Case Report | Open Access
Primary Renal Leiomyosarcoma: Case Report and Review of the Literature
Sunil V Jagtap1, Shubham S. Jagtap2, kaushiki Varshney3, Yogesh Jadhav4, Pranjal Shah31Professor, Department of Pathology, Krishna Institute of Medical Sciences Deemed University, Karad, India.
2Assistant Lecturer, Department of Medicine, Krishna Institute of Medical Sciences Deemed University, Karad, India.
3Assistant Lecturer, Department of Pathology, Krishna Institute of Medical Sciences Deemed University, Karad, India.
4Assistant Professor, Department of Surgery, Krishna Institute of Medical Sciences Deemed University, Karad, India.
Correspondence: Sunil Vitthalrao Jagtap (Department of Pathology, Krishna Institute of Medical Sciences, Deemed University, karad 415110, Maharashtra, India; Email: drsvjagtap@gmail.com).
Annals of Urologic Oncology 2023, 6(1): 32-38. https://doi.org/10.32948/auo.2023.01.28
Received: 14 Jan 2023 | Accepted: 29 Jan 2023 | Published online: 09 Feb 2023
Key words renal leiomyosarcoma, mesenchymal tumors of kidney, sarcomatoid renal carcinoma, kidney tumors
On the abdomino-pelvis sonography, left kidney measured 7.8 x 4.0 cm with an exophytic cortical solid mass having partially cystic areas at midpolar to hilar region. The mass measured 4.0 x 3.8 cm with septations. Right kidney measured 7.2 x 4.0 cm which was normal in size, shape and architecture. Urinary bladder was mildly dilated. Uterus showed intramural fibroid measuring 1.2 cm in maximum diameter. On sonography impression given was left renal mass suggestive of renal neoplasm with cyst and hematoma.
MRI Abdomen and pelvis (plain) showed a well defined non encapsulated exophytic predominantly solid mass lesion measuring 4.3 x 4 x 4.5 cm. It was located in the anterior cortex of left kidney at interpolar region (Figure 1A, B). It was isointense on T1, hypointense on T2. The lesion was seen in close proximity of left renal vein with hilar lymph node enlargement. The venous thrombosis was absent. A bone scan revealed the absence of bone metastasis. Her Right kidney was normal. Other systemic evaluations showed there was no evidence of any metastasis or any other malignancy. Other organs- urinary bladder, ovaries, liver, gall bladder, spleen, adrenals appeared normal. On MRI reported as malignant neoplastic lesion of left kidney likely to be renal cell carcinoma and advised histopathological correlation.
Patient underwent left radical nephrectomy. No adjuvant chemotherapy or radiotherapy was provided. Our patient recovered well after nephrectomy and advised follow up.
We received specimen of left radical nephrectomy along with left renal hilar lymph nodes. On gross examination specimen of left radical nephrectomy totally measured 12 x 10.5 x 4.5 cm. A solid mass at mid pole of kidney extending to the renal pelvis was noted. The cut section revealed a large, well circumscribed, lobulated, greyish white firm, fleshy tumor mass with focal areas of hemorrhage and necrosis (Figure 2). Tumor was totally measured 5.1 x 4 x 3 cm and solid area measured 2.3 x 2 cm. Cystic area was filled with blood and largest cyst measured 2.8 x 2 cm. The three left renal hilar lymph nodes were dissected, the largest measured 2.0 x 1.0 x 0.3 cm and smallest lymph node measured 1.8 x 1 x 1 cm. The cut section of which was unremarkable.
On microscopy multiple sections were studied showed tumor composed of interlacing fascicles, whirling pattern and in sheets of neoplastic cells exhibiting spindle shaped morphology, mildly pleomorphic hyperchromatic vesicular nuclei with occasional nucleoli and eosinophilic fibrillar cytoplasm (Figure 3, 4, 5). Tumor showed increased mitotic figures (12/10 hpf). Tumor was infiltrating adjacent renal parenchyma. An areas of hemorrhage, necrosis was also evident. Adjacent renal parenchyma showed chronic interstitial nephritis, focal cyst formation and vascular proliferation. Renal capsule, pelvis perinephric fat, renal vessels, hilar lymph nodes and ureter were free from tumor. Microscopic features were of Malignant spindle cell tumor, histomorphology diagnosed as Primary Renal Sarcoma - Leiomyosarcoma. In our case tumor differentiation was - score 1 mitotic count score 2 (12 mitoses per 10 hpf, tumor necrosis - score 2, Histologic grade-2 total score (5) as per French Federation of Cancer Centers Sarcoma Group. The special stains were done which showed, Van Gieson's and Masson's trichrome staining confirmed a smooth muscle origin. Pathological TNM staging was T2N0M0 with a negative resection margins. The immunohistochemistry for confirmation and typing was done, which showed smooth muscle actin diffusely and desmin focally positive. The patient was thoroughly investigated for any primary sarcoma elsewhere. The final diagnosis of primary renal LMS was given. On follow up patient was asymptomatic and there was no evidence of any metastasis.
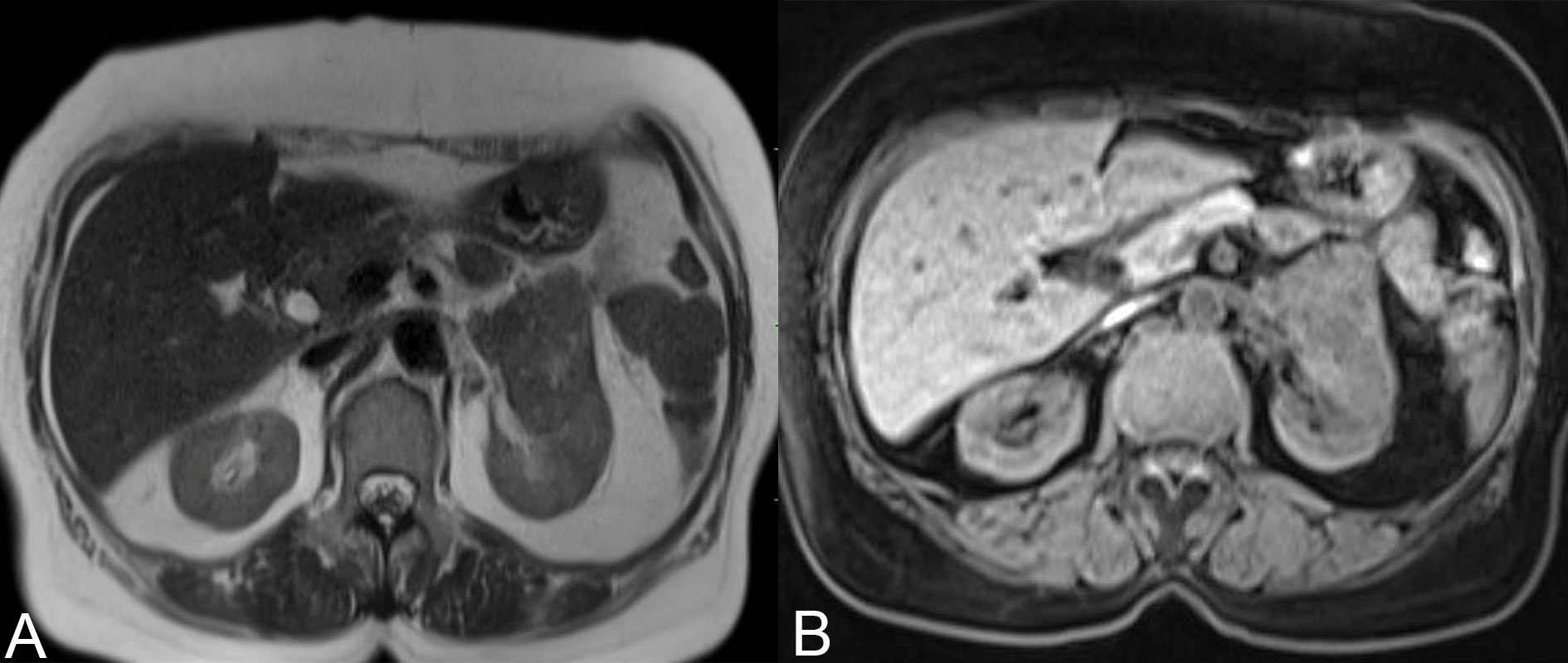 Figure 1 A, B. MRI Abdomen and pelvis (plain) shows a well defined non encapsulated solid mass in the anterior cortex of left kidney at interpolar region.
Figure 1 A, B. MRI Abdomen and pelvis (plain) shows a well defined non encapsulated solid mass in the anterior cortex of left kidney at interpolar region.
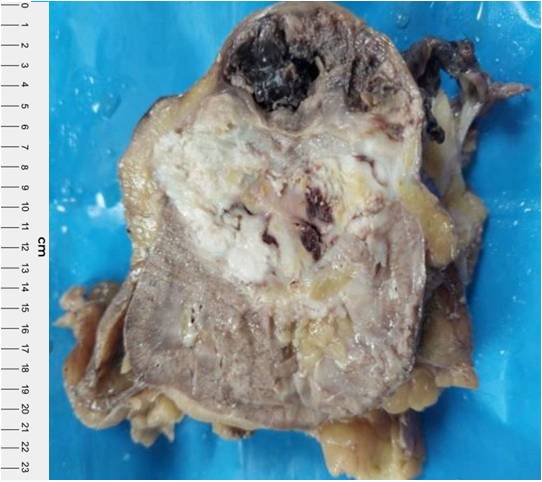 Figure 2. Left radical nephrectomy cut section showing a large, well circumscribed, lobulated, greyish white, firm , fleshy tumor mass with areas of necrosis and haemorrahges.
Figure 2. Left radical nephrectomy cut section showing a large, well circumscribed, lobulated, greyish white, firm , fleshy tumor mass with areas of necrosis and haemorrahges.
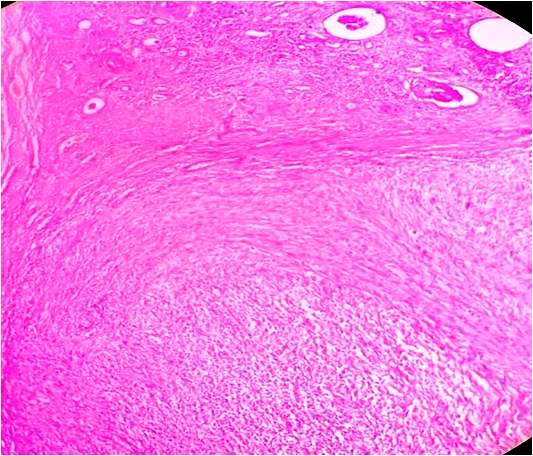 Figure 3. Photographmicrograph showing neoplastic spindle cells arranged in interlacing fascicles having pleomorphic nuclei with blunt ends and eosinophilic cytoplasm (Haematoxyline and eosin stain x40).
Figure 3. Photographmicrograph showing neoplastic spindle cells arranged in interlacing fascicles having pleomorphic nuclei with blunt ends and eosinophilic cytoplasm (Haematoxyline and eosin stain x40).
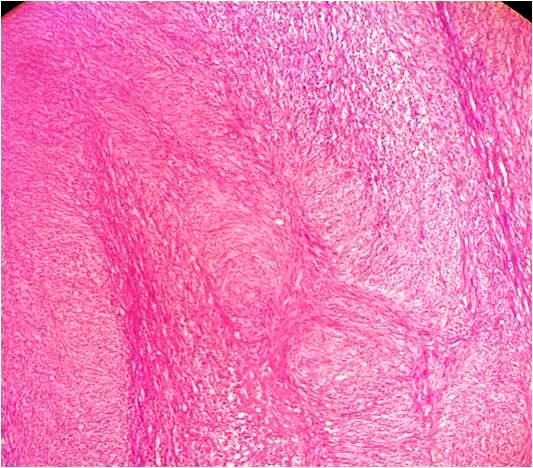 Figure 4. Tumor composed of neoplastic spindle cells having pleomorphic, hyperchromatic nuclei with occasional nucleoli and eosinophilic fibrillar cytoplasm (Haematoxyline and eosin stain x100).
Figure 4. Tumor composed of neoplastic spindle cells having pleomorphic, hyperchromatic nuclei with occasional nucleoli and eosinophilic fibrillar cytoplasm (Haematoxyline and eosin stain x100).
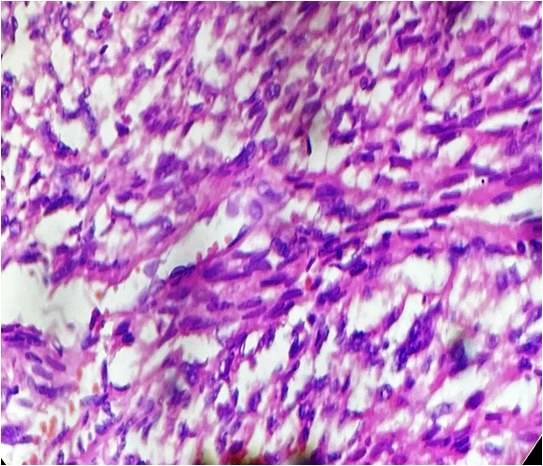 Figure 5. Spindle shape tumor cells with high cellularity, pleomorphic, hyperchromatic nuclei with occasional nucleoli and eosinophilic cytoplasm (Haematoxyline and eosin stain x400).
Figure 5. Spindle shape tumor cells with high cellularity, pleomorphic, hyperchromatic nuclei with occasional nucleoli and eosinophilic cytoplasm (Haematoxyline and eosin stain x400).
Renal LMS are rare tumors of the kidney that were first reported by Berry in 1919 [3]. They comprise 0.5% to 1.5% of all malignant renal tumors in adults [4]. Prior to 1997, only 11 cases were reported in the English literature [5]. Improved diagnostic methods make current reports more frequent.
Primary sarcomas are rare in the kidney and represent 1%-2% of all malignant renal tumors with only 0.12% of renal malignancies confirmed as leiomyosarcoma [6]. On histological subtype of renal sarcomas of pure mesenchymal origin, LMS is the most common variant accounting to 50-60%, followed by fibrosarcoma and liposarcoma. The renal LMS appear to arise from renal capsule or smooth muscle tissue of the vessels or renal pelvic wall [7, 8].
On clinical presentation the symptoms are nonspecific and often overlap with other renal pathologies. The mean age at presentation is 50-60 years with a female preponderance. The cases may present with classic triad of symptoms as an abdominal mass, flank pain, and haematuria. Rare cases presenting with spontaneous retroperitoneal hemorrhage and hypotension have been reported. The cases of renal leiomyosarcoma presents clinically and radiographically as to the common renal malignancies. On radio imaging computed tomography of renal sarcomas usually reveals a solid infiltrating mass as other common renal malignancies [9].
Radio imaging evaluation
Advances in the imaging techniques have created great improvements in the diagnosis of renal cell carcinoma, however, renal is rare and does LMS not have known specific imaging characteristics. The computed tomography(CT) demonstrate imaging features usually reveals a solid infiltrating mass which is often very difficult to indistinguishable from renal sarcomas [10]. Due to this limitation, CT image-guided percutaneous renal mass biopsy is strongly recommended for histopathological diagnosis of the tumor with a high degree of diagnostic accuracy.
On CT renal sarcomas appearance is variable from solid masses, low attenuating to cystic. The renal LMS appear as expansile, heterogeneously-enhancing, and well-circumscribed solid masses that usually project exophytically from the kidney [11]. Large renal LMS may present as multilocular cystic masses with peripheral enhancement [9, 12].
Pathological findings
Usually renal LMS are larger in size when detected. Miller et al. observed the mean tumor size was 13.4?cm having a range from 4 to 26 cm [13]. The 60% of tumors are located on the right kidney.
On gross morphology, the tumor is well-circumscribed. On cut surface tumor show fleshy, grey white, whorled appearance with areas of haemorrhage necrosis and cystic degeneration.
The diagnosis is usually post-operative and requires a thorough sampling of the tumor. The careful evaluation for the site of tumor origin, histopathological type, its subtypes and to rule out an epithelial component especially to sarcomatoid renal cell carcinoma should be done. The common malignant renal neoplasms of pure mesenchymal origin are LMS, fibrosarcoma and liposarcoma.
On microscopically, LMS show characteristics of smooth muscle tumor. The neoplastic cells are arranged in sheaths and fascicles of spindle shaped cells having blunt ended nuclei and mild to moderate amount of eosinophilic cytoplasm. The features of malignancy are tumor necrosis, nuclear pleomorphic nucleli, and increased mitotic figures (more than 1 mitoses/10 HPF).
Histopathological diagnosis and Differential diagnosis
According to the classification by the French Federation of Cancer Centers Sarcoma Group (FNCLCC) the pathological diagnosis and grade of the tumor was evaluated. The grade was scored based in the level of differentiation, presence of mitosis, and necrosis in each high power field [14]. The most of the renal LMS had significantly higher mitotic activity at the time of diagnosis(mean 8.6 mitoses/10 hpf), with most being FNCLCC grade 2 [15].
On histopathology the LMS has to be differentiated from sarcomatoid renal cell carcinoma, leiomyoma and renal angiomyolipoma [16, 17]. Retroperitoneal LMS, involving the kidney should be ruled out before making the diagnosis of primary renal LMS.
The low-grade LMS requires to be distinguished from a leiomyoma. In leiomyoma shows the fascicular arrangement of benign smooth muscles with no atypia the tumor cells lack of cytologic atypia and there is no necrosis. The mitotic activity is low i.e 0-1 mitoses/10 hpf.
In sarcomatoid variant of renal cell carcinoma shows a mixture of both epithelial and sarcomatoid components. The sarcomatous tumor cells are highly pleomorphic, with high mitotic activity. In tumor carefully look for any foci of classic renal cell carcinoma. Also the tumor morphologically lacks the alternating fascicles. Immunohistochemical positivity for Keratin and EMA expression.
The epithelioid angiomyolipoma, a variant of angiomyolipoma, histologically can be mistaken for a LMS. The histological finding of mature adipose tissue with thick hyalinized blood vessels favors angiomyolipoma.
The other differential is primary monophasic synovial sarcoma of the kidney. It shows neoplastic spindle cells with hypercellular fascicular pattern, scant intervening stroma along with focal staghorn, branching vascular pattern. Also the fibrosarcoma and malignant peripheral nerve sheath tumor remains differential diagnosis.
Immunohistochemistry analysis
Leiomyosarcomas on immunohistochemical analysis showed that the tumor cells were diffusely positive for smooth muscle actin. Also express positivity for calponin, desmin, and H-caldesmon. Tumor are negative for cytokeratin, S-100 protein, and HMB45, CD117 and S-100.
In the sarcomatoid renal cell carcinoma, the epithelial markers CKAE1/AE3, EMA stain positive while the like smooth muscle actin, H-caldesmon, and desmin are negative [6, 18].
The Ki-67 proliferation index, and p16 and p53 tumor suppressor proteins are over-expressed in LMS, so it could be used as a prognostic marker.
Treatment for renal LMS
The currently recommended treatment for resectable tumors is complete surgical excision of the tumor with negative margins. Radical nephrectomy is the treatment of choice for primary renal LMS [19]. The early and complete resection of the tumor improve the overall prognosis. There is no consensus on the role of neoadjuvant or adjuvant radio/chemotherapy in the management of renal LMS [20]. Demir A, et al. observed that significant proportion of aggressive LMS that tend to recur locally, radical nephrectomy is a superior option that provides better oncologic control [21]. If the surgeon anticipates a grossly incomplete resection, intraoperative radiation therapy results in excellent local control and survival, with acceptable morbidity. Sharma et al. prescribed chemotherapy with mesna, adriamycin, ifosphamide and dacarbazine regimen and sandwich radiotherapy with a dose of 44 Gy/22#/4.5 weeks to the renal bed and adjoining lymphatics [22].
Novak M et al, stated that the neoadjuvant radiotherapy is generally administered while it is not indicated postoperatively in R0 (tumor resection with negative macro and microscopic margins) adjuvant chemotherapy is recommended in cases of R2 resections (tumors with positive macro and microscopic resection margins) as well as in unresectable and metastatic disease [20]. The regular follow-up play important role in the treatment of renal LMS.
Prognosis
Dhamne SA et al. reported that the small tumor size (<5 cm), low histologic grade, and renal-limited disease are some favorable prognostic factors for LMS [23]. Kendal et al. reported the favorable prognostic markers are size < 4 cm, low grade, absence of nodal metastasis, and radical surgical treatment having favorable survival outcomes in the largest renal LMS cohort [1]. Novak M et al. observed that the renal LMS frequently metastasizes to distant body organs via hematogenous spread and carries an overall poor prognosis with a median overall survival of 25 months [20]. The distant metastases were identified in 90% of the patient primary LMS of the kidney in Miller et al study [13].
The study by Novak M et al. observed that the distant metastasis occurred in 30% of patients, local in 4.5%, while 15% of the patients experienced combined local recurrence and distant metastasis [20].
In Indians, renal primary leiomyosarcomas are represented mostly as case reports in the literature or as components of series of renal sarcomas as we summarized of recent cases in table 1 [24-37]. As primary renal leiomyosarcoma is an exceedingly uncommon tumor, advances in the techniques will be helpful for improvements in the diagnosis and future plans.
| Table 1. Summary of primary renal leiomyosarcoma in Indians (by Dr S V Jagtap , 2023). | ||||||||||
| S No. | Author et al /Reference | Year | Patient Age (Years) | Gender | Main Clinical symptoms | Renal Side | Radioimaging USG/CT/MRI | Gross Tumor Size (cm) | Surgery | Adjuveant Rx |
| 1 | Jagtap SV present study | 2023 | 59 | F | hematuria, left-sided flank pain | Left | Renal Mass RCC | 4.3x 4 x 4.5 | Radical Nephrectomy | No |
| 2 | Karri M [24] | 2022 | 48 | F | Flank pain | Right | Renal Mass with peritoneal metastasis | 7x5x4 | Radical Nephrectomy | No |
| 3 | Nandi S [25] | 2021 | 68 | F | Abdominal pain | Right | Renal Mass | 8x8x7 | Radical Nephrectomy | No |
| 4 | Jagtap SV [8] | 2021 | 68 | M | Abdominal pain Hematuria | Right | Renal pelvis mass | 3x1.9x2.4 | Radical Nephrectomy | No |
| 5 | Beigh A [26] | 2018 | 35 | M | Flank Pain | Right | Renal Mass | 12x5.5x4 | Radical Nephrectomy | No |
| 6 | 65 | F | Hematuria | Left | Renal Mass | 5x4x2.5 | Radical Nephrectomy | No | ||
| 7 | 55 | F | Flank pain | Left | Renal Mass | 7x4x2.4 | Radical Nephrectomy | No | ||
| 8 | 64 | M | Flank pain | Left | Renal Mass | 4x3x1 | Radical Nephrectomy | No | ||
| 9 | Kundu R [27] | 2019 | 65 | F | Abdominal pain | Left | RCC | 8x7x8 | Radical Nephrectomy | No |
| 10 | Malik A [28] | 2017 | 50 | F | Flank pain | Left | RCC | 8x4x2 | Nephrectomy | |
| 11 | Vasudevan S [29] | 2016 | 57 | F | Flank pain | Right | RCC | 22x15x10 | Radical | No |
| 12 | Rupali B [30] | 2015 | 39 | M | Abdominal lump | Left | Renal Mass | 16x14x12 | Partial Nephrectomy | No |
| 13 | Narula V [31] | 2015 | 39 | F | Abdominal lump | Right | Renal Mass | 11x7x6 | Radical Nephrectomy | No |
| 14 | Srivastav P [32] | 2015 | 50 | F | Flank pain | Left | RCC | - | - | - |
| 15 | Suresh B [33] | 2014 | 65 | F | Abdominal lump | Right | Renal Mass | 18x8x7 | Nephrectomy | No |
| 16 | Dhawan S [6] | 2012 | 62 | F | Abdominal pain | Left | Retroperitoneal sarcoma | 10x9x8 | Radical Nephrectomy | No |
| 17 | Suresh K [37] | 2011 | 65 | M | Flank pain | Right | Renal mass | - | Radical Nephrectomy | No |
| 18 | Bhat GS [34] | 2011 | 68 | M | Abdominal pain | Right | Renal mass | 5.6x5.1x5 | Radical Nephrectomy | No |
| 19 | Venkatesh K [35] | 2010 | 55 | F | Abdominal mass | Left | Renal mass | 20x16x12 | Radical Nephrectomy | No |
| 20 | Choudhury M [36] | 2009 | 65 | F | Abdominal lump | Right | Renal exophytic mass | 15x11x8 | Radical Nephrectomy | No |
| 21 | Dhamne S [23] | 2009 | 60 | M | Increased frequency mituration | Right | Renal hilum pelvis mass | 10x8x5.6 | Radical Nephrectomy | No |
| 22 | Sharma D [22] | 2007 | - | - | Flank pain | Left | RCC | - | Radical Nephrectomy | Adjvent chemo and radiotherapy |
| RCC: Renal Cell Carcinoma, USG: ultrasonography, CT: computed tomography, MRI: Magnetic resonance imaging, Adjuvant Rx: Adjuvant treatment, M: male, F: female. | ||||||||||
Acknowledgements
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
SVJ carried out review of various articles and texts and them compiling, proof reading, editing and final drafting of manuscript. All authors contributed to the conception, case study, analysis, interpretation, drafting the work and substantively revising it.
Competing interests
All authors declare no competing interests.
Funding
None.- Kendal WS: The comparative survival of renal leiomyosarcoma. Can J Urol 2007, 14: 3435-3442.
- Grignon DJ, Ayala AG, Ro JY, El-Naggar A, Papadopoulos NJ: Primary sarcomas of kidney: A clinicopathologic and DNA flow cytometric study of 17 cases. Cancer 1990, 65: 1611-1618.
- Berry FB: Report of three cases of combined tumors of the kidney in adults. J Med Res 1919, 40(3): 459-470.
- Yoshikawa M, Hayashi Y, Sanma S, Maruyama Y, Hirao Y, Okajima E: Spontaneous rupture of renal leiomyosarcoma: report of a case. Hinyokika Kiyo 1986, 32(9): 1282-1287.
- Chen JH, Lee SK: Renal leiomyosarcoma mimicking transitional cell carcinoma. AJR Am J Roentgenol 1997, 169(1): 312-313.
- Dhawan S, Chopra P, and Dhawan S: Primary renal leiomyosarcoma: a diagnostic challenge. Urol Ann 2012, 4(1): 48-50.
- Niceta P, Lavengood RW, Jr, Fernandes M, Tozzo PJ: Leiomyosarcoma of kidney. Review of the literature. Urology 1974, 3: 270-277.
- Jagtap SV, Jagtap SS, Agarwal G, Huddedar A: Leiomyosarcoma of the renal pelvis: Rare mesenchymal tumor. J Datta Meghe Inst Med Sci Univ 2021, 16: 746-748.
- Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR: Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics 2010, 30(6): 1525-1540.
- Karaosmanoğlu AD, Onur MR, Shirkhoda A, Ozmen M, Hahn PF: Unusual Malignant Solid Neoplasms of the Kidney: Cross-Sectional Imaging Findings. Korean J Radiol 2015, 16(4): 853-859.
- Lalwani N, Prasad SR, Vikram R, Katabathina V, Shanbhogue A, Restrepo C: Pediatric and adult primary sarcomas of the kidney: a cross-sectional imaging review. Acta Radiol 2011, 52: 448-457.
- SV Jagtap: Multilocular cystic renal neoplasm-low malignant potential (MCRN-LMP) - review. Ann Urol Oncol 2020, 3(2): 97-102.
- Miller JS, Zhou M, Brimo F, Guo CC, Epstein JI: Primary Leiomyosarcoma of the Kidney: A Clinicopathologic Study of 27 Cases. Am J Surg Pathol 2010, 34(2): 238-242.
- Coindre JM, Terrier P, Bui NB, Bonichon F, Collin F, Le Doussal V, Mandard AM, Vilain MO, Jacquemier J, Duplay H: Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 1996, 14: 869-877.
- Deyrup AT, Montgomery E, Fisher C: Leiomyosarcoma of the kidney: a clinicopathologic study. Am J Surg Pathol 2004, 28(2): 178-182.
- Jagtap SV, Nikumbh DB, Desai SR, Kshirsagar AY, Khedkar J, Chavan SH: Unusual large sporadic angiomyolipoma coexisting with huge simple renal cyst. Online J Health Allied Scs 2011, 10(2): 29.
- Mai KT, Perkins DG, Collins JP: Epithelioid cell variant of renal angiomyolipoma. Histopathology 1996, 28: 277-280.
- Chow LT, Chan SK, Chow WH: Fine needle aspiration cytodiagnosis of leiomyosarcoma of the renal pelvis. A case report with immunohistochemical study. Acta Cytologica 1994, 38(5): 759-763.
- Kwon YS, Salmasi A, Han CS, Hon JD, Singer EA: Renal Leiomyosarcoma: Case Report and Review of the Literature. World J Nephrol Urol 2015, 4(2): 213-217.
- Novak M, Perhavec A, Maturen KE, Pavlovic Djokic S, Jereb S, Erzen D: Leiomyosarcoma of the renal vein: analysis of outcome and prognostic factors in the world case series of 67 patients. Radiol Oncol 2017, 51(1): 56-64.
- Demir A, Yazici CM, Eren F, Türkeri L: Case report: good prognosis in leiomyosarcoma of the kidney. Int Urol Nephrol 2007, 39(1): 7-10.
- Sharma D, Pradhan ES, Aryya NC, Shukla VK: Leiomyosarcoma of kidney: a case report with long term result after radiotherapy and chemotherapy. Int Urol Nephrol 2007, 39(2): 397-400.
- Dhamne SA, Gadgil NM, Padmanabhan A: Leiomyosarcoma of the renal pelvis. Indian J Pathol Microbiol 2009, 52(4): 549-551.
- Karri MD, Konkay K, Mandava VS, Panchakarla GV, Chaganti PD: A rare case of primary renal leiomyosarcoma with peritoneal sarcomatosis - A case report. J NTR Univ Health Sci 2022, 11: 152-154.
- Nandi S, Das S, Das C, Mukhopadhyay M: Primary renal leiomyosarcoma: A rare case report. Indian J Case Reports 2021, 7(11): 503-505.
- Beigh A, Sheikh J, Sheikh B, Mujo SP, Summyia F: Primary renal leiomyosarcoma: a rare entity. Int J Res Med Sci 2018, 6: 1292-1296.
- Kundu R, Chandra M, Punia RS, Aggarwal P: Primary renal leiomyosarcoma arising from renal vein: A case report of rare entity with review of literature. Indian J Med Paediatr Oncol 2019, 40: S139-S141.
- Malik A, Kumar R, Shankar A, Chumber S, Bakhshi S, Kaushal S, Thirunavukkarasu B: Report of primary leiomyosarcomaof renal pelvis and literature review. J Cancer Metastasis Treat 2017, 3: 111-115.
- Vasudevan S, Kumar V, Krishna B: Leiomyosarcoma of Kidney: A Rare Case Report. Kerala Med J 2016, 9(2): 73-75.
- R Bavikar and S Deshmukh: Primary Leiomyosarcoma of Kidney in a Young Male Treated with Partial Nephrectomy - A Case Report. Sarcoma Res Int 2015, 2(1): 1013.
- Narula V, Siraj F, Bansal A: Renal leiomyosarcoma with soft tissue metastasis: An unusual presentation. Can Urol Assoc J 2015, 9: E139 141.
- Srivastava P, Prasad R, Khanna G: Primary leiomyosarcoma of kidney: A rare case report. Muller J Med Sci Res 2015, 6: 157-159.
- Babu S, Singhai A, Hussain N, Singh V: Renal Leiomyosarcoma - A Rare Entity. JCR 2014, 4: 29-32.
- GS Bhat, GG Nelivigi, M Shivalingaiah and C S: Case Report Primary Renal Leiomyosarcoma: Case Report and Literature Review. Afr J Urol 2011, 17(1): 15-17.
- Choudhury M, Singh SK, Pujani M, Pathania OP: A case of leiomyosarcoma of kidney clinically and radiologically misdiagnosed as renal cell carcinoma. Indian J Cancer 2009, 46(3): 241-243.
- Venkatesh K, Lamba Saini M, Niveditha SR, Krishnagiri C, Babu S: Primary leiomyosarcoma of the kidney. Pathol Res Int 2010, 2010: 652398.
- Kumar S, Jeet P, Kumar DR, Kumar KA, Gupta S: Primary Renal Leiomyosarcoma: A Brief Case Report. Urol Today Int J 2011, 4(3): art 34.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript