Review Article | Open Access
Treatment Options for Renal Cell Carcinoma: Mechanisms and Outcomes
Benjamin Ptasienski1, 2, Jake Myers2, Weston Krenn2, Rex T. Perry1, Trenton G. Mayberry1, 2, Qian Bai2, Mark R. Wakefield2, Yujiang Fang1, 2
1Department of Microbiology, Immunology & Pathology, Des Moines University, Des Moines, IA, 50312, USA.
2Department of Surgery, University of Missouri School of Medicine, Columbia, MO, 65212, USA.
Correspondence: Yujiang Fang (Department of Microbiology, Immunology & Pathology, Des Moines University College of Osteopathic Medicine, Des Moines, Iowa 50312, USA; Email: yujiang.fang@dmu.edu).
Annals of Urologic Oncology 2023, 6(2): 63-69. https://doi.org/10.32948/auo.2023.06.28
Received: 24 Jun 2023 | Accepted: 28 Jun 2023 | Published online: 28 Jun 2023
Key words renal cell carcinoma, nephrectomy, immunotherapy, angiogenesis
Nephrectomy treatment outcomes
The outcome of Nephrectomy procedures can be classified into a number of subgroups: radical nephrectomies (RN), nephron saving surgeries (NSS), Open nephrectomies (ON), and laparoscopic nephrectomies (LN). RNs are a surgical procedure that takes out the whole kidney while PNs are similar to NSSs that only take out the cancerous/damaged tissue and save healthy nephrons. ONs are comparable to LN procedures and RNs to PN procedures. While nephrectomy treatments can be classified, many sources conclude that there are minimal differences in outcomes after ON and LN procedures. These differences include mean body mass index, tumor size, mean estimated blood loss, rate of blood transfusion, and mean length of stay after surgery. Patients in the LN group had a higher mean body mass index (31.9 vs 28.1), smaller tumors (7.7 cm vs 9.1 cm), lower mean estimated blood loss (277 vs 1429), lower rate of blood transfusion (4.7% vs 45.5%), and a shorter mean length of stay (3.5 days vs 5.7 days) compared with patients who underwent ON procedures (all above data had significance values of p ≤ 0.008). At a median follow-up of 32.8 months, there was no significant difference in overall survival (p = 0.8) between the two groups [14].
RNs and PNs are compared differently than ONs and LNs. RNs and PNs are compared in terms of overall survival, cancer-specific survival and recurrence-free survival. A study that merged six studies involving 19,580 patients concluded that those who underwent PN had a better overall survival compared to those treated with RN (HR: 0.81, 95% CI: 0.74–0.89; P < 0.001). The same study merged six studies involving 18,540 patients when comparing the cancer-specific survival and found there was little difference between those who were treated with PNs in comparison to those treated with RNs (HR: 0.85, 95% CI: 0.73–1.01; P = 0.060). For recurrence free survival, four studies were merged involving 3752 patients and also found similar results between patients who underwent PNs to RNs (HR: 0.66, 95% CI: 0.34–1.31; P = 0.239) [15].
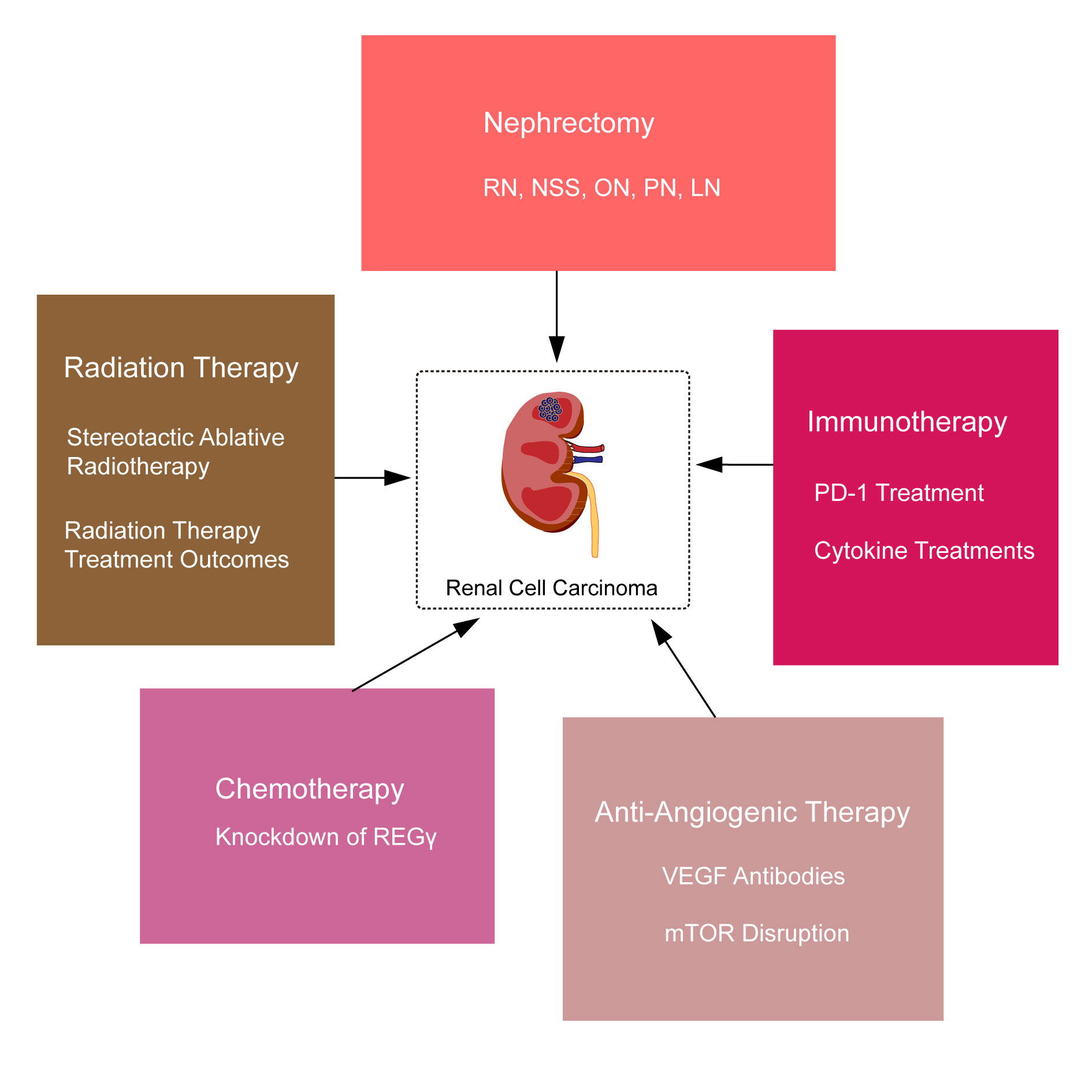 Figure 1. Five treatment options for renal cell carcinoma in the review.
Figure 1. Five treatment options for renal cell carcinoma in the review.
PD-1 treatment
PD-1 is a protein expressed on a number of immune cells throughout the body, including activated T-cells, B-cells, and monocytes. It functions to hold back the immune system during times of infection, limiting autoimmunity and damage to the healthy body. Many RCC tumors express ligands of PD-1 (often PD-L1) on their surfaces, limiting the overall immune response towards them [17]. Early studies have begun to demonstrate that molecules which target the PD-1 pathway (both PD-1 and its ligands) on tumor cells can increase activity of T-cells in the targeted regions. The targeting of this pathway can occur through either blockading the PD-1 on the T-cells directly or blockading the PD-L1. Blockading the PD-1 proteins on T-cells directly with antibodies can increase T-cell activity and reduce downregulation, increasing activity against tumor cells. There are two general mechanisms through which anti-PD-L1 treatments can be utilized. First, similar to the anti-PD-1 mechanism, antibodies are bound to PD-L1 on tumor cells, limiting their effectiveness. Second, some immune checkpoint monoclonal antibodies (MAb) have demonstrated an ability to mediate antibody-dependent cell-mediated cytotoxicity (ADCC) activity along with blocking PD-1 interaction [18]. ADCC is the process by which antibodies can recruit additional immune cells to induce cell death by binding to Fc receptors [19] and MAbs capable of both functions are of increased interest clinically. Overall, Pd-1/PD-L1 treatments work to increase the activity of T-cells and other immune cells against tumors. However, it should be noted that because the activity of the immune system is enhanced, a number of side effects are also consistent with PD-1 treatment, often autoimmune-like symptoms such as fatigue, chills, and gastrointestinal issues [20-23].
Cytokine treatments
Cytokine treatments have been in use clinically longer than immune checkpoint therapies (PD-1) with varying degrees of success. These treatments involve introducing cytokines into the body, prompting changes within the immune system which have been seen to increase tumor responses. IL-2 is a cytokine occurring in the body which promotes the growth and proliferation of T-cells, effectively boosting the activity of the immune system, which is then able to act against RCC. High dose IL-2 treatment has resulted clinically in positive tumor regression, but unfortunately has been associated with high toxicity rates resulting from increased immune reactions [24]. IFN-α is a cytokine present in the body which functions to facilitate the differentiation of monocytes into dendritic cells, which are far more capable of recognizing more complex antigens, such as RCC. IFN-α treatment has seen more mild tumor reduction results clinically, and does still contain a level of toxicity, but the effects are milder and resemble that of the flu [25]. Other types of cytokines, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), which generally drives the formation of additional immune cells in the body [26], have been investigated for use for RCC but have not been seen to be as effective as IL-2 and IFN-α. Thus, these two molecules remain the most prominent cytokine treatments used for RCC [27, 28].
Immunotherapy treatment outcomes
There were two main groups of patients with immunotherapy treatment: one group was the use of immunotherapy after a nephrectomy procedure and one group with just the use of immunotherapy. Of the group of starting treatment with immunotherapy, there has been positive results for long-term survival and response to treatment for some drugs used in immunotherapy. A study which followed 1,096 randomized patients with 42 months' minimum follow-up in patients with RCC and intermediate/poor-risk disease, median OS (95% CI) favored nivolumab plus ipilimumab ( NIVO+IPI) versus sunitinib; n = 65; HR, 0.45 (95% CI, 0.3–0.7; P = 0.0004); Progression-free survival was also recorded for NIVO+IPI (median 26.5 vs. 5.1 months; HR, 0.54 (95% CI, 0.33–0.86; P = 0.0093). The confirmed objective response rate was 60.8% for patients in the NIVO+IPI group versus 23.1% with those in the sunitinib group, with complete response rates of 18.9% versus 3.1%, respectively [29].
Immunotherapy has also been used after a partial or radical nephrectomy has been done; a study of 310 patients found that there was no difference in reoccurrence-free survival or overall survival between a group that was observed after partial or radical nephrectomy and one where immunotherapy was given after PN or RN [30].
Nivolumab, Ipilimumab and Sunitinib are all common drugs used in immunotherapy for RCC. Nivolumab and Ipilimumab are also commonly used together, so the side effects associated with the two drugs are often compiled as side effects for both drugs together; the most common side effects include fatigue, diarrhea, pruritus, rash, and elevated aspartate aminotransferase [31]. Common side effects for patients undergoing therapy with sunitinib are Anorexia, diarrhea, nausea and vomiting, oral changes (sensitivity, taste changes, dry mouth, stomatitis or mucositis), hypothyroidism, hepatotoxicity, hypertension, bleeding, cardiac toxicity, fatigue, skin toxicity, and changes in skin or hair color [32].
Anti-angiogenic therapy
As is the case with any growing tumor, RCC requires a large and constant supply of blood to support its high rate of growth. As a result, RCC contains a method of creating new blood vessels leading in and out of the tumor itself, a process known as angiogenesis [33]. Treatments that specifically target these generated blood vessels and their origins have seen some clinical success and are known as anti-angiogenic treatments.
VEGF antibodies
Tumor development and growth in RCC is driven by mutations in the von Hippel-Lindau tumor suppressor gene (VHL), which prevents angiogenesis in wild-type cells by promoting the breakdown of the transcription factor hypoxia-inducible factor-1α (HIF-1α). In mutated renal tumor cells, breakdown of HIF-1α does not occur, and it builds up. This buildup causes production of angiogenic growth factors such as vascular endothelial growth factor (VEGF), which binds to endothelial cell surface receptors (VEGFR) and activates a signaling pathway which ultimately leads to the proliferation of blood vessels in the tumor. Knowing this mechanism of angiogenesis, researchers have been able to create antibodies for VEGF which bind to these growth factors and neutralize them, preventing them from binding to VEGFR [34-36].
mTOR disruption
A pathway which has undergone extensive research in broad cancer research is the rapamycin (mTOR) pathway. mTOR in normally functioning cells is associated with signaling pathways that regulate cell apoptosis, autophagy, and cell proliferation. However, in cancer cells, overactivation of mTOR plays a role in angiogenesis and tumor metabolism. Functionally, mTOR is a serine and threonine kinase which is formed of two main complexes, TORC1 and TORC2. TORC1 is involved in regulation of the aforementioned HIF-1, while TORC2 phosphorylates the Akt oncogene, which is involved in the stimulation of angiogenesis [37]. Drugs such as Temsirolimus are able to bind to one of the complexes and thus disrupt mTOR signaling, which then works to slow the process of angiogenesis in RCC [38].
Anti-angiogenic therapy treatment outcomes
Pazopanib is a tyrosine kinase inhibitor that targets several different proteins involved in the process of angiogenesis. By inhibiting these proteins, Pazopanib blocks the formation of new blood vessels, which can slow or stop the growth and spread of cancer cells. A clinical study was done on 435 patients with kidney cancer, of which 233 had not received treatment before and 202 had received cytokine treatment. The study found that Pazopanib was effective in improving overall PFS. Patients who received Pazopanib had a median PFS of 9.2 months, while those who received a placebo had a median PFS of 4.2 months. The study also found that the Pazopanib group had a higher response rate of 30% compared to the placebo group, which only had a 3% response rate. Overall, there were no observed differences in quality of life between the two groups, but it was concluded that Pazopanib is an effective treatment option for RCC [39].
Knockdown of REGγ
While RCC is typically resistant to chemotherapy, there is hope that new treatments may soon be able to increase its chemosensitivity. A study has recently demonstrated that knockdown of the Wnt/beta catenin pathway in RCC tumors can induce apoptosis and alter the tumor biology to make chemotherapy more effective. microRNA-195-5p is a regulatory microRNA which binds to the 3’ untranslated UTR of target genes and has been demonstrated to be downregulated in many tumors. It is proposed that miR-195-5p binds to REGγ, an oncogene which is expressed in the Wnt/beta catenin pathway in RCC, and knocks down its activity, leading to beneficial results, including chemosensitivity to the chemotherapeutic drug sorafenib. A hope of researchers is that these findings may be able to be used in the future to develop treatments centered around microRNA-195-5p and its tumor suppressing qualities [43].
Chemotherapy treatment outcomes
While chemotherapy is a very commonly used treatment for many types of cancer, it is not generally used as the main treatment for RCC. Many clinical studies analyzing the effectiveness of chemotherapy on metastatic RCC suggest that this treatment on its own is not very successful. Several drugs have been used in various clinical studies such as Gemcitabine and Capecitabine. Overall, results from these single agent chemotherapy treatments show generally low response rate, progression free survival (PFS), and overall survival (OS) values. Studies conducted in 1993 and 1996 used Gemcitabine to treat mRCC, and the observed response rates were 6-10%, the average PFS length was 3.7 months, and the average OS length was 12 months. Different studies from 1994, 2002, and 2007 utilized Capecitabine, but the results were not promising whatsoever, with only about 10% of patients having modest responses [44]. Due to generally unsuccessful results from studies like these, other treatments and combinations of treatments are now more commonly used to treat RCC.
Stereotactic ablative radiotherapy
Stereotactic Ablative Radiotherapy (SABR) is a technology that is highly promising for the treatment of RCC that has been detected in early stages. SABR is guided by the use of computerized tomography (CT) that is built into the radiation therapy machine and allows for more specific doses and limits damage done to healthy tissue. This feature allows for minimal side effects for the patients. As well, the doses of radiation given are far more intense than traditional radiation therapy, meaning both that fewer treatments are given, allowing for SABR to be used in conjunction with other treatments, and that the treatment is often able to overcome the resistance that RCC traditionally has for radiation therapy [46]. Unfortunately, as mentioned earlier in this paper, there is a smaller percentage of RCC diagnoses that occur in the early stages of the disease when compared to other cancers, meaning that there are fewer instances when this treatment is indicated. However, when indicated SABR is a compelling option for treatment [47-49].
Radiation therapy treatment outcomes
Similar to chemotherapy, radiation therapy is not usually used as a front-line treatment for RCC. It can be used with other treatment plans or in special cases, but studies have shown that on its own, it is generally not an effective or reliable treatment. One type of treatment, stereotactic ablative body radiotherapy (SABR), is a more recent treatment that is currently being studied. One retrospective review of 16 patients who received SABR treatment analyzed the outcomes to attempt to get an accurate understanding of its effectiveness. These patients were treated from 2012 to 2015 and were not eligible for surgery. After early follow ups following the SABR treatment, the local control rate was determined to be 100% overall with minimal toxicities. Although SABR does seem to be successful early on, the study notes that further trials should be conducted [50]. Although RCC is generally thought to be radioresistant, newer treatments like this should be studied further and could be useful for patients who are not able to receive surgical treatment.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
YF initiated the idea. BP, JM and WK wrote the manuscript draft. YF, RTP, TGM, QB, MRW prepared the figure. RTP, TGM, QB, MRW edited the draft. YF made critical revision to the final manuscript and YF approved the final manuscript.
Competing interests
The authors report no conflict of interest.
Funding
This study was partially supported by the grant from Des Moines University for Yujiang Fang, M.D., Ph.D. (IOER 112-3119).
- Song X, Tian Y, Li H, Liu B, Zhang A, and Hong Y: Research progress on advanced renal cell carcinoma. J Int Med Res 2020, 48(6): 300060520924265.
- Motzer RJ, Bander NH, and Nanus DM: Renal-Cell Carcinoma. N Engl J Med 1996, 335: 865-875.
- Bahadoram S, Davoodi M, Hassanzadeh S, Bahadoram M, Barahman M, Mafakher L: Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol 2022, 39(3): 2022-vol3.
- Barata PC, Rini BI: Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin 2017, 67(6): 507-524.
- Bian Z, Fan R, Xie L: A Novel Cuproptosis-Related Prognostic Gene Signature and Validation of Differential Expression in Clear Cell Renal Cell Carcinoma. Genes (Basel) 2022, 13(5): 851.
- Cairns P: Renal cell carcinoma. Cancer Biomark 2010, 9(1-6): 461-473.
- Garfield K, LaGrange CA: Renal Cell Cancer. Treasure Island (FL): StatPearls Publishing 2023, Available from: https://www.ncbi.nlm.nih.gov/books/NBK470336/
- Kumbla RA, Figlin RA, Posadas EM: Recent Advances in the Medical Treatment of Recurrent or Metastatic Renal Cell Cancer. Drugs 2016, 77: 17-28.
- Muglia VF, Prando A: Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras 2015, 48(3): 166-74.
- Mayo Foundation for Medical Education and Research: Nephrectomy (Kidney Removal). Mayo Clinic 2018. https://www.mayoclinic.org/tests-procedures/nephrectomy/about/pac-20385165
- Surgery for Kidney Cancer: Surgery for Kidney Cancer. Johns Hopkins Medicine 2021. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/surgery-for-kidney-cancer
- Krabbe LM, Bagrodia A, Margulis V, Wood C: Surgical management of renal cell carcinoma. Seminars in Interventional Radiology 2014, 31(01): 027-032.
- Venkatramani V, Swain S, Satyanarayana R, Parekh DJ: Current Status of Nephron-Sparing Surgery (NSS) in the Management of Renal Tumours. Indian J Surg Oncol 2017, 8(2): 150-155.
- Bragayrac LA, Abbotoy D, Attwood K, Darwiche F, Hoffmeyer J, Kauffman EC, Schwaab T: Outcomes of Minimal Invasive vs Open Radical Nephrectomy for the Treatment of Locally Advanced Renal-Cell Carcinoma. J Endourol 2016, 30 (8): 871-876.
- Gu L, Ma X, Li H, Chen L, Xie Y, Li X, Gao Y, Zhang Y, Zhang X: Comparison of oncologic outcomes between partial and radical nephrectomy for localized renal cell carcinoma: A systematic review and meta-analysis. Surg Oncol 2016, 25(4): 385-393.
- Chang AJ, Zhao L, Zhu Z, Boulanger K, Xiao H, Wakefield MR, Bai Q, Fang Y: The Past, Present and Future of Immunotherapy for Metastatic Renal Cell Carcinoma. Anticancer Res 2019, 39(6): 2683-2687.
- Massari F, Santoni M, Ciccarese C, Santini D, Alferi S, Martignoni G, Brunelli M, Piva F, Berardi R, Montironi R, et al: PD-1 Blockade Therapy in Renal Cell Carcinoma: Current Studies and Future Promises. Cancer Treat Rev 2015, 41(2): 114-121.
- Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J: Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res 2015, 3(10): 1148-1157.
- Zahavi D, AlDeghaither D, O'Connell A, Weiner LM: Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther 2018, 1(1): 7-12.
- American Cancer Society Medical and Editorial Content Team. (n.d.): Immunotherapy for Kidney Cancer. Biologic Therapy 2023, https://www.cancer.org/cancer/kidney-cancer/treating/immunotherapy.html
- Kammerer-Jacquet SF, Deleuze A, Saout J, Mathieu R, Laguerre B, Verhoest G, Dugay F, Belaud-Rotureau MA, Bensalah K, Rioux-Leclercq N: Targeting the PD-1/PD-L1 Pathway in Renal Cell Carcinoma. Int J Mol Sci 2019, 20(7): 1692.
- Linehan WM, Ricketts CJ: The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol 2019, 16(9): 539-552.
- Su C, Wang H, Liu Y, Guo Q, Zhang L, Li J, Zhou W, Yan Y, Zhou X, Zhang J: Adverse Effects of Anti-PD-1/PD-L1 Therapy in Non-small Cell Lung Cancer. Front Oncol 2020, 10: 554313.
- Bleumer I, Oosterwijk E, & De Mulder P: Immunotherapy for Renal Cell Carcinoma. Eur Urol 2003, 44(1): 65-75.
- Ross K, Jones RJ: Immune checkpoint inhibitors in renal cell carcinoma. Clin Sci (Lond) 2017, 131(21): 2627-2642.
- Kumar A, Taghi Khani A, Sanchez Ortiz A, Swaminathan S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front Immunol 2022, 13: 901277.
- Aggen DH, Ager CR, Obradovic AZ, Chowdhury N, Ghasemzadeh A, Mao W, Chaimowitz MG, Lopez-Bujanda ZA, Spina CS, Hawley JE, et al: Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin Cancer Res 2021, 27(2): 608-621.
- Vuong L, Kotecha RR, Voss MH, Hakimi AA: Tumor Microenvironment Dynamics in Clear-Cell Renal Cell Carcinoma. Cancer Discov 2019, 9(10): 1349-1357.
- Tannir NM, Signoretti S, Choueiri TK, McDermott DF, Motzer RJ, Flaifel A, Pignon JC, Ficial M, Frontera OA, George S, et al: Efficacy and Safety of Nivolumab Plus Ipilimumab versus Sunitinib in First-line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma. Clin Cancer Res 2021, 27(1): 78-86.
- Gul A, Rini B: Adjuvant Therapy in Renal Cell Carcinoma. Cancer 2019, 125(17): 2935-2944.
- Somekawa K, Horita N, Kaneko A, Tagami Y, Fukuda N, Matsumoto H, Namkoong H, Fujiwara Y, Minegishi K, Fukumoto T, et al: Adverse events induced by nivolumab and ipilimumab combination regimens. Ther Adv Med Oncol 2022, 14: 17588359211058393.
- Kollmannsberger C, Soulieres D, Wong R, Scalera A, Gaspo R, Bjarnason G: Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J 2007, 1(2 Suppl): S41-54.
- Larroquette M, Peyraud F, Domblides C, Lefort F, Bernhard JC, Ravaud A, Gross-Goupil M: Adjuvant therapy in renal cell carcinoma: Current knowledges and future perspectives. Cancer Treat Rev 2021, 97: 102207.
- Ambrosetti D, Coutts M, Paoli C, Durand M, Borchiellini D, Montemagno C, Rastoin O, Borderie A, Grepin R, Rioux-Leclercq N, et al: Cancer-associated fibroblasts in renal cell carcinoma: implication in prognosis and resistance to anti-angiogenic therapy. BJU Int 2022, 129(1): 80-92.
- Choueiri TK, Kaelin WG Jr: Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med 2020, 26(10): 1519-1530.
- Dutcher JP: Recent developments in the treatment of renal cell carcinoma. Ther Adv Urol 2013, 5(6): 338-53.
- Rini B, Srinivas S: Antiangiogenic Therapy for Advanced Renal Cell Carcinoma. Targeting Tumor Angiogenesis 2023. https://angio.org/pdf/Angio_RCC_Final_Publication.pdf
- Zou Z, Tao T, Li H, Zhu X: mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci 2020, 10: 31.
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, et al: Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol 2023, 41(11): 1957-1964.
- Buti S, Bersanelli M, Sikokis A, Maines F, Facchinetti F, Bria E, Ardizzoni A, Tortora G, Massari F: Chemotherapy in metastatic renal cell carcinoma today? A systematic review. Anticancer Drugs 2013, 24(6): 535-554.
- Hartmann JT, Bokemeyer C: Chemotherapy for renal cell carcinoma. Anticancer Research 1999, 19(2C): 1541-1543.
- Amato RJ: Chemotherapy for renal cell carcinoma. Semin Oncol 2000, 27(2): 177-186.
- Chen S, Wang L, Yao X, Chen H, Xu C, Tong L, Shah A, Huang T, Chen G, Chen J, et al: miR-195-5p is critical in REGγ-mediated regulation of wnt/β-catenin pathway in renal cell carcinoma. Oncotarget 2017, 8(38): 63986-64000.
- Diamond E, Molina AM, Carbonaro M, Akhtar NH, Giannakakou P, Tagawa ST, Nanus DM: Cytotoxic chemotherapy in the treatment of advanced renal cell carcinoma in the era of targeted therapy. Crit Rev Oncol Hematol 2015, 96(3): 518-526.
- Spyropoulou D, Tsiganos P, Dimitrakopoulos F-I, Tolia M, Koutras A, Velissaris D, Lagadinou M, Papathanasiou N, Gkantaifi A, Kalofonos H: Radiotherapy and renal cell carcinoma: A continuing saga. In Vivo 2021, 35(3): 1365-1377.
- Ali M, Mooi J, Lawrentschuk N, McKay RR, Hannan R, Lo SS, Hall WA, Siva S: The Role of Stereotactic Ablative Body Radiotherapy in Renal Cell Carcinoma. Eur Urol 2022, 82(6): 613-622.
- Chang JH, Cheung P, Erler D, Sonier M, Korol R, Chu W: Stereotactic ablative body radiotherapy for primary renal cell carcinoma in non-surgical candidates: Initial clinical experience. Clin Oncol 2016, 28(9): e109-114.
- Christensen M, Hannan R: The Emerging Role of Radiation Therapy in Renal Cell Carcinoma. Cancers 2022, 14(19): 4693.
- Rühle A, Andratschke N, Siva S, Guckenberger M: Is there a role for stereotactic radiotherapy in the treatment of renal cell carcinoma? Clin Transl Radiat Oncol 2019, 18: 104-112.
- Siva S, Ali M, Correa RJM, Muacevic A, Ponsky L, Ellis RJ, Lo SS, Onishi H, Swaminath A, McLaughlin M, et al: 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: an individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol 2022, 23(12): 1508-1516.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript