Review Article | Open Access
Recent Progress on Exosomes in the Diagnosis of Prostate Cancer
Juanzi Wang1, Tianlong Liu2, Minna Liu31Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi’an, China.
2Department of Pharmacy, The 940th Hospital of Joint Logistic Support Force of PLA, Lanzhou, China.
3Department of Nephrology, The 940th Hospital of Joint Logistic Support Force of PLA, Lanzhou, China.
Correspondence: Tianlong Liu (No.333 Binhe Road, Qilihe District, Lanzhou City, Gansu Province 730050; Email: liutl_0318@126.com ) and Minna Liu (No.333 Binhe Road, Qilihe District, Lanzhou City, Gansu Province 730050; Email: lmn2010@foxmail.com).
Annals of Urologic Oncology 2023, 6(1): 18-26. https://doi.org/10.32948/auo.2023.03.14
Received: 05 Mar 2023 | Accepted: 14 Mar 2023 | Published online: 18 Mar 2023
Prostate cancer (Pca) is the second common cancer in men worldwide. Although prostate-specific antigen (PSA) screen can serve as a diagnostic marker in most of the patients with Pca, its diagnostic specificity is insufficient, and the false positive rate can result in unnecessary biopsy increasing pain and treatment costs in patients. Exosomes are source for mRNA, microRNA, non-encoded RNA, protein, and lipids. In recent years, exosome are used for assessment of tumors and serve as tumor markers for early diagnosis and disease prognosis. This article highlights the application of exosomes in connection with diagnosis, treatment and prognosis of Pca.
Key words prostate cancer, exosome, tumor marker, diagnosis
CircRNA organisms mainly include four models: Splice looping of the exon lariat, Looping of intron pairs, Splice looping of the inner ring daughter lariat, Splice looping of internal ring rope, cyclization driven by RNA-binding proteins or trans factors [17]. Most circRNAs are encoded by known protein-coding genes and fall into three categories based on their structural composition: exon circRNA(EcircRNA), annular intron RNA(ciRNA), and exon-intron circRNA(EIciRNA) [18].
Exosomes as a biomarker
Transmembrane serine proteinase 2 (TMPRSS2) in Pca and the erythroblastic virus E26 carcinogen (ERG) fusion gene has been shown to be related to poor cancer prognosis [28]. Three RNAs (ERG, PC3, SP-DEF) in the urine exosomes of patients with the PSA gray area were detected to avoid unnecessary needle biopsy [29]. As a double-edged sword, the exosomes can not only help the clinical detection of disease. It not only induces apoptosis of immune cells but also reduce the function of the human immune system. The FAS ligand in the Pca exosomes induce the apoptosis of CD8 + T cells, inhibit T cell reactions, and completes the immune escape of Pca cells [30]. PCa patients through PKM2 induces CXCL12 in bone marrow mesenchymal stem cells [31], indicating that exosomes may be potential tumor marks of Pca. As a biomarker, the exosome have the following advantages: 1) exosomes released by tumor cells contains tumor-related molecules, which exist in blood [32], urine [33], semen [34], and breast milk [35] and other body fluids; 2) the lipid bilayer structure of exosomes can well maintain the stability of the nucleic acid, and will not be degraded by RNase; 3) exosomes can be extracted from the patient's urine and semen through non-invasive methods; 4) specific protein on the surface of the exosome membrane is conducive to reflect the physiological and pathological functions of its parent cells [36]. For some proteins, mRNAS, miRNAs, and lipids contained in exosomes have been identified as potential Pca biomarkers.
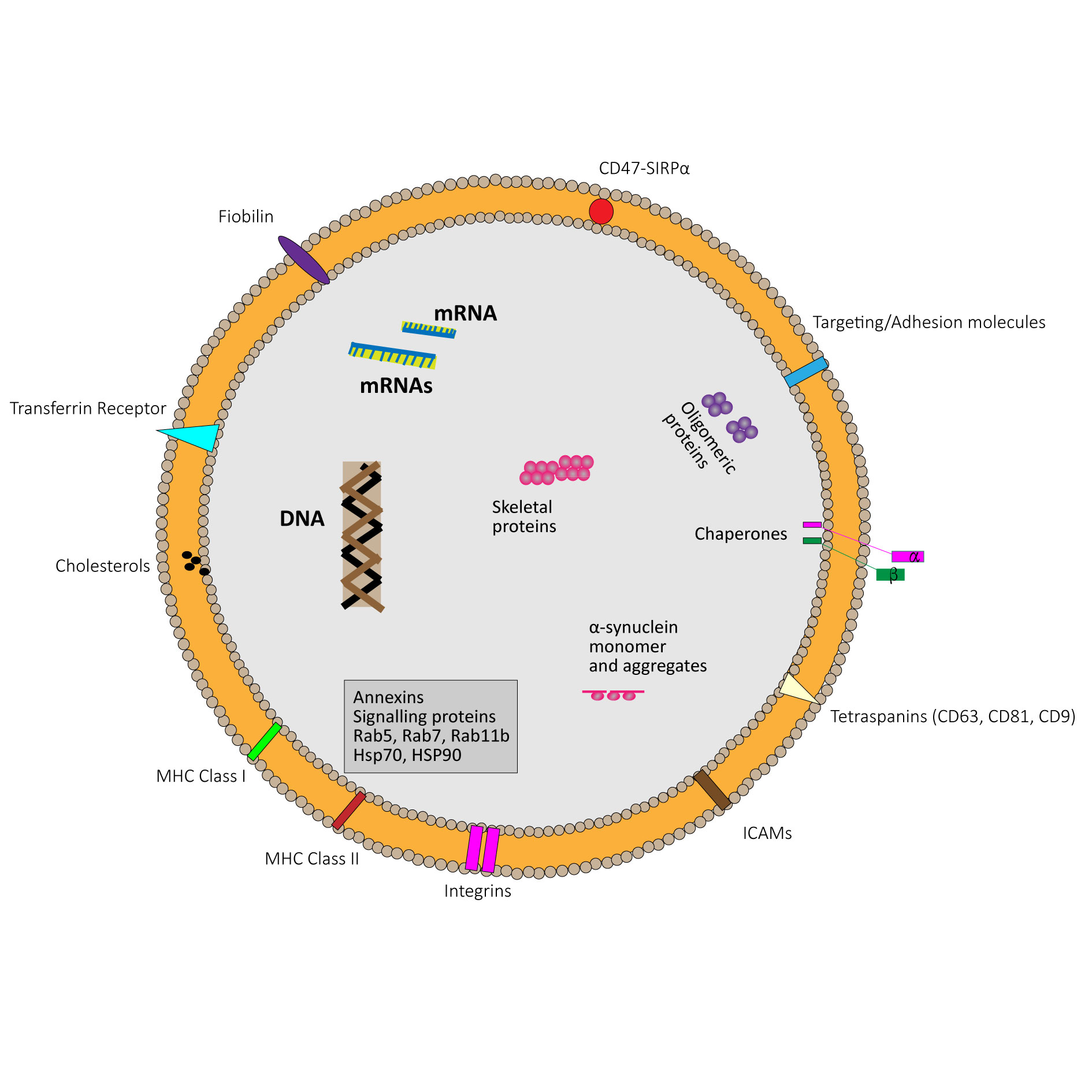 Figure 1. Schematic diagram of an exosome.
Figure 1. Schematic diagram of an exosome.
Two genes kallikrein 3 (KLK3) and androgen receptor splicing variant 7 (AR-V7) encode PSA and AR-V7, respectively. Exosome KLK3 has a strong correlation with serum PSA value. AR-V7 is a splice variant of AR mRNA. The results obtained from the exosome show that among CRPC patients, the expression level of AR-V7 is related to hormone concentrations, as well as the adverse prognosis and the response to Abiraterone and Enzalutamide [37]. The study found that in patients with advanced Pca, AR-V7 mRNA level and AR-V7 /AR-FL ratio value is higher [38]. As a typical Pca biomarker, the expression levels of PSA mRNA and PCA3 mRNA are significantly increased in prostate-specific membrane antigen (PSMA) in urine. Therefore, capturing urine PSMA positive exosome detection PSA and PCA3 mRNA levels can improve the diagnostic efficiency of Pca [6, 39].
miRNA
miRNAs is a single-chain non-encoded RNA with a length of 18 ~ 22 nucleotides. It often blocks gene expression by combining with its target protein encoding mRNA, participating in translation inhibition and degradation of mRNA [7]. There are about 41.72 % of mature miRNA for all miRNA in the exosome [40], miRNA can affect tumorigenesis through oncogene or tumor suppressors, therefore the exosomes miRNA is of great significance to the early diagnosis and prognosis of tumor. The application of miRNA in the exosome to liquid biopsy for prostate cancer can improve the specificity of PSA test. miR-196A-5p and miR-501-3p expressed downgrade in the urine exosome samples of prostate cancer [41], while miR-145 significantly increased compared to BPH patients (P = 0.018). miR-145 is significantly related to Gleason scores. Compared with the Gleason score ≤ 7 points, patients with Gleason score ≥8 points have increased miR-145 expression. miR-145 and serum PSA can better distinguish between Pca and BPH with PSA indicator alone. In addition, there are researches [42] reported that the combined expression of miRNA-21 and miRNA-375 in the urine exosome in Pca patients has a great advantage to identify prostate cancer. The area under ROC curve (AUC) is 0.863 and 0.805 respectively. miRNA levels are also closely related to the staging of tumors. The exosome miRNA has the potential to predict invasion or local metastasis. Expression of miR-888 in CRPC patients is significantly higher than normal healthy person and BPH patients [43]. The exosome miRNA-1246 expressed disorders in the invasive Pca, and in the invasive prostate cancer, miRNA-1246 is 31 times of the normal person (P = 0. 026), which is 23 times of BPH patients (P = 0.035) [44]. Combined with the detection of PSA and semen exosome miR-142-3p, miR-142-5p, miR-223-3p, patients with Pca and BPH can be accurately divided into 91.7 % sensitivity, and 42.9 % specificity [34, 45]. Thereby the specificity of the PSA screening test is increasing.
lncRNA
LncRNA (long-chain non-encoded RNA) can regulate tumor suppression, metastasis, and progress [46]. LncRNA-Myu can be transported by an exosome. It plays a role in the extracellular environment of Pca cells, LncRNA-Myu has raised c-Myc expression by competitive combination of miR-184, thereby inducing the prostate cancer cells, and the knockout of the MyU gene will decline the growth and migration of prostate cancer cells [47]. The level of exosome LncRNA may help diagnose BPH and Pca [46]. Evidence proves that the expression of LncRNA in different prostate diseases will also be different. The level of LncRNA-SAP30L-AS1 in the BPH group is significantly higher than Pca group (P <0.05) and the control group (P <0.00001), in the Pca group LncRNA-SCHLAP1 level is significantly higher than the BPH group (P <0.0000) and healthy people (P <0.000), and LNCRNA-SCHLAP1 levels will increase with the progress of Pca [48]. It was found thatn exosomal long noncoding RNA HOXD-AS1 promotes prostate cancer metastasis via miR-361-5p/FOXM1 axis [49]. Li Q et al reported that exosomal lncAY927529 enhances prostate cancer cell proliferation and invasion through regulating bone microenvironment [50]. In addition, an experimental study by Ozgur et. provides evidence that H19 might be involved in androgen receptor pathway [51]. And Young X et al. found that the long non-coding RNA PCSEAT exhibits an oncogenic property in prostate cancer and functions as a competing endogenous RNA that associates with EZH2 [52].
cirCRNA
circRNA (circular RNA) is a single-chain closed RNA, without 5’ end hats and 3’ end Poly (a) tail. It forms a closed-loop structure with covalent bonds, which is more stable than linear RNA [53]. circRNA has an important connection with the proliferation and migration of tumors [54]. In patients with prostate cancer, the expression of circRNA-0044516 in the exosome can be observed significantly, and the lowering of circRNA-0044516 will inhibit the proactive and transfers of prostate cancer cells [55]. There were some studies involving in the exosomal circRNAs and prostate caner recently. It were found that exosome-derived circTFDP2 promotes prostate cancer progression by preventing PARP1 from caspase-3-dependent cleavage [56]. Huang et found that exosomal circKDM4A Induces CUL4B to promote prostate cancer cell malignancy in a miR-338-3p-dependent manner [57]. In addition, it was reported that exosomal circRNA HIPK3 knockdown inhibited cell proliferation and metastasis in prostate cancer by regulating miR-212/BMI-1 pathway [58]. circ0081234 was inhibited is able to reduce prostate cancer tumor growth and metastasis via the miR-1/MAP 3 K1 axis [59].
Proteins
PSA in the exosome may become a new tool for the early screening and diagnosis for Pca. In contrast to patients with BPH, only Pca patients have nanovesicle expressed with PSA and CD81 at the same time [60]. Compared with healthy people, in the plasma of Pca patients, the expression of the exosome CAIX (carbonic anhydrase 9) is elevated [61], because the Pca exosome is acidic, and the acidity of the tumor micro-environment results in increasing the CAIX activity in Pca exosomes, and raising expression levels [62]. The activity of serum exosomes GGT (gamma-glutamyltransferase) may be potential Pca biomarkers. The chemical analysis of immunohistochemicals shows that the expression of GGT1 in cancer tissue is significantly higher than the BPH group (P <0.01), and the activity of serum GGT in Pca is higher than those of patients with BPH (P <0.05) [63]. The horizontal CLDN 3 (closed protein 3) in plasma increases with the increasing tumor grade. CLDN3 is a surface protein of Pca exosome. Gleason 8 and higher levels of tumor patients with exosome surface proteins CLDN 3 are significantly increased compared with BPH or Gleason scores 6 to 7 points (P = 0.029) [64].
The exosome from blood separation in Pca patients, αvβ3 integrin, αvβ3 transferred from exosome to β3 negative receptor cells, which is much related to the disease [65], suggesting that exosome to detect Pca may be a clinical biomarker to track the progress of Pca.
Lipids and fats
The lipids in the exosome may become a potential Pca biomarker. Endosomal sorting transport complex (ESCRT) plays an important role in the formation and release of the exosome [66], while some exosomes are generated by the non-dependent mechanism of ESCRT. Neutral sphingomyelin enzyme can not rely on ESCRT to form an exosome, and ceramides can form exosomes independent of ESCRT, which is one of the key lipids formed by the exosome [67, 68]. On the analysis of the urinary exosome of 15 Pca patients and 13 healthy person, it was found that phosphatidylserine (PS) 18:1/18:1 and Lactose ceramide (LACCER) (D18: 1/16: 0) were the most of significant diference (P <0.001), the latter is also the highest proportion in the samples of Pca patients and health groups, which is 95 %. A specific lipid combination can improve prostate cancer diagnosis 1/16:0. The sensitivity of the combination of PS18:1/18:1 and PS18:0/18:2 and PS18:1/18:1 is 93 %, the specificity is 100 %, and AUC is 0.989 [69]. See Table 1 in the related tumor markers of Pca.
Table 1 shows that a summary on exosome tumor markers in prostate cancer.
|
Table 1. Exosome tumor markers in prostate cancer. |
||||
|
Exosome contents |
Tumor biomarkers |
Expression levels |
Exosome source |
References |
|
mRNA |
KLK3 |
Increased |
plasma |
[81] |
|
|
AR-V7 |
Increased |
Plasma, urine |
[80, 82] |
|
|
PSA |
Increased |
urine |
[60] |
|
|
PCA3 |
Increased |
urine |
[29] |
|
miRNA |
miR-196A-5p |
Decreased |
urine |
[83] |
|
|
miR-501-3p |
Decreased |
urine |
[83] |
|
|
miR-145 |
Increased |
urine |
[84] |
|
|
miRNA-21 |
Increased |
urine |
[42] |
|
|
miRNA-375 |
Increased |
urine |
[85] |
|
|
miR-888 |
Increased |
urine |
[43] |
|
|
miR-1246 |
Increased |
urine |
[44] |
|
|
miRNA-142-3p |
Increased |
seminal fluid |
[34, 86] |
|
|
miRNA-142-5p |
Increased |
seminal fluid |
[34, 86] |
|
|
MiR-223-3p |
Increased |
seminal fluid |
[34, 86] |
|
LncRNA |
LncRNA-Myu |
Increased |
tissue |
[47] |
|
|
LncRNA-SChLAP1 |
Increased |
plasma |
[48] |
|
|
LncRNA-SAP30L-AS1 |
Increased |
plasma |
[48] |
|
Protein |
CAIX |
Increased |
plasma |
[61] |
|
|
GGT |
Increased |
Serum |
[63] |
|
|
CLDN3 |
Increased |
plasma |
[64] |
|
|
αvβ3 |
Increased |
blood |
[65] |
|
Lipid |
LacCer(d18:1/16:0) |
Increased |
urine |
[69] |
|
|
PS 18:1/18:1 |
Increased |
urine |
[69] |
|
|
PS 16:0/18:1 |
Increased |
urine |
[69] |
|
|
PS 18:0/18:1 |
Increased |
urine |
[69] |
Excessive expression of LNCaP cellular exosomes miR-26A can inhibit proliferation, migration, and invasion capabilities of PC-3 cells [76]. Human bone marrow derived stem cells derivative miR-205 high expression help inhibit Pca cells and enhance their apoptosis [77]. With the increasing clinical stage of Pca, the expression level of exosome miR-141 is increased [78], and the exosome miR-141-3P can promote the osteoblast bone metastasis and shorten the time of survival. Exosome of Pca cells MDA-PCA-2B, miR-141-3p can adjust the microenvironment of the bone metastasis and enhance the activity of osteoblasts [79]. The above studies have shown that the detection of exosome contents such as Pca may provide new possibilities for the treatment and prognosis of Pca bone metastasis.
Figure 2 shows that the role on exosomes in prostate cancer.
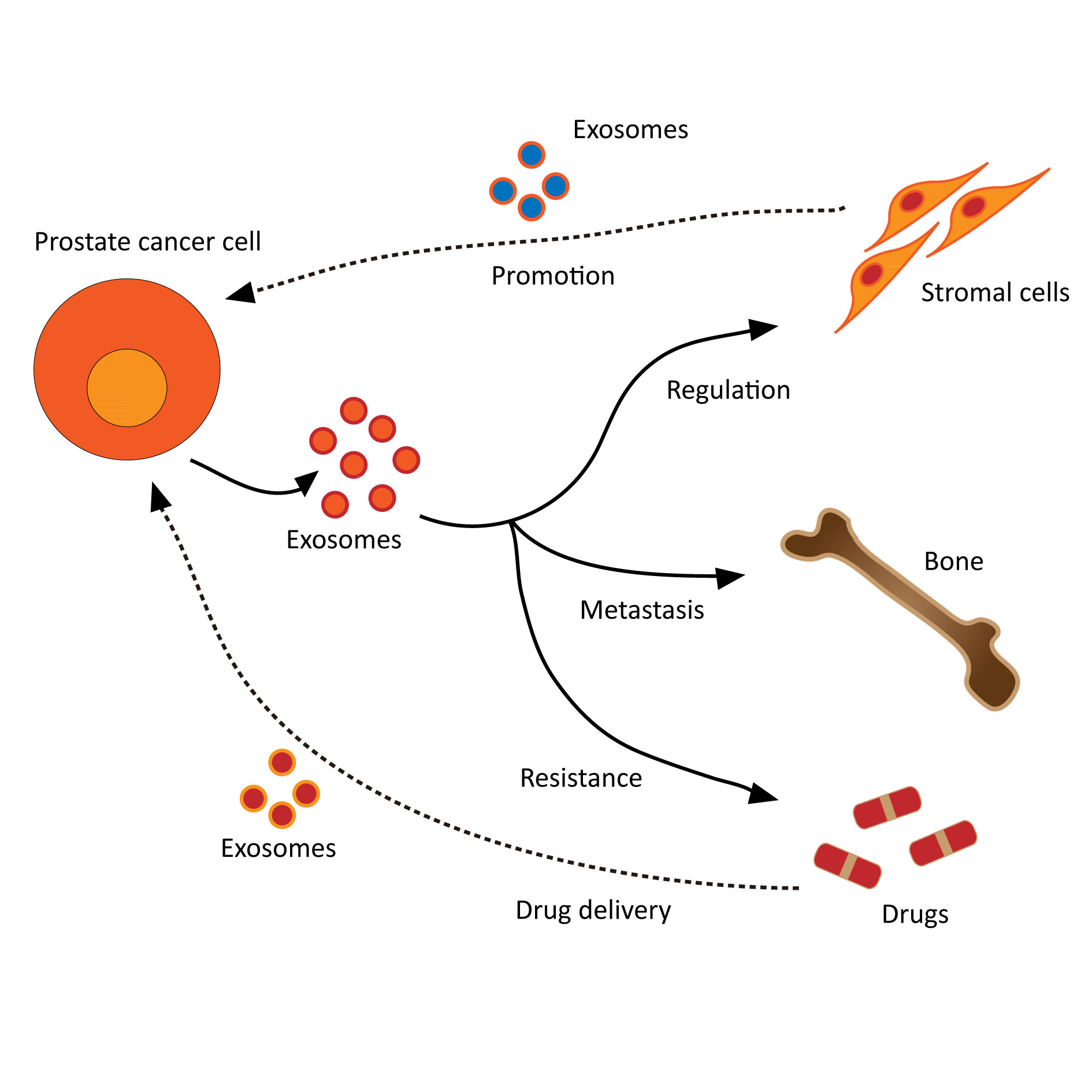 Figure 2. The roles on exosomes in prostate cancer.
Figure 2. The roles on exosomes in prostate cancer.
PSA combined with the detection of exosome content to improve the specificity and sensitivity of Pca diagnosis, and makeup for the deficiency of PSA testing in false positive, diagnosis, and specificity. It can also become a carrier of cancer-targeted drugs, increasing medicinal effects, indicating that the exosome is not only a promising tumor biomarker, but also provide a new mode to treat cancer in the future. At present, the extraction method of exosome is mainly super-speed centrifugal method. It is an advantage that the specimens of various types of body fluids can be applied, but the disadvantage is the cost of the instrument during operation, and requirement of large samples. Exosome sedimentation method (suitable for large samples, simple operation, but long cycle and low purity), immunoaffinity capture method (high purity, high cost, low yield), ExoQuick kits (the operation is simple and efficient but the use cost is high), ultrafiltration method (average purity and affected if blockage of the filter film) [39, 80], has enormous potential but not yet developed a set of high efficiency, good specificity, low cost, and applicable to clinical standard protocols. The standard scheme is necessary to further explore the extraction solution of the exosome. There is currently a new type of micro-current system that can efficiently extract the exosomes. It is convenient, fast, high, and low in cost, however needs to be validated. If these exogenous biomarkers can be integrated into a screening plan, it may be able to improve Pca diagnosis to avoid unnecessary invasive biopsy, help patients reduce pain, reduce psychological pressure, and costs. In summary, it is worth looking for more high-specific tumor biomarkers in the exosome content to improve the diagnostic of Pca.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
JZW designed the study and was responsible for the writing of the original draft. TLL and MNL edited and approved the final manuscript.
Competing interests
All authors declare no competing interests.
Funding
This study was supported by National Natural Science Foundation of China (82204746), the Special Cultivation Project of the 940th Hospital (2021yxky026), and Natural Science Foundation of Shaanxi (2023GHZD43).
- Brook MN, Ni Raghallaigh H, Govindasami K, Dadaev T, Rageevakumar R, Keating D, Hussain N, Osborne A, Lophatananon A, Collaborators U et al: Family History of Prostate Cancer and Survival Outcomes in the UK Genetic Prostate Cancer Study. Eur Urol 2023, 83(3): 257-266.
- Adeleke S, Latifoltojar A, Sidhu H, Galazi M, Shah TT, Clemente J, Davda R, Payne HA, Chouhan MD, Lioumi M et al: Localising occult prostate cancer metastasis with advanced imaging techniques (LOCATE trial): a prospective cohort, observational diagnostic accuracy trial investigating whole-body magnetic resonance imaging in radio-recurrent prostate cancer. BMC Med Imaging 2019, 19(1): 90.
- Naseer F, Ahmad T, Kousar K, Anjum S: Advanced Therapeutic Options for Treatment of Metastatic Castration Resistant Prostatic Adenocarcinoma. Front Pharmacol 2021, 12: 728054.
- Zhang X, Zhang G, Wang J, Bi J: The efficacy and adverse events of conventional and second-generation androgen receptor inhibitors for castration-resistant prostate cancer: A network meta-analysis. Front Endocrinol (Lausanne) 2023, 14: 1131033.
- Cui X, Fu Q, Wang X, Xia P, Cui X, Bai X, Lu Z: Molecular mechanisms and clinical applications of exosomes in prostate cancer. Biomark Res 2022, 10(1): 56.
- Dijkstra S, Birker IL, Smit FP, Leyten GH, de Reijke TM, van Oort IM, Mulders PF, Jannink SA, Schalken JA: Prostate cancer biomarker profiles in urinary sediments and exosomes. J Urol 2014, 191(4): 1132-1138.
- Movahedpour A, Khatami SH, Karami N, Vakili O, Naeli P, Jamali Z, Shabaninejad Z, Tazik K, Behrouj H, Ghasemi H: Exosomal noncoding RNAs in prostate cancer. Clin Chim Acta 2022, 537: 127-132.
- Zhan F, Shen J, Wang R, Wang L, Dai Y, Zhang Y, Huang X: Role of exosomal small RNA in prostate cancer metastasis. Cancer Manag Res 2018, 10:4029-4038.
- Li Y, Li Q, Gu J, Qian D, Qin X, Li D: Exosomal prostate-specific G-protein-coupled receptor induces osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Transl Cancer Res 2020, 9(10): 5857-5867.
- Liu CM, Hsieh CL, Shen CN, Lin CC, Shigemura K, Sung SY: Exosomes from the tumor microenvironment as reciprocal regulators that enhance prostate cancer progression. Int J Urol 2016, 23(9): 734-744.
- Chowdhury SG, Ray R, Karmakar P: Exosomal miRNAs-a diagnostic biomarker acting as a guiding light in the diagnosis of prostate cancer. Funct Integr Genomics 2022, 23(1): 23.
- Gao Z, Pang B, Li J, Gao N, Fan T, Li Y: Emerging Role of Exosomes in Liquid Biopsy for Monitoring Prostate Cancer Invasion and Metastasis. Front Cell Dev Biol 2021, 9: 679527.
- Kim J, Shim JS, Han BH, Kim HJ, Park J, Cho IJ, Kang SG, Kang JY, Bong KW, Choi N: Hydrogel-based hybridization chain reaction (HCR) for detection of urinary exosomal miRNAs as a diagnostic tool of prostate cancer. Biosens Bioelectron 2021, 192: 113504.
- Leslie SW, Soon-Sutton TL, R IA, Sajjad H, Siref LE: Prostate Cancer. In: StatPearls. Epub ahead of print., edn. Treasure Island (FL); 2022.
- Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S: Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27(17).
- Hu M, Mao Y, Guan C, Tang Z, Bao Z, Li Y, Liang G: Dynamic changes in PSA levels predict prognostic outcomes in prostate cancer patients undergoing androgen -deprivation therapy: A multicenter retrospective analysis. Front Oncol 2023, 13: 1047388.
- Barlow M, Down L, Mounce LTA, Merriel SWD, Watson J, Martins T, Bailey SER: Ethnic differences in prostate-specific antigen levels in men without prostate cancer: a systematic review. Prostate Cancer Prostatic Dis 2022, https://doi.org/10.1038/s41391-022-00613-7. Epub ahead of print.
- Liao X, Tang Z, Ai J, Xu H, Zhang S, Liu L, Qiu S, Tan P, Fan Y, Yang L et al: Detection of Prostatic Inflammation From Peripheral Lymphocyte Count and Free/Total PSA Ratio in Men With LUTS/BPH. Front Pharmacol 2020, 11: 589.
- Grayling MJ, McMenamin M, Chandler R, Heer R, Wason JMS: Improving power in PSA response analyses of metastatic castration-resistant prostate cancer trials. BMC Cancer 2022, 22(1): 111.
- Tidd-Johnson A, Sebastian SA, Co EL, Afaq M, Kochhar H, Sheikh M, Mago A, Poudel S, Fernandez JA, Rodriguez ID et al: Prostate cancer screening: Continued controversies and novel biomarker advancements. Curr Urol 2022, 16(4): 197-206.
- Huang Y, Li ZZ, Huang YL, Song HJ, Wang YJ: Value of free/total prostate-specific antigen (f/t PSA) ratios for prostate cancer detection in patients with total serum prostate-specific antigen between 4 and 10 ng/mL: A meta-analysis. Medicine (Baltimore) 2018, 97(13):e0249.
- Denham JW, Bender R, Paradice WE: It's time to depolarise the unhelpful PSA-testing debate and put into practice lessons from the two major international screening trials. Med J Aust 2010, 192(7): 393-396.
- Mizutani K, Terazawa R, Kameyama K, Kato T, Horie K, Tsuchiya T, Seike K, Ehara H, Fujita Y, Kawakami K et al: Isolation of prostate cancer-related exosomes. Anticancer Res 2014, 34(7): 3419-3423.
- Bertokova A, Svecova N, Kozics K, Gabelova A, Vikartovska A, Jane E, Hires M, Bertok T, Tkac J: Exosomes from prostate cancer cell lines: Isolation optimisation and characterisation. Biomed Pharmacother 2022, 151: 113093.
- Pan J, Ding M, Xu K, Yang C, Mao LJ: Exosomes in diagnosis and therapy of prostate cancer. Oncotarget 2017, 8(57): 97693-97700.
- Lee YJ, Shin KJ, Jang HJ, Ryu JS, Lee CY, Yoon JH, Seo JK, Park S, Lee S, Je AR et al: GPR143 controls ESCRT-dependent exosome biogenesis and promotes cancer metastasis. Dev Cell 2023, 58(4): 320-334 e328.
- Cheng J, Wang X, Yuan X, Liu G, Chu Q: Emerging roles of exosome-derived biomarkers in cancer theranostics: messages from novel protein targets. Am J Cancer Res 2022, 12(5): 2226-2248.
- Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan DW, Regan MM, Groskopf J, Chipman J, Patil DH et al: Association Between Combined TMPRSS2:ERG and PCA3 RNA Urinary Testing and Detection of Aggressive Prostate Cancer. JAMA Oncol 2017, 3(8): 1085-1093.
- Donovan MJ, Noerholm M, Bentink S, Belzer S, Skog J, O'Neill V, Cochran JS, Brown GA: A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis 2015, 18(4): 370-375.
- Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP: Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 2005, 35(2): 169-173.
- Gharibpoor F, Kamali Zonouzi S, Razi S, Rezaei N: AMPK's double-faced role in advanced stages of prostate cancer. Clin Transl Oncol 2022, 24(11): 2064-2073.
- Chen H, Luo D, Shang B, Cao J, Wei J, Chen Q, Chen J: Immunoassay-type biosensor based on magnetic nanoparticle capture and the fluorescence signal formed by horseradish peroxidase catalysis for tumor-related exosome determination. Mikrochim Acta 2020, 187(5): 282.
- Cimmino I, Bravaccini S, Cerchione C: Urinary Biomarkers in Tumors: An Overview. Methods Mol Biol 2021, 2292: 3-15.
- Barcelo M, Castells M, Bassas L, Vigues F, Larriba S: Semen miRNAs Contained in Exosomes as Non-Invasive Biomarkers for Prostate Cancer Diagnosis. Sci Rep 2019, 9(1): 13772.
- Le Doare K, Holder B, Bassett A, Pannaraj PS: Mother's Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front Immunol 2018, 9: 361.
- Wu D, Yan J, Shen X, Sun Y, Thulin M, Cai Y, Wik L, Shen Q, Oelrich J, Qian X et al: Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat Commun 2019, 10(1): 3854.
- Cattrini C, Rubagotti A, Zinoli L, Cerbone L, Zanardi E, Capaia M, Barboro P, Boccardo F: Role of Circulating Tumor Cells (CTC), Androgen Receptor Full Length (AR-FL) and Androgen Receptor Splice Variant 7 (AR-V7) in a Prospective Cohort of Castration-Resistant Metastatic Prostate Cancer Patients. Cancers (Basel) 2019, 11(9).
- Woo HK, Park J, Ku JY, Lee CH, Sunkara V, Ha HK, Cho YK: Urine-based liquid biopsy: non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab Chip 2018, 19(1): 87-97.
- Li P, Yu X, Han W, Kong Y, Bao W, Zhang J, Zhang W, Gu Y: Ultrasensitive and Reversible Nanoplatform of Urinary Exosomes for Prostate Cancer Diagnosis. ACS Sens 2019, 4(5): 1433-1441.
- Yu MY, Jia HJ, Zhang J, Ran GH, Liu Y, Yang XH: Exosomal miRNAs-mediated macrophage polarization and its potential clinical application. Int Immunopharmacol 2023, 117: 109905.
- Li K, Zhu X, Li L, Ning R, Liang Z, Zeng F, Su F, Huang S, Yang X, Qu S: Identification of non-invasive biomarkers for predicting the radiosensitivity of nasopharyngeal carcinoma from serum microRNAs. Sci Rep 2020, 10(1): 5161.
- Foj L, Ferrer F, Serra M, Arevalo A, Gavagnach M, Gimenez N, Filella X: Exosomal and Non-Exosomal Urinary miRNAs in Prostate Cancer Detection and Prognosis. Prostate 2017, 77(6): 573-583.
- Hasegawa T, Glavich GJ, Pahuski M, Short A, Semmes OJ, Yang L, Galkin V, Drake R, Esquela-Kerscher A: Characterization and Evidence of the miR-888 Cluster as a Novel Cancer Network in Prostate. Mol Cancer Res 2018, 16(4): 669-681.
- Bhagirath D, Yang TL, Bucay N, Sekhon K, Majid S, Shahryari V, Dahiya R, Tanaka Y, Saini S: microRNA-1246 Is an Exosomal Biomarker for Aggressive Prostate Cancer. Cancer Res 2018, 78(7): 1833-1844.
- Mercadal M, Herrero C, Lopez-Rodrigo O, Castells M, de la Fuente A, Vigues F, Bassas L, Larriba S: Impact of Extracellular Vesicle Isolation Methods on Downstream Mirna Analysis in Semen: A Comparative Study. Int J Mol Sci 2020, 21(17).
- Isin M, Uysaler E, Ozgur E, Koseoglu H, Sanli O, Yucel OB, Gezer U, Dalay N: Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet 2015, 6:168.
- Wang J, Yang X, Li R, Wang L, Gu Y, Zhao Y, Huang KH, Cheng T, Yuan Y, Gao S: Long non-coding RNA MYU promotes prostate cancer proliferation by mediating the miR-184/c-Myc axis. Oncol Rep 2018, 40(5): 2814-2825.
- Wang YH, Ji J, Wang BC, Chen H, Yang ZH, Wang K, Luo CL, Zhang WW, Wang FB, Zhang XL: Tumor-Derived Exosomal Long Noncoding RNAs as Promising Diagnostic Biomarkers for Prostate Cancer. Cell Physiol Biochem 2018, 46(2): 532-545.
- Jiang Y, Zhao H, Chen Y, Li K, Li T, Chen J, Zhang B, Guo C, Qing L, Shen J et al: Exosomal long noncoding RNA HOXD-AS1 promotes prostate cancer metastasis via miR-361-5p/FOXM1 axis. Cell Death Dis 2021, 12(12): 1129.
- Li Q, Hu J, Shi Y, Xiao M, Bi T, Wang C, Yan L, Li X: Exosomal lncAY927529 enhances prostate cancer cell proliferation and invasion through regulating bone microenvironment. Cell Cycle 2021, 20(23): 2531-2546.
- Ozgur E, Gezer U: Investigation of lncRNA H19 in prostate cancer cells and secreted exosomes upon androgen stimulation or androgen receptor blockage. Bratisl Lek Listy 2020, 121(5): 362-365.
- Yang X, Wang L, Li R, Zhao Y, Gu Y, Liu S, Cheng T, Huang K, Yuan Y, Song D et al: The long non-coding RNA PCSEAT exhibits an oncogenic property in prostate cancer and functions as a competing endogenous RNA that associates with EZH2. Biochem Biophys Res Commun 2018, 502(2): 262-268.
- Bach DH, Lee SK, Sood AK: Circular RNAs in Cancer. Mol Ther Nucleic Acids 2019, 16: 118-129.
- Shi X, Wang B, Feng X, Xu Y, Lu K, Sun M: circRNAs and Exosomes: A Mysterious Frontier for Human Cancer. Mol Ther Nucleic Acids 2020, 19: 384-392.
- Li T, Sun X, Chen L: Exosome circ_0044516 promotes prostate cancer cell proliferation and metastasis as a potential biomarker. J Cell Biochem 2020, 121(3): 2118-2126.
- Ding L, Zheng Q, Lin Y, Wang R, Wang H, Luo W, Lu Z, Xie H, Ren L, Lu H et al: Exosome-derived circTFDP2 promotes prostate cancer progression by preventing PARP1 from caspase-3-dependent cleavage. Clin Transl Med 2023, 13(1): e1156.
- Huang G, Jiang Z, Zhu W, Wu Z: Exosomal circKDM4A Induces CUL4B to Promote Prostate Cancer Cell Malignancy in a miR-338-3p-Dependent Manner. Biochem Genet 2023, 61(1): 390-409.
- Tang Y, Liu J, Li X, Wang W: Exosomal circRNA HIPK3 knockdown inhibited cell proliferation and metastasis in prostate cancer by regulating miR-212/BMI-1 pathway. J Biosci 2021, 46.
- Zhang G, Liu Y, Yang J, Wang H, Xing Z: Inhibition of circ_0081234 reduces prostate cancer tumor growth and metastasis via the miR-1/MAP 3 K1 axis. J Gene Med 2022, 24(8): e3376.
- Logozzi M, Angelini DF, Giuliani A, Mizzoni D, Di Raimo R, Maggi M, Gentilucci A, Marzio V, Salciccia S, Borsellino G et al: Increased Plasmatic Levels of PSA-Expressing Exosomes Distinguish Prostate Cancer Patients from Benign Prostatic Hyperplasia: A Prospective Study. Cancers (Basel) 2019, 11(10).
- Logozzi M, Mizzoni D, Capasso C, Del Prete S, Di Raimo R, Falchi M, Angelini DF, Sciarra A, Maggi M, Supuran CT et al: Plasmatic exosomes from prostate cancer patients show increased carbonic anhydrase IX expression and activity and low pH. J Enzyme Inhib Med Chem 2020, 35(1): 280-288.
- Logozzi M, Capasso C, Di Raimo R, Del Prete S, Mizzoni D, Falchi M, Supuran CT, Fais S: Prostate cancer cells and exosomes in acidic condition show increased carbonic anhydrase IX expression and activity. J Enzyme Inhib Med Chem 2019, 34(1): 272-278.
- Kawakami K, Fujita Y, Matsuda Y, Arai T, Horie K, Kameyama K, Kato T, Masunaga K, Kasuya Y, Tanaka M et al: Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer 2017, 17(1): 316.
- Worst TS, von Hardenberg J, Gross JC, Erben P, Schnolzer M, Hausser I, Bugert P, Michel MS, Boutros M: Database-augmented Mass Spectrometry Analysis of Exosomes Identifies Claudin 3 as a Putative Prostate Cancer Biomarker. Mol Cell Proteomics 2017, 16(6): 998-1008.
- Krishn SR, Singh A, Bowler N, Duffy AN, Friedman A, Fedele C, Kurtoglu S, Tripathi SK, Wang K, Hawkins A et al: Prostate cancer sheds the alphavbeta3 integrin in vivo through exosomes. Matrix Biol 2019, 77: 41-57.
- Hornung T, O'Neill HA, Logie SC, Fowler KM, Duncan JE, Rosenow M, Bondre AS, Tinder T, Maher V, Zarkovic J et al: ADAPT identifies an ESCRT complex composition that discriminates VCaP from LNCaP prostate cancer cell exosomes. Nucleic Acids Res 2020, 48(8): 4013-4027.
- Chiasserini D, Mazzoni M, Bordi F, Sennato S, Susta F, Orvietani PL, Binaglia L, Palmerini CA: Identification and Partial Characterization of Two Populations of Prostasomes by a Combination of Dynamic Light Scattering and Proteomic Analysis. J Membr Biol 2015, 248(6): 991-1004.
- Worst TS, Meyer Y, Gottschalt M, Weis CA, von Hardenberg J, Frank C, Steidler A, Michel MS, Erben P: RAB27A, RAB27B and VPS36 are downregulated in advanced prostate cancer and show functional relevance in prostate cancer cells. Int J Oncol 2017, 50(3): 920-932.
- Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K, Llorente A: Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer 2017, 70: 122-132.
- Ishizuya Y, Uemura M, Narumi R, Tomiyama E, Koh Y, Matsushita M, Nakano K, Hayashi Y, Wang C, Kato T et al: The role of actinin-4 (ACTN4) in exosomes as a potential novel therapeutic target in castration-resistant prostate cancer. Biochem Biophys Res Commun 2020, 523(3): 588-594.
- Kumar A, Kumar P, Sharma M, Kim S, Singh S, Kridel SJ, Deep G: Role of extracellular vesicles secretion in paclitaxel resistance of prostate cancer cells. Cancer Drug Resist 2022, 5(3): 612-624.
- Shan G, Gu J, Zhou D, Li L, Cheng W, Wang Y, Tang T, Wang X: Cancer-associated fibroblast-secreted exosomal miR-423-5p promotes chemotherapy resistance in prostate cancer by targeting GREM2 through the TGF-beta signaling pathway. Exp Mol Med 2020, 52(11): 1809-1822.
- Duan Y, Tan Z, Yang M, Li J, Liu C, Wang C, Zhang F, Jin Y, Wang Y, Zhu L: PC-3-Derived Exosomes Inhibit Osteoclast Differentiation by Downregulating miR-214 and Blocking NF-kappaB Signaling Pathway. Biomed Res Int 2019, 2019: 8650846.
- Hashimoto K, Ochi H, Sunamura S, Kosaka N, Mabuchi Y, Fukuda T, Yao K, Kanda H, Ae K, Okawa A et al: Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc Natl Acad Sci U S A 2018, 115(9): 2204-2209.
- Sanchez CA, Andahur EI, Valenzuela R, Castellon EA, Fulla JA, Ramos CG, Trivino JC: Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016, 7(4): 3993-4008.
- Wang X, Wang X, Zhu Z, Li W, Yu G, Jia Z, Wang X: Prostate carcinoma cell-derived exosomal MicroRNA-26a modulates the metastasis and tumor growth of prostate carcinoma. Biomed Pharmacother 2019, 117: 109109.
- Guo Z, Lu X, Yang F, Qin L, Yang N, Cai P, Han C, Wu J, Wang H: The Expression of miR-205 in Prostate Carcinoma and the Relationship with Prognosis in Patients. Comput Math Methods Med 2022, 2022: 1784791.
- Li W, Dong Y, Wang KJ, Deng Z, Zhang W, Shen HF: Plasma exosomal miR-125a-5p and miR-141-5p as non-invasive biomarkers for prostate cancer. Neoplasma 2020, 67(6):1314-1318.
- Ye Y, Li SL, Ma YY, Diao YJ, Yang L, Su MQ, Li Z, Ji Y, Wang J, Lei L et al: Exosomal miR-141-3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Oncotarget 2017, 8(55): 94834-94849.
- Foroni C, Zarovni N, Bianciardi L, Bernardi S, Triggiani L, Zocco D, Venturella M, Chiesi A, Valcamonico F, Berruti A: When Less Is More: Specific Capture and Analysis of Tumor Exosomes in Plasma Increases the Sensitivity of Liquid Biopsy for Comprehensive Detection of Multiple Androgen Receptor Phenotypes in Advanced Prostate Cancer Patients. Biomedicines 2020, 8(5).
- Wu YP, Ke ZB, Lin F, Wen YA, Chen S, Li XD, Chen SH, Sun XL, Huang JB, Zheng QS et al: Identification of key genes and pathways in castrate-resistant prostate cancer by integrated bioinformatics analysis. Pathol Res Pract 2020, 216(10): 153109.
- Wang C, Liu X, Li H, Zhao L, Kong G, Chen J, Li Z, Qi J, Tian Y, Zhang F: Urinary exosome-based androgen receptor-variant 7 detection in metastatic castration-resistant prostate cancer patients. Transl Androl Urol 2022, 11(2): 202-212.
- Rodriguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, Sandvig K, Line A, Llorente A: Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer 2017, 16(1): 156.
- Takahara K, Ii M, Inamoto T, Nakagawa T, Ibuki N, Yoshikawa Y, Tsujino T, Uchimoto T, Saito K, Takai T et al: microRNA-145 Mediates the Inhibitory Effect of Adipose Tissue-Derived Stromal Cells on Prostate Cancer. Stem Cells Dev 2016, 25(17): 1290-1298.
- Lee J, Kwon MH, Kim JA, Rhee WJ: Detection of exosome miRNAs using molecular beacons for diagnosing prostate cancer. Artif Cells Nanomed Biotechnol 2018, 46(sup3): S52-S63.
- Li S, Zeng M, Yang L, Yang J, Tan J, Guan H, Kuang M: EDNRB Reverses Methylprednisolone-Mediated Decrease in Neural Progenitor Cell Viability via Regulating PI3K/Akt Pathway and lncRNA Expression. J Mol Neurosci 2020, 70(3): 403-412.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript