Research Article | Open Access
Diet, Physical Activity, and Smoking in Patients Living with Non-Muscle Invasive Bladder Cancer
Elizabeth Y. Wang1, 3, Manuel Armas-Phan1, 4, Maxwell V. Meng1, Sima P. Porten1*, Stacey A. Kenfield1, 2*
1Department of Urology, University of California, San Francisco (UCSF), San Francisco, CA, USA.
2Department of Epidemiology & Biostatistics, University of California, San Francisco (UCSF), San Francisco, CA, USA.
3University of Hawaii, Honolulu, HI, USA (Present).
4Department of Urology, The Emory Clinic, Emory University, Atlanta, GA, USA (Present).
*Shared senior authors.
Correspondence: Sima P. Porten and Stacey A. Kenfield (Department of Urology, University of California, San Francisco (UCSF), 550 16th St, 6th floor, Box #0560, San Francisco, CA 94158, USA; Sima P. Porten's Email: sima.porten@ucsf.edu, Stacey A. Kenfield's Email: stacey.kenfield@ucsf.edu).
Annals of Urologic Oncology 2023, 6(4): 145-153. https://doi.org/10.32948/auo.2023.10.26
Received: 24 Oct 2023 | Accepted: 26 Oct 2023 | Published online: 02 Nov 2023
Objective We aim to describe modifiable behaviors (smoking, diet, and physical activity) in a cohort of patients with NMIBC.
Methods We conducted an observational cross-sectional study in patients undergoing surveillance for NMIBC. A comprehensive survey comprised of validated measures of physical activity, diet, and mutagen consumption was administered. We assessed a “healthy lifestyle score” consisting of body mass index (BMI) <25, smoking status, and physical activity (aerobic exercise minutes). We examined differences in lifestyle factors by stage, grade, recurrence, BMI, age, gender, and education level.
Results In a cohort of 113 NMIBC patients, median age was 67 years (IQR: 59, 73) and median time since initial diagnosis was 26 months (IQR: 9, 42). Low levels of healthy habits are common among patients with NMIBC. Those with a BMI of ≥25 had greater red and processed meat intake, higher mutagen intake, and lower physical activity. Older patients reported more supplement use and lower physical activity. Low education level was associated with a less healthy lifestyle (higher processed meat consumption, higher Meiqx mutagen consumption, and lower physical activity).
Conclusions In patients undergoing surveillance for NMIBC, 25% had all three components reflecting a healthy lifestyle. Older age, lower education, and higher body mass index were associated with fewer healthy habits. Education on healthy lifestyle changes should be a key component in NMIBC survivorship care.
Key words modifiable behaviors, bladder cancer, epidemiology, observational
Modifying behavioral factors such as diet and physical activity in patients with bladder cancer may improve bladder cancer survivorship. A study examining a wide range of modifiable factors is warranted to inform future management strategies in patients with NMIBC. We aimed to describe modifiable behaviors in patients with NMIBC and explore whether these differed by clinical and sociodemographic characteristics. We hypothesized that modifiable behaviors may differ by grade, stage, recurrence status, healthy body mass index (BMI), age, gender, and education level. To our knowledge, this is the first study to examine behaviors by these characteristics in NMIBC patients.
We conducted an IRB-approved study (#10-04057) at the University of California, San Francisco (UCSF) using validated questionnaires to assess lifestyle habits in patients undergoing surveillance for NMIBC using a cross-sectional study design. We identified participants with histopathologic diagnosis of NMIBC (Ta or T1 urothelial carcinoma, CIS). Patients were excluded if their preferred language was not English, had predominant variant histology, or had prior radical cystectomy. All consecutive new and follow-up patients with an eligible diagnosis were prospectively offered participation during in-person clinic visits and consented. Consenting participants independently completed surveys electronically via Research Electronic Data Capture (REDCap) or paper questionnaire between 03/23/19 and 03/27/2020. Additional clinical information was abstracted via medical record review.
Physical activity assessment
Participants were asked how often (never to 11+ h/wk) on average over the past year they participated in walking for exercise, transportation, or errands; jogging (>10 min/mile); running (10 min/mile or faster); calisthenics, aerobics, rowing, Nordic track; bicycling; tennis, squash, racquetball; lap swimming; weightlifting; and other aerobic exercise (e.g., heavy outdoor work), as well as their usual walking pace (based on a validated questionnaire) [8, 9]. A metabolic equivalent task (MET) value was assigned to each activity on the basis of the energy required by that activity relative to the resting metabolic rate [8]. Activities were classified as vigorous if they required 6 or more METs [10]. The following relevant variables were derived for statistical analyses: total activity in MET-hr/week, moderate to vigorous aerobic activity in MET-hr/week, which are standard physical activity measures, and whether one was meeting the United States (US) exercise recommendation of ≥150 minutes of aerobic exercise per week (yes/no), [11] which aligns with the guidelines for cancer survivors [12].
Dietary intake assessment
The validated food frequency questionnaire (FFQ) [13] included 127 food and beverage items plus supplements. A portion size was specified for each item, and participants were asked how often they had consumed that amount of the item on average over the past year (never, <1 time/mo, 1 time/mo, 2-3 times/mo 1 time/wk, 2-4 times/wk, 5-6 times/wk, 1 time/d, 2+ times/d). Intake of each food item was calculated by multiplying the frequency of consumption by the portion size specified. Additional questions addressed the type of fat used when cooking, the frequency of fried food consumption, and an open-ended section for any foods eaten frequently that were not included in the multiple-choice section. We defined 14 food groups for this analysis: cruciferous vegetables, cooked tomatoes, healthy fats, fish, processed meats, dairy, fruits, vegetables, lean protein, red meat, breads/cereals/starches, non-alcoholic liquids, alcohol, and sweets/desserts. Foods that were included in each of the categories are included in Supplementary (Suppl.),Table 1.
Mutagen intake assessment
The Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease (CHARRED) questionnaire was developed in 2006 and includes data on heterocyclic amines (HCA) and meat-derived mutagenicity (MDM) [14]. Details on the typical size, cooking modality and doneness of these foods are shown in Suppl. Table 1. The frequency of cooked meat intake in these categories was multiplied by the measured heterocyclic amine (ng/g meat) or MDM levels (revertant colonies/g meat) using the CHARRED software (version 1.7, 2006) [15]. Outputs from this calculation included intake of 3 HCAs: 2-Amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP), 2-amino-3,8-dimethylimidazo [4, 5-f] quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo [4, 5-f] quinoxaline (DiMeIQx); actual and predicted total mutagenicity (variable incorporating mutagenicity of all meat-related mutagens); and Benzo[a]pyrene (B[a]p), a measure of overall PAH exposure from meat [16, 17]. Mann-Whitney and Kruskal-Wallace tests were used to examine differences in distributions between groups.
Other exposures of interest
Medical history, demographics, smoking history, food security, and supplement use were also self-reported. Clinical data about bladder cancer history were collected from the electronic medical record (EMR). Slight modifications were piloted at 7 months to address the updated scientific literature pertaining to diet and supplements. i.e. raw versus cooked broccoli consumption and probiotic supplement consumption. A healthy behavior score was generated that included 3 factors consistent with evidence-based recommendations for cancer survivorship: not smoking, BMI, and physical activity. Participants were considered ‘healthy’ if they had a BMI less than 25 kg/m2, reported a level of physical activity meeting the US physical activity guidelines of 150 minutes of moderate-vigorous activity/week, [11] and were a never smoker or had quit smoking 10 or more years ago, which follow guidance from national organizations such as the American Cancer Society for patients diagnosed with cancer [18] and the Centers of Disease Control and Prevention [19].
Our primary objective was to describe the study population’s lifestyle behaviors using medians and interquartile (IQR) range for continuous variables and N and % for categorical variables, overall, and report on the percentage of patients with a ‘healthy’ score. In exploratory analyses we present the data by subgroups determined a priori, including initial stage and grade (LGTa, HGTa, HGT1, or CIS with/without concurrent papillary tumor), BMI <25 or ≥25 kg/m2, age (<65 or ≥65 years), gender (male or female), and education level (completion of 2-year college or higher (high) or high school completion or lower (low) to develop hypotheses for future studies and also to inform clinical practice (e.g., if healthful behaviors were considerably lower in certain patient subgroups compared with others). In exploratory analyses, we conducted non-parametric Mann-Whitney U or Kruskal Wallis tests to assess differences in the distribution of the data among the groups, and chi-square test or Fisher’s exact test were used to examine differences between categorical variables. Statistical tests were 2-sided and were performed at the 0.05 level of significance. Statistical analyses were performed using SAS version 9.4.
Lifestyle-related characteristics are summarized in Table 1 and Suppl. Table 2. Median level of total activity was 28.5 MET-hr/wk, with 2.5 hours/week classified as walking and 0 min/week of strength training. Median intake levels over 1 year were the following: fruit, 2.0 servings/day, vegetables, 3.6 servings/day, red meat, 0.3 servings/day, and processed meat, 0.2 servings/day. 40% of participants used multivitamins, while 35% reported taking probiotics. Few participants reported food insecurity; most participants prepared their own meals from groceries. 56% of participants were eating out at restaurants one or more times a week and 24% were eating take out from restaurants one or more times per week.
For NMIBC characteristics, no statistically significant differences were found by grade or stage at diagnosis or history of recurrence for any lifestyle risk factors (data not shown). BMI ≥25kg/m2 was associated with greater red meat intake (p= 0.0007), processed meat intake (p= 0.03), and lower physical activity levels (total MET-hr/wk, p= 0.046; hr/wk of walking, p= 0.02; and higher mutagen intake (B[a]p intake, p= 0.02) (Table 2). Supplement use was high overall, but age ≥65 years was associated with greater supplement use compared with those age <65 (86% vs 69%, respectively, p= 0.04). Age ≥65 years was also associated with markedly lower physical activity levels (MET-hr/wk of moderate-vigorous aerobic activity, p= 0.04; percentage meeting national exercise recommendations, p= 0.04). (Suppl. Table 3). Women consumed more dairy than men (p= 0.03), while men consumed more alcohol than women (p= 0.04). Otherwise, men and women had similar lifestyle profiles (Suppl. Table 3). Higher education was associated with lower processed meat (p= 0.04), more healthy fat (p= 0.045), lower Meiqx intake (p= 0.02), and lower predicted total mutagen intake (p= 0.04). Those with higher education levels consumed qualitatively more fruit and vegetables (~15 additional servings combined per week). Higher education was associated with more physical activity (total MET-hr/wk, p= 0.045; walking, p= 0.003; and higher percentage meeting national exercise recommendations, p= 0.01) (Suppl. Table 3).
Healthy Behavior Score
The percentage of patients who had behaviors consistent with recommended behaviors is shown in Figure 1. Across levels of stage/grade, recurrence, age, and gender, only 20-30% met these recommendations. Among those with a BMI <25 (one of the three healthful behaviors comprising the score), 77% had a lifestyle consistent with all three recommended behaviors. Among those reporting high education, 27% met all three healthy lifestyle recommendations, while 0% of those reporting low education met these three recommendations (p= 0.02).
|
Table 1. Participant characteristics of patients living with NMIBC (n=113)* |
||
|
Items |
Characteristic, Median (IQR) or n (%) |
|
|
Total |
n = 113* |
|
|
Age at survey start |
67 (59, 73) |
|
|
Sex |
Male |
76 (67%) |
|
Female |
37 (33%) |
|
|
Grade at initial diagnosis |
High-grade |
72 (65%) |
|
Low-grade |
38 (34%) |
|
|
PUMLMP |
1 (1%) |
|
|
Stage at initial diagnosis |
Ta |
66 (58%) |
|
T1 |
29 (26%) |
|
|
CIS only |
4 (3%) |
|
|
Papillary tumor with CIS |
9 (8%) |
|
|
Ta with CIS |
6 (5%) |
|
|
T1 with CIS |
2 (2%) |
|
|
Ta and T1 with CIS |
1 (1%) |
|
|
Missing initial pathology |
2 (2%) |
|
|
Highest level of education |
Grade school |
1 (1%) |
|
High school |
14 (12%) |
|
|
2-year college |
16 (14%) |
|
|
4-year college |
29 (26%) |
|
|
Graduate/professional |
53 (47%) |
|
|
History of occupational exposure(s) |
Agent Orange |
1 (1%) |
|
Smoking statusa,b |
Never |
62 (55%) |
|
Former |
49 (43%) |
|
|
Pack-yearsc |
10 (4, 21) |
|
|
Current |
2 (2%) |
|
|
Pack-yearsc |
16 (13, 19) |
|
|
Body mass index (BMI) at survey starta,d |
26 (24, 29) |
|
|
Physical activity |
Total MET-hr/week |
28.5 (7.5, 51.2) |
|
Moderate-Vigorous Aerobic, MET- hr/week |
23.0 (4.6, 49.5) |
|
|
Walking, hr/week |
2.5 (1.0, 5.0) |
|
|
Strength Training, min/week |
0 (0, 60) |
|
|
Servings/day, over past year, Median (IQR) |
Total fruits |
2.0 (1.2, 3.5) |
|
Total vegetables |
3.6 (2.4, 5.4) |
|
|
Total broccoli |
1 (1, 3) |
|
|
% cookede |
90 (70, 100) |
|
|
% uncookede |
10 (0, 25) |
|
|
Cruciferous vegetables |
0.6 (0.3, 1.1) |
|
|
Lean protein |
1.1 (0.6, 1.6) |
|
|
Healthy fats |
1.6 (1.0, 2.4) |
|
|
Processed meats |
0.2 (0, 0.4) |
|
|
Red meat |
0.3 (0.1, 0.5) |
|
|
Dairy |
1.3 (0.6, 2.1) |
|
|
Sweets |
1 (.3, 1.4) |
|
|
Total liquids |
3.4 (2, 5.1) |
|
|
Drinks with alcohol |
0.4 (0.1, 1) |
|
|
Drinks without alcohol |
2.7 (1.3, 3.9) |
|
|
Supplements |
Multivitamins |
41 (40%) |
|
Vitamin B9 (folate) |
3 (3%) |
|
|
Vitamin E (Current) |
6 (6%) |
|
|
Vitamin E (Past) |
32 (32%) |
|
|
Probioticsa |
17 (35%) |
|
|
aSelf-reported bCurrent or former smoking status defined as 20 packs or more over lifetime cCalculated from packs/day and years smoked, with less than ½ pack/day approximated as ¼ pack/day dCalculated from self-reported height and weight eSlight modifications were made to the questionnaire at 7 months to be more inclusive of the diet and supplement literature (i.e., what proportion of consumed broccoli is raw, probiotics, glyburide); thus, only 48 of 113 participants contributed responses to these questions. *Includes all patients with clinical data who completed at least one survey. |
||
|
Table 2. Lifestyle characteristics by body mass index (BMI)* |
|||
|
Subgroup |
BMI <25 (N=36) |
BMI ³25 (N=77) |
|
|
Food Groups |
Vegetables, s/d |
3.3 (2.4, 4.1) |
3.8 (2.4, 5.7) |
|
Cruciferous Vegetables, s/d |
0.5 (0.4, 1.0) |
0.6 (0.3, 1.1) |
|
|
Fruit, s/d |
2.4 (1.2, 3.2) |
2.0 (1.1, 3.6) |
|
|
Lean Protein, s/d |
0.9 (0.6, 1.3) |
1.1 (0.6, 1.8) |
|
|
Red Meat, s/d |
0.1 (0, 0.3) |
0.4 (0.2, 0.6) |
|
|
Processed Meat, s/d |
0.1 (0, 0.4) |
0.3 (0.1, 0.5) |
|
|
Healthy Fat, s/d |
1.8 (1.1, 2.6) |
1.6 (1.0, 2.3) |
|
|
Dairy, s/d |
1.1 (0.5, 2.1) |
1.3 (0.9, 2.1) |
|
|
Alcohol, s/d |
0.4 (0.1, 1.0) |
0.4 (0.1, 1.0) |
|
|
Broccoli, s/d |
1.0 (1.0, 3.0) |
1.0 (0.5, 3.0) |
|
|
Cooked, s/d |
97.5 (77.5, 100.0) |
90.0 (70.0, 100.0) |
|
|
Uncooked, s/d |
2.5 (0.0, 22.5) |
10.0 (0.0, 30.0) |
|
|
Mutagens |
Phip, ng/g |
24.1 (1.6, 107.8) |
67.1 (14.3, 171.5) |
|
Meiqx, ng/g |
5.3 (1.4, 23.3) |
12.8 (4.0, 31.1) |
|
|
Dimeiqx, ng/g |
0.4 (0, 1.6) |
1.0 (0.3, 3.0) |
|
|
Actual total mutagenicity, ng/g |
1676.7 (217.5, 3951.4) |
2878.1 (910.2, 5866.7) |
|
|
Predicted total mutagenicity, ng/g |
968.4 (543.3, 3182.1) |
2496.4 (988.1, 5269.7) |
|
|
B[a]p, ng/g |
3.1 (0.1, 23.6) |
26.3 (0.9, 52.5) |
|
|
Supplement Use |
Multivitamin use, yes, % |
33.3 |
42.9 |
|
Single supplement use, yes, % |
78.8 |
80.9 |
|
|
Physical Activity |
Total, MET-hr/week |
41.8 (19.9, 56.2) |
21.8 (6.4, 51.2) |
|
Moderate-Vigorous Aerobic, MET-hr/week |
34.2 (10.8, 49.5) |
21.0 (3.3, 48.7) |
|
|
Walking, hr/week |
3.8 (1.3, 5.0) |
2.5 (0.7, 5.0) |
|
|
Strength Training, min/week |
0 (0, 52.8) |
0 (0, 78.0) |
|
|
Meeting exercise recommendation, % |
85.3 |
68.0 |
|
|
*Bolded results denotes p-values of <0.05 based on Mann-Whitney U Test or Kruskal-Wallis Test for continuous variables testing differences in the distribution of the data among the groups, and the Chi-Square test or Fisher’s Exact Test for categorical variables. |
|||
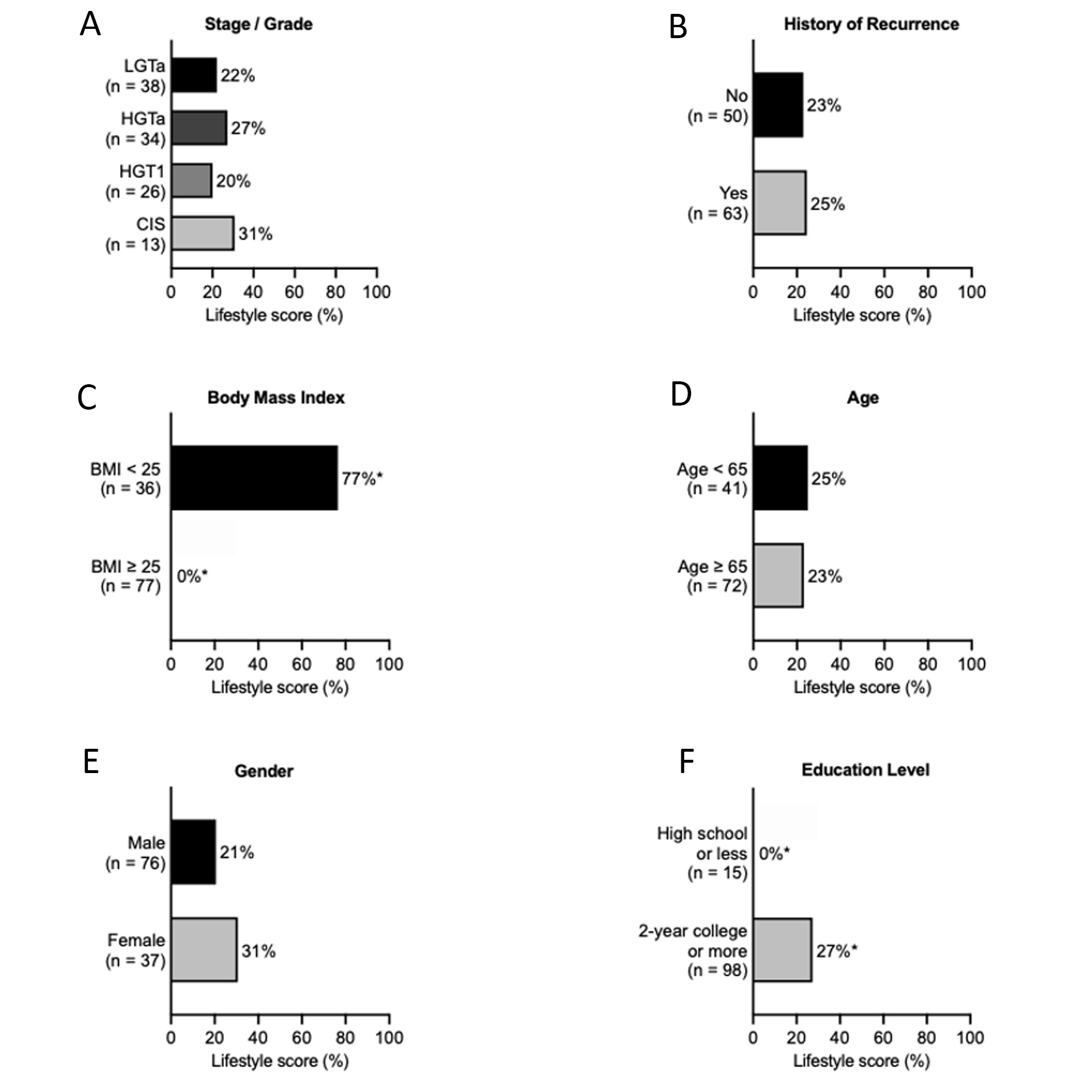 Figure 1. Percentage of participants meeting all three criteria of the Lifestyle Score: Body mass index <25, never smoker or quit at least 10 years ago, and meeting the national physical activity guidelines of ≥150 minutes of aerobic exercise per week. *p-value<0.05.
Figure 1. Percentage of participants meeting all three criteria of the Lifestyle Score: Body mass index <25, never smoker or quit at least 10 years ago, and meeting the national physical activity guidelines of ≥150 minutes of aerobic exercise per week. *p-value<0.05.
Stratifying by education revealed statistically significant differences in dietary and physical activity habits. Most notably, those with lower education demonstrated higher processed meat consumption, less healthy fat consumption, higher Meiqx mutagen intake, and higher predicted total mutagen intake, while patients classified as overweight had elevated B[a]p mutagen levels. Associations among red meat, processed meat, and mutagen intake and bladder cancer carcinogenesis remain unclear. A “western diet” consisting of high quantities of red meat and fried foods has been previously associated with bladder cancer recurrence, [20] while for bladder cancer incidence, a meta-analysis reported a summary relative risk estimate of 1.22 (95% CI: 1.04, 1.43) for high vs. low processed meat intake [21] and a separate prospective observational study reported elevated hazard ratios of 1.29 (95% CI: 1.00, 1.67) and 1.19 (95% CI: 0.95, 1.48) between the first and fifth quintiles of nitrate and nitrite from processed meat, and PhIP mutagen levels, respectively [16]. In addition, we also report that no one reporting low levels of education met all three non-dietary healthy lifestyle recommendations, compared to 27% reporting a high level of education. A handful of prior studies suggest an association between education level and worse bladder cancer outcomes, [22-24] with one study using education as a proxy for SES [22]. However, none had investigated associations with composite diet and physical activity habits in patients with NMIBC as in our study.
Importantly, physical activity appeared to decrease with increasing age and BMI, and increase with higher education level. While current Physical Activity Guidelines for Americans recommending that engaging in physical activity may be associated with lower risks of several cancers including bladder cancer, [25] no studies have evaluated physical activity and clinical outcomes after NMIBC diagnosis. The pending Bladder cancer and exeRcise trAining during intraVesical thErapy (BRAVE) trial (NCT04593862) will examine the safety and feasibility of a 12-week exercise intervention in patients with bladder cancer undergoing intravesical therapy for NMIBC and may shed some light on impact to cancer outcomes (at 3-month and 1-year follow-up by cystoscopy) [26].
Limitations include the cross-sectional design and small sample size; however, to our knowledge this is the largest study to describe lifestyle factors, including mutagen intake, across demographic characteristics in patients with NMIBC. Similar to our study, a Canadian study reported that bladder cancer survivors (NMIBC and MIBC) were not meeting guidelines for important lifestyle behaviors, [27] and one other study in NMIBC patients has reported changes in behaviors over time, noting that consumption of red and processed meat, fruit and vegetables, and current smoking status, all declined over time in the first 15 months after diagnosis [28]. Our study population was comprised of highly educated, mostly White bladder cancer patients with intermediate- and high-risk bladder cancer. To improve generalizability to the general clinical population, a concerted effort to recruit diverse study participants will be required in future studies. The self-reported nature of this questionnaire predisposes our findings to recall bias. In addition, the study duration overlapped with the COVID-19, which may have impacted food sources; however, surveys were completed before 03/27/2020, which coincided with the start of COVID-19 shelter-in-place in the San Francisco Bay Area starting 03/17/2020. This study was exploratory to generate hypotheses for future research. Future longitudinal studies should include larger sample sizes to further examine these preliminary findings and determine the impact of lifestyle factors (e.g., diet, physical activity) and the risk of bladder cancer recurrence or progression, and whether certain pre-diagnosis behaviors are linked with poorer stage or grade at diagnosis of NMIBC. Future qualitative studies should be conducted to examine the context, attitudes, and rationale for specific lifestyle habits in this population, particularly as dietary and exercise behaviors are heavily contingent upon social factors. Clinical trials to assess efficacy of various diet interventions are needed to definitively guide dietary management for these patients.
Nevertheless, this study contributes to an understanding of modifiable lifestyle factors in bladder cancer prognosis and which sociodemographic characteristics may be associated with healthier behaviors. Providing clinical guidance to NMIBC patients on general healthy lifestyle recommendations, especially to those who are overweight/obese, older, and those with less education, may be warranted to improve outcomes for cancer and overall health.
Supplemental Table 2 Additional participant characteristics in patients with NMIBC (n = 113).
Supplementary Table 3 Lifestyle characteristics in patients with NMIBC by age, gender, and education level.
We thank the participants for their valuable contributions.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
SPP, SAK: Conception; EYW, MAP, SAK: Performance of work; EYW, MAP, MVM, SPP, SAK: Interpretation of data; EYW, SPP, SAK: Writing the article.
Competing interests
We have no disclosures that are related to the current study.
Funding
Dr. Kenfield is supported by the Helen Diller Family Chair in Population Science for Urologic Cancer at the University of California, San Francisco.
- Siegel RL, Miller KD, Wagle NS, Jemal A: Cancer statistics, 2023. CA Cancer J Clin 2023, 73(1): 17-48.
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD et al: Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016, 196(4): 1021-1029.
- Rink M, Furberg H, Zabor EC, Xylinas E, Babjuk M, Pycha A, Lotan Y, Karakiewicz PI, Novara G, Robinson BD et al: Impact of smoking and smoking cessation on oncologic outcomes in primary non-muscle-invasive bladder cancer. Eur Urol 2013, 63(4): 724-732.
- van Osch FH, Jochems SH, van Schooten FJ, Bryan RT, Zeegers MP: Significant Role of Lifetime Cigarette Smoking in Worsening Bladder Cancer and Upper Tract Urothelial Carcinoma Prognosis: A Meta-Analysis. J Urol 2016, 195(4 Pt 1): 872-879.
- Zuniga KB, Graff RE, Feiger DB, Meng MV, Porten SP, Kenfield SA: Lifestyle and Non-muscle Invasive Bladder Cancer Recurrence, Progression, and Mortality: Available Research and Future Directions. Bladder Cancer 2020, 6(1): 9-23.
- Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, Pendleton EM, Celaya MO, Zens MS, Karagas MR et al: Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer 2014, 120(3): 408-414.
- Liss MA, White M, Natarajan L, Parsons JK: Exercise Decreases and Smoking Increases Bladder Cancer Mortality. Clin Genitourin Cancer 2017, 15(3): 391-395.
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr., Schmitz KH, Emplaincourt PO et al: Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000, 32(9 Suppl): S498-504.
- Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC: Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996, 7(1): 81-86.
- Physical Activity Guidelines Advisory Committee: Physical Activity Advisory Committee Report, 2008. In. Epub ahead of print. Washington, DC: Department of Health and Human Services; 2008.
- Physical Activity Guidelines for Americans, 2nd edition.
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M et al: Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012, 62(4): 243-274.
- Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC: Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993, 93(7): 790-796.
- CHARRED: Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease [https://dceg.cancer.gov/tools/design/charred].
- Sinha R, Cross A, Curtin J, Zimmerman T, McNutt S, Risch A, Holden J: Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res 2005, 49(7): 648-655.
- Ferrucci LM, Sinha R, Ward MH, Graubard BI, Hollenbeck AR, Kilfoy BA, Schatzkin A, Michaud DS, Cross AJ: Meat and components of meat and the risk of bladder cancer in the NIH-AARP Diet and Health Study. Cancer 2010, 116(18): 4345-4353.
- Wu JW, Cross AJ, Baris D, Ward MH, Karagas MR, Johnson A, Schwenn M, Cherala S, Colt JS, Cantor KP et al: Dietary intake of meat, fruits, vegetables, and selective micronutrients and risk of bladder cancer in the New England region of the United States. Br J Cancer 2012, 106(11): 1891-1898.
- Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, Neuhouser ML, Bandera EV, Wang Y, Robien K et al: American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 2022, 72(3): 230-262.
- CDC: Cancer Care Settings and Smoking Cessation. [https://www.cdc.gov/tobacco/patient-care/care-settings/cancer/].
- Westhoff E, Wu X, Kiemeney LA, Lerner SP, Ye Y, Huang M, Dinney CP, Vrieling A, Tu H: Dietary patterns and risk of recurrence and progression in non-muscle-invasive bladder cancer. Int J Cancer 2018, 142(9): 1797-1804.
- Li F, An S, Hou L, Chen P, Lei C, Tan W: Red and processed meat intake and risk of bladder cancer: a meta-analysis. Int J Clin Exp Med 2014, 7(8): 2100-2110.
- Russell B, Hemelrijck MV, Gardmark T, Holmberg L, Kumar P, Bellavia A, Haggstrom C: A mediation analysis to explain socio-economic differences in bladder cancer survival. Cancer Med 2020, 9(20): 7477-7487.
- Weiner AB, Keeter MK, Manjunath A, Meeks JJ: Discrepancies in staging, treatment, and delays to treatment may explain disparities in bladder cancer outcomes: An update from the National Cancer Data Base (2004-2013). Urol Oncol 2018, 36(5): 237 e239-237 e217.
- Golombos DM, O'Malley P, Lewicki P, Nguyen DP, Stone BV, Al Hussein Al Awamlh B, Scherr DS: The impact of socioeconomic status on perioperative complications and oncologic outcomes in patients undergoing radical cystectomy. World J Urol 2017, 35(7): 1063-1071.
- U.S. Department of Health and Human Services: Physical Activity Guidelines for Americans. In. Epub ahead of print., 2nd edn. Washington, DC; 2018.
- Arthuso FZ, Fairey AS, Boule NG, Courneya KS: Bladder cancer and exeRcise trAining during intraVesical thErapy-the BRAVE trial: a study protocol for a prospective, single-centre, phase II randomised controlled trial. BMJ Open 2021, 11(9): e055782.
- Chung J, Kulkarni GS, Bender J, Breau RH, Guttman D, Maganti M, Matthew A, Morash R, Papadakos J, Jones JM: Modifiable lifestyle behaviours impact the health-related quality of life of bladder cancer survivors. BJU Int 2020, 125(6): 836-842.
- Beeren I, Goeij L, Dandis R, Vidra N, Zutphen MV, Witjes JA, Kampman E, Kiemeney L, Vrieling A: Limited Changes in Lifestyle Behaviours after Non-Muscle Invasive Bladder Cancer Diagnosis. Cancers (Basel) 2022, 14(4): 960.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript