ethical committee. The study was performed in accordance with the Declaration of Helsinki. Patients gave their informed consent for their participation.
Author contributions
All authors have equally contributed to the manuscript.
Competing interests
NIL
Funding
NIL
- Narang S, Handa U, Nanda A, Bansal R, Nahar R, Sood S: Primary intravitreal hydatid cyst: diagnosis on cytological examination. Ann Trop Med Parasitol 2006, 100(4): 371-374.
- Zmerli S, Ayed M, Horchani A, Chami I, El Ouakdi M, Ben Slama MR: Hydatid cyst of the kidney: diagnosis and treatment. World J Surg 2001, 25(1): 68-74.
- Bandyopadhyay A, Khatua S, Das S, Bose K, Konar K: A rare case of primary renal hydatid cyst presenting with hydatiduria. J Parasit Dis 2013, 39(3): 577-580.
- Angulo JC, Sanchez-Chapado M, Diego A, Escribano J, Tamayo JC, Martin L: Renal echinococcosis: Clinical study of 34 cases. J Urol 1997, 157(3): 787-794.
- Brunetti E, Kern P, Vuitton DA: Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Tropica 2010, 114(1): 1-16.
- Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W: Diagnosing and Staging of Cystic Echinococcosis: How Do CT and MRI Perform in Comparison to Ultrasound? PLoS Neglected Tropical Diseases 2012, 6(10): e1880.
- Bhat GS: Isolated Renal Hydatid Cyst Masquerading as Cystic Renal Cell Carcinoma: A Case Report. J Clin Diagn Res 2015, 57-60.
- A Cushieri, RJC Steele, and AR Moosa: Treatment of Hydatid Cyst, Essential Surgical Practice, Arnold, 4th edition.
- Shahait M, Saoud R, El Hajj A: Laparoscopic treatment of giant renal cystic echinococcosis. Int J Infect Dis 2016, 42: 58-60.
- Tryfonas GJ, Avtzoglou PP, Chaidos C, Zioutis J, Gavopoulos S, Limas C: Renal hydatid disease: diagnosis and treatment. J Pediatr Surg 1993, 28(2): 228-231.
- Durakbasa CU, Tireli GA, Sehiralti V, Sander S, Tosyali AN, Mutus M: An audit on pediatric hydatid disease of uncommon localization: Incidence,diagnosis, surgical approach, and outcome. J Pediatr Surg 2006, 41(8): 1457-1463.
- A Basiri, M Nadjafi-Semnani, A Nooralizadeh: Laparoscopic partial nephrectomy for isolated renal hydatid disease. J Endourol 2006, 20(1): 24-26.
- Ozden E, Bostanci Y, Mercimek MN, Yakupoglu YK, Yilmaz AF, Sarıkaya S: Renal hydatid cyst treatment: Retroperitoneo-scopic ‘‘closed cyst’’ pericystectomy. Int J Urol 2011, 18(3): 237-239.
- Rabii R, Mezzour MH, Essaki H, Fekak H, Joual A, Meziane F: Laparoscopic Treatment for Renal Hydatid Cyst. J Endourol 2006, 20(3): 199-201.
- Desai MR, Shah KJ, Ganpule AP: Isolated renal hydatid cyst managed by laparoscopic transperitoneal nephrectomy. Indian J Urol 2009, 25(4): 531-533.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript