Research Article | Open Access
Oncolytic and Immunotherapeutic CG0070 Adenovirus for High-Risk Bacillus Calmette-Guerin Unresponsive Bladder Cancer
Evan Austin1, Debra Mobley2, Jamaka Tarajkowski2, Donald Lamm2, 1
1University of Arizona College of Medicine-Phoenix, Phoenix, Arizona 85004, United States of America.
2Bladder Cancer Genitourinary Oncology, Phoenix, Arizona 85032, United States of America.
Correspondence: Donald Lamm (University of Arizona College of Medicine-Phoenix, Phoenix, Arizona 85004, United States of America; Email: dlamm@bcgoncology.com).
Annals of Urologic Oncology 2021; 4(2): 80-86. https://doi.org/10.32948/auo.2021.12.22
Received: 17 Jan 2021 | Accepted: 20 Dec 2021 | Published online: 31 Dec 2021
Methods 15 patients with residual high grade BCG-unresponsive CIS +/- Ta/T1/T2 bladder cancer received one or more 6-week instillations of intravesical CG0070 and were retrospectively reviewed. Overall response including the number, location, grade and stage of recurrences, were recorded. Side effects of intravesical instillation of CG0070 were also investigated. 11 of the 15 patients had at least 2.5 years of follow up both before and after treatment, permitting statistical chi-square analysis for the 2.5 year pre- and post-CG0070 periods.
Results Of the 15 patients, 5 had Ta + Cis, 4 had T1 + CIS, 4 had CIS alone, and 2 had T2 + CIS prior to CG0070 instillation. Complete response of CIS was seen in 60% at 6 months, 47% at 12 months, and 40% at 24 months. Overall, 40% of patients remained tumor free and none progressed. For the 11 patients amenable to statistical analysis, 32 recurrences were noted within 2.5 years before therapy and 13 2.5 years after (p <0.01). 40% of patients experienced no adverse events as a result of treatment. Most common side effects were hematuria (33.3%), malaise/fatigue (33.3%), and urgency/frequency (26.7%).
Discussion Treatment with intravesical CG0070 for high-risk BCG-unresponsive bladder cancer appears to be a promising salvage regimen worthy of further investigation.
Key words non-muscle invasive bladder cancer, oncolytic adenovirus, intravesical therapy, BCG unresponsive
To address this problem, viral gene therapy has been introduced as a potential solution for patients with NMIBC and BCG failure who do not undergo radical cystectomy. These viruses have the theoretical advantage of selective replication in tumor cells, leading to cell lysis and increased immune response to both oncolytic virions and tumor-specific antigens [9]. One such virus, CG0070, is a conditionally replicating adenovirus with an Rb promoter and human granulocyte macrophage colony-stimulating factor (GM-CSF) gene designed to take advantage of the defects in the retinoblastoma (Rb) pathway found in most cancers including urothelial carcinoma [10]. Here we present our institution’s experience and response results in 15 patients with high-risk BCG–unresponsive NMIBC who were treated with at least one six-week course of intravesical CG0070.
Study population and design
Patients who received at least one six-week course of intravesical CG0070 were identified (n=15) and retrospectively reviewed. Post CG0070 number, location, grade and stage of recurrences, progression to muscle invasion/metastasis, and overall length of follow up were recorded, as well as all cause and bladder-cancer specific survival. Side effects occurring post intravesical instillation were noted. Inclusion criteria specified patients with pathologically-confirmed NMIBC who had received at least two or more prior courses of intravesical therapy per recommended schedules, of which at least one therapy was BCG. The first course of BCG required at least 6 weekly treatments, and the second course required at least 2 weekly treatments. The term BCG un-responsive is defined as patients with BCG refractory disease (failure to achieve disease-free state at 6 months following initial BCG therapy with either maintenance of retreatment at 3 months because of persistent or rapidly recurrent tumor), BCG-resistant (rapid recurrence/persistence at 3 months), or BCG-relapsing disease (recurrence of disease after achieving disease free-state by 6 months). Patients with previous systemic chemotherapy or radiation therapy for bladder cancer, those with a history of an immunocompromised state, those on immunosuppressive or immunomodulatory agents, and those with a history of other clinically-significant malignancy were excluded. This IRB-approved study is a part of a larger single-arm, phase II, multicenter study (clinicaltrials.gov NCT02365818) assessing the safety and efficacy of CG0070 in patients with NMIBC who have failed BCG therapy and refused cystectomy [11]. These 15 patients were included in the latest interim analysis of the multicenter study [12]. Special permission was obtained from Cold Genesys to retrospectively review the patients from our institution who had received intravesical CG0070.
Study variables
The following variables were collected from patients’ medical records: gender, age, ethnicity, tobacco exposure, the initial date and stage/grade of bladder cancer diagnosis, the number and stage/grade of recurrences before CG0070 treatment, date of CG0070 treatment(s), side effects experienced while undergoing CG0070 therapy, the number and stage/grade of recurrences after CG0070 treatment, involvement (if any) of bladder cancer in bladder diverticula, deBruhn nest, prostatic urethra or upper tract in initial diagnosis of bladder cancer or on subsequent recurrences, length of follow up from date of CG0070 treatment to last cystoscopy, the length of follow up from date of CG0070 treatment to last documented patient encounter, and date and cause of mortality.
CG0070 treatment protocol
Patients received 6 weekly instillations of intravesical CG0070 on days 1, 8, 15, 22, 29, and 36 at dose levels ranging from 1 * 1012 to 3 *1013 viral particles (vp), using the same dose for all 6 treatments. Pretreatment of 5% DDM, a nonionic surfactant transduction agent that acts as a mild detergent and solubilizing agent, was given via a 100% silicone catheter. Pretreatment consisted of an intravesical wash with 100 ml normal saline, followed by an intravesical wash with 75 mL of 0.1% DDM. Patients then received intravesical instillation of 100 mL of 0.1% DDM, which was retained in the bladder for 15 minutes, followed by a subsequent rinse with 100 mL of saline. Following pretreatment, CG0070 in100 mL saline was instilled for dwelling time of 45-50 minutes, during which the patient was repositioned from left side, right side, abdomen, and back every 10-12 minutes to maximize bladder surface exposure to CG0070. Patients were advised to limit fluid intake, hold medications unless medically necessary, and avoid caffeine for 8-24 hours prior to instillation and were asked to retain the medication in bladder for recommended time period.
Surveillance
Following initiation of 6 weekly instillations, patients were evaluated at 3 months with cystoscopy, bladder biopsy if lesions were seen, and urine cytology. At 6 months, patients underwent cystoscopy and random biopsies if no visible tumor was present to evaluate for treatment response. Patients were then followed every 3 months with cystoscopy, biopsy if lesions seen, and urine cytology. After initial demonstration of safety, patients received further 6 week courses of maintenance intravesical CG0070 following the 6 month evaluation. If recurrence was found further treatment could include but was not limited to CG0070. Recurrence was defined by pathologically confirmed disease following bladder biopsy, TURBT, or office ablation for small tumors with a positive urine cytology. A positive urine cytology alone was not criteria for recurrence.
Statistical analysis
Statistical analysis and methodology was loosely based on Morales et al. original documentation of intravesical BCG in the treatment of superficial bladder tumors [13]. Similar to their problem, wide variation in pre-and post-CG0070 treatment periods invalidates traditional statistical analysis for all 15 subjects in our study. To address this, 11 of the 15 patients had at least 2.5 years of follow up both before and after treatment of CG0070. As such, an analysis was done on these 11 patients for recurrences 2.5 years before and after instillation of CG0070. A chi-square test was conducted for these 11 patients for the 2.5 year pre-CG0070 and 2.5 year post-CG0070 periods.
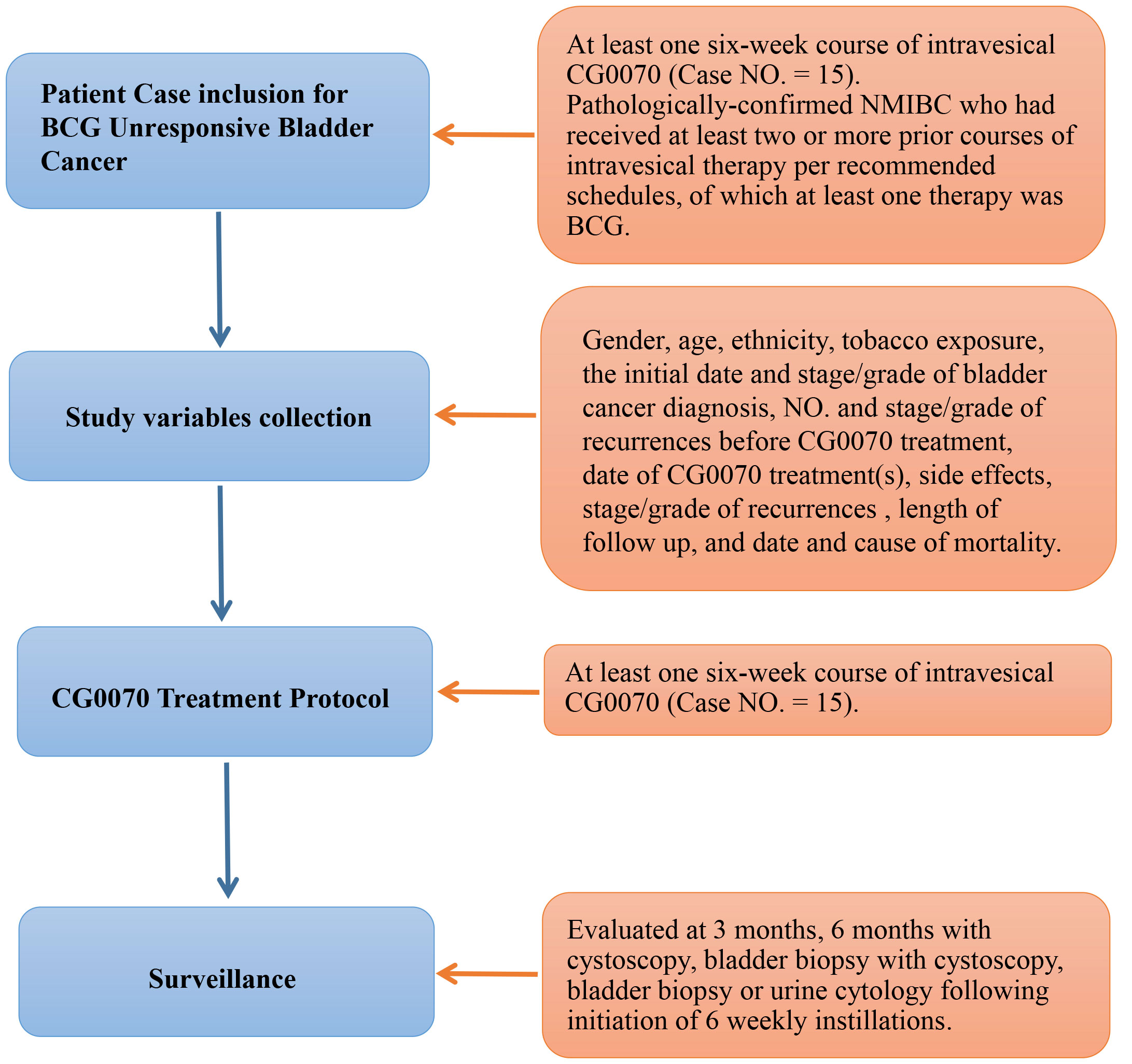 Figure 1. Progressing Flow-chart of the study.
Figure 1. Progressing Flow-chart of the study.
The total number of recurrences before and after CG0070 treatment are described in Table 2. Greater than 92 recurrences were noted for all 15 patients prior to CG0070 exposure. 100% of patients had CIS prior to CG0070 administration. 4 patients (27%) had CIS alone, 5 (33%) had Ta + CIS, 4 (27%) had T1 + CIS, and 2 (13%) had prior T2 and persistent CIS. Following treatment of CG0070 there were 22 recurrences, a 76% decrease in bladder cancer recurrences overall. Six patients (40%) had no recurrence following therapy, 5 (33%) had recurrence of CIS alone, 2 (13%) had HG Ta + CIS. Three had these recurrences in prostatic tissue. Two (13%) had HG T1 + CIS recurrence but no patient had progression of their bladder cancer. Complete response of CIS was seen in 9 patients (60%) at 6 months, 7 (46.7%) at 12 months, and 6 (40%) at 24 months.
Despite these findings, wide variation in pre-and post-CG0070 treatment periods precludes traditional statistical analysis for the data described above. To address this, 11 of the 15 (73%) patients had at least 2.5 years of follow up both before and after treatment of CG0070. Of the four patients that did not have at least 2.5 years of follow up, 1 was lost to follow up and 3 patients died, 2 of which died of a cause unrelated to bladder cancer and 1 died of an unknown cause. Considering the patients who were evaluable for 2.5 year follow up, 5 (45%) patients had highest stage of recurrence being CIS alone, 3 (27%) had Ta + CIS, 2 (18%) had T1 + CIS, and 1 (9%) had T2 + CIS prior to CG0070 treatment. 32 recurrences were noted before therapy whereas only 13 recurrences were recorded after, accounting for a statistically significant difference (p <0.01). After treatment 5 (45%) patients experienced no recurrence, 3 (27%) experienced CIS alone, 2 (18%) experienced stage of Hg Ta + CIS, and only 1 (9%) had HG T1 + CIS.
A complete breakdown regarding the time points stage of recurrences 2.5 years before and after CG0070 therapy are demonstrated in Table 3.
Side effects experienced during the 6 week treatment period are described in figure 4. Six patients (40%) experienced no symptoms related to the treatment. Hematuria and generalized malaise or fatigue were the most common side effects experienced with 33.3%, followed by urgency or frequency (26.7%) and dysuria (20%). All 15 patients completed the 6 week therapy. For those that did experience side effects, none had to interrupt the therapy because of the severity of side effects (Table 4).
|
Table 1. Baseline Characteristics. |
|
|
Items |
Number |
|
Number of patients |
15 |
|
Age, years (median) |
71.5 |
|
Caucasian race, n (%) |
14 (93%) |
|
Smoke Exposure, n (%) |
12 (80%) |
|
Table 2. Results of CG0070. |
|||
|
Pre-CG0070 |
Post-CG0070 |
||
|
Total Recurrences Before |
92 + |
Total Recurrences After |
22 |
|
Highest Stage, n (%) |
|
Highest Stage, n (%) |
|
|
Ta + CIS |
5 (33%) |
No recurrence Overall |
6 (40%) |
|
T1 + CIS |
4 (27%) |
at 6 months |
9 (60%) |
|
CIS alone |
4 (27%) |
at 12 months |
7 (47%) |
|
T2 + CIS |
2 (13 %) |
at 24 months |
6 (40%) |
|
|
|
CIS alone |
5 (33%) |
|
|
|
HG T1 + CIS |
2 (13%) |
|
|
|
Prostatic HG Ta + CIS |
2 (13%) |
|
|
|
|
|
|
|
|
Progression |
0 |
|
|
|
Deaths unrelated to bladder cancer |
2 |
|
|
|
Death of unknown cause |
1 |
|
|
|
|
|
|
Recurrences 2.5 years before* |
32 |
Recurrences 2.5 years after* |
13 (p<0.01) |
|
|
|
|
|
|
Highest Stage, n (%) |
|
Highest stage, n (%) |
|
|
CIS alone |
5 (45%) |
No recurrence |
5 (45%) |
|
Ta + CIS |
3 (27%) |
CIS alone |
3 (18%) |
|
T1 + CIS |
2 (18%) |
Prostatic HG Ta + CIS |
2 (18%) |
|
T2 + CIS |
1 (9%) |
HG T1 + CIS |
1 (9%) |
|
*Amenable to statistical analysis |
|||
|
Table 3. 2.5 year CG0070 timetable. |
|||||||||||
|
|
Pre-CG0070 |
|
Post-CG0070 |
||||||||
|
Pt |
-2.5 Years |
-2.0 Years |
-1.5 Years |
-1.0 Years |
-0.5 Years |
CG0070 |
0.5 Years |
1.0 Years |
1.5 Years |
2.0 Years |
2.5 Years |
|
1 |
|
|
HG T1 |
|
HG CIS |
|
|
|
|
|
|
|
2 |
|
|
|
HG Ta* |
HG CIS bladder & prostate HG CIS prostate |
|
|
HG CIS Prostate |
|
|
|
|
3 |
|
HG T2/CIS |
|
HG Ta |
HG CIS R UO |
|
|
HG T1 |
|
|
LG Ta bladder/HG Ta Prostate Positive cytology CIS***** |
|
4 |
HG CIS |
|
HG CIS |
|
LG Ta/CIS** |
|
|
|
|
|
|
|
5 |
|
|
In-office ablation HG Ta |
|
HG CIS |
|
|
|
|
|
|
|
6 |
|
HG T1 |
HG T1 |
HG T1/CIS |
HG T1/Ta CIS |
|
|
|
HG Ta + CIS HG Ta |
CIS |
|
|
7 |
|
|
|
|
HG CIS bladder & prostate |
|
CIS |
|
|
|
|
|
8 |
|
|
HG Ta HG CIS |
HG CIS |
HG CIS |
|
|
|
|
|
|
|
9 |
|
|
HG Ta HG Ta & CIS Bladder, CIS prostate |
HG Ta/CIS |
HG Ta Bladder & L UO |
|
HG Ta/CIS |
CIS L UO |
HG Ta/CIS Bladder and Prostate*** HG CIS R Kidney**** |
|
|
|
10 |
HG T1/CIS, HG CIS |
|
|
|
HG CIS |
|
|
|
|
|
|
|
11 |
|
|
|
|
CIS |
|
CIS |
|
|
|
|
|
* HG Ta in bladder and prostatic urethra; |
|||||||||||
|
**LG Ta and CIS with involvement of prostate and Left Ureteral Orrifice; |
|||||||||||
|
***S/P Radical cystectomy with neoadjuvant chemo; |
|||||||||||
|
****S/P nephroureterectomy; |
|||||||||||
|
*****S/P in-office ablation. |
|||||||||||
|
Table 4. Side Effects. |
|
|
CG0070 Side Effects |
Number (% Frequency) |
|
None |
6 (40%) |
|
Hematuria |
5 (33.3%) |
|
Malaise/fatigue |
5 (33.3%) |
|
Urgency/Frequency |
4 (26.7%) |
|
Dysuria |
3 (20%) |
|
Flank pain |
2 (13.3%) |
|
Fever |
2 (13.3%) |
|
Flu like symptoms |
1 (6.7%) |
|
Abd pain |
1 (6.7%) |
|
Nausea |
1 (6.7%) |
|
Abdominal Rash |
1 (6.7%) |
CG0070, a conditionally replicated GM-CSF-armed oncolytic adenovirus, takes advantage of the RB pathway defect in urothelial carcinoma to induce cytotoxicity of tumor cells while simultaneously increasing the immune response to both oncolytic virions and tumor-specific antigens [9, 10]. Our experience with CG0070 suggests that this form of treatment may alter the recurrence pattern of high-risk, treatment resistant disease. Considering the recurrent shortages of BCG and the observation that to date CG0070 appears to have no significant adverse or long-lasting side effects, this treatment could become a welcome advance in the treatment of NMIBC.
When compared to other emerging treatments for BCG unresponsive NMIBC, particularly among other viral gene therapies, our results demonstrate comparable or superior efficacy despite a higher risk patient population with 100% of patients having CIS, and others having prostatic, upper tract and even prior muscle involvement. Instiladrin (rAD-IFN[alpha]/Syn3) is another adenovirus currently in phase III trials that reported 35% of 43 patients with high grade BCG refractory bladder cancer remained disease free at one year [17]. Another viral gene therapy, ALT-801, a recombinant fusion protein consisting of IL-2 linked to a T-cell-receptor domain that can recognize a peptide of the p53 antigen, is currently being tested in combination with gemcitabine in a phase Ib/II trial with preliminary results demonstrating a complete durable response >12 months in 2 of 6 patients [16].
This study is limited by its retrospective design and lack of a comparison control. Furthermore, it is important to note that our results reflect just our single institution’s experience with CG0070 and therefore are not necessarily reflective of other institutions using CG0070. Our relatively small cohort size reduces the power of statistical analysis. Additionally, 93% of our patients were Caucasian and 93% were male, creating a fairly restricted distribution of sex and ethnicity.
We would like to thank and acknowledge all of the staff from the BCG Oncology office, especially Javon Freeman, for all of their help and support.
We would also like to acknowledge Cold Genesys for their special permission and support for this study.
Ethical policy
This IRB approved study is a part of a larger single-arm, phase II, multicenter study (clinicaltrials.gov NCT02365818) assessing the safety and efficacy of CG0070 in patients with NMIBC who have failed BCG therapy and refused cystectomy. These 15 patients were included in the latest interim analysis of the multicenter study. Special permission was obtained from Cold Genesys to retrospectively review the patients from our institution who had received intravesical CG0070.
Author contributions
EA: performed the majority of the retrospective review, data analysis, and drafted the manuscript. DM: assisted with the retrospective review and methodology and regulatory compliance for the study. JT: also assisted with the retrospective review and assisted in the initial CG0070 treatment protocol in compliance with the original single-arm, phase II, multicenter study (clinicaltrials.gov NCT02365818). DL: is the principal investigator for the project and oversaw all aspects of the project and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
All authors declare no competing interests.
Funding
All authors declare that they do not receive grants or financial support for this study.
- Soukup V, Čapoun O, Cohen D, Hernández V, Babjuk M, Burger M, Compérat E, Gontero P, Lam T, MacLennan S, et al: Prognostic Performance and Reproducibility of the 1973 and 2004/2016 World Health Organization Grading Classification Systems in Non-muscle-invasive Bladder Cancer: A European Association of Urology Non-muscle Invasive Bladder Cancer Guidelines Panel Systematic Review. Eur Urol 2017, 72(5): 801-813.
- Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M, et al: EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017, 71: 447-461.
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, Wolf JS Jr, Schellhammer PF: Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007, 178: 2314-2330.
- Brausi M, Witjes JA, Lamm D, Persad R, Palou J, Colombel M, Buckley R, Soloway M, Akaza H, Böhle A: A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 2011; 186: 2158-2167.
- Böhle A, Bock P: Intravesical bacille calmette-guérin versus mitomycin c in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology 2004, 63(4): 682-686.
- Sylvester RJ, van der Meijden APM, Lamm DL: Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 2002, 168: 1964-1970.
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, Pruthi R, Quale DZ, Ritch CR, Seigne JD, et al: Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016, 196(4): 1021-1029.
- Kamat AM, Colombel M, Sundi D, Lamm D, Boehle A, Brausi M, Buckley R, Persad R, Palou J, Soloway M, et al: BCG-unresponsive non-muscle-invasive bladder cancer: recommendations from the IBCG. Nat Rev Urol 2017, 14(4): 244-255.
- Ramesh N, Ge Y, Ennist DL, Zhu M, Mina M, Ganesh S, & Seshidhar Reddy P: CG0070, a Conditionally Replicating Granulocyte Macrophage Colony-Stimulating Factor -- Armed Oncolytic Adenovirus for the Treatment of Bladder Cancer. Clin Cancer Res 2006, 12(1): 305-313.
- Sherr CJ, McCormick F: The Rb and p53 pathways in cancer. Cancer Cell 2002, 2(2): 103-112.
- Burke JM, Lamm DL, Meng MV, Nemunaitis JJ, Stephenson JJ, Arseneau JC, Aimi J, Lerner S, Yeung AW, Kazarian T, et al: A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol 2012, 188(6): 2391-2397.
- Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL 3rd, Clark W, Kroeger M, Dumbadze I, Chamie K, et al: An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol 2018, 36(10): 440-447.
- Morales A, Eidinger D, & Bruce AW: Intracavitary Bacillus Calmette-Guerin in the Treatment of Superficial Bladder Tumors. J Urol 2017, 197(2): S142-S145.
- Harland SJ, Kynaston H, Grigor K, Wallace DM, Beacock C, Kockelbergh R,Clawson S, Barlow T, Parmar MK, Griffiths GO: National Cancer Research Institute Bladder Clinical Studies Group. A randomized trial of radical radiotherapy for the management of pT1G3 NXM0 transitional cell carcinoma of the bladder. J Urol 2007, 178(3 Pt 1): 807-813.
- Steinberg G(1), Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M: Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol 2000, 163(3): 761-767.
- Hassler MR, Shariat SF, & Soria F: Salvage therapeutic strategies for bacillus Calmette–Guerin failure. Current Opinion in Urology 2019, 29(3): 239-246.
- Shore ND, Boorjian SA, Canter DJ, Ogan K, Karsh LI, Downs TM, Gomella LG, Kamat AM, Lotan Y, Svatek RS, et al: Intravesical rAd-IFNα/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol 2017, 35(30): 3410-3416.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript