REVIEW | Open Access
Research Progress of Neoadjuvant Chemotherapy in Advanced Bladder Cancer
Haijun Hu1, Xianghui Wu1
1Department of Urology, Wushan County People's Hospital, Chongqing, China.
Annals of Urologic Oncology 2024, 7(3): 110-117. https://doi.org/10.32948/auo.2024.09.02
Received: 20 Aug 2024 | Accepted: 02 Sep 2024 | Published online: 09 Sep 2024
Key words bladder cancer, systemic treatment, neoadjuvant chemotherapy, muscle-invasive bladder cancer
An early randomized controlled trial (RCT) found that among 976 patients with muscle-invasive bladder cancer scheduled for radical cystectomy or full-dose external radiotherapy, 491 patients received three cycles of neoadjuvant chemotherapy with Cisplatin, Methotrexate, and Vinblastine (CMV). After a median follow-up of 4 years, the 3-year survival rate for patients receiving CMV neoadjuvant chemotherapy was 55.5%, compared to 50.0% in the control group (P=0.075). The median survival in the neoadjuvant chemotherapy group was 44 months compared to 37.5 months in the control group, with a pathological complete response rate of 32.5% following neoadjuvant chemotherapy [14]. Long-term follow-up with a median duration of 8 years showed that the CMV neoadjuvant chemotherapy group exhibited a 16% higher overall survival rate (HR 0.84, 95% CI 0.72-0.99), with a 10-year survival rate of 36%, highlighting its critical role in improving the prognosis of patients with muscle-invasive bladder cancer (MIBC). A meta-analysis [15] encompassing 11 RCTs and a total of 3005 patients demonstrated a significant improvement in prognosis for patients receiving Cisplatin-based neoadjuvant chemotherapy (HR=0.86, 95% CI 0.77-0.95, p=0.003). The 5-year overall survival rate and the disease-free survival rate were notably better for these patients, highlighting the important role of neoadjuvant chemotherapy in bladder cancer treatment [16].
Cisplatin is the cornerstone of all bladder cancer chemotherapy regimens and is used in combination with other chemotherapeutic agents to enhance efficacy and mitigate side effects. Two prominent regimens include Methotrexate, Vinblastine, Doxorubicin, and Cisplatin (MVAC) and Gemcitabine plus Cisplatin (GC). In a large Phase III RCT conducted by von der Maase et al., the efficacy and safety of the GC regimen were compared with the MVAC regimen in 405 patients with locally advanced or metastatic bladder cancer [17]. The findings revealed no significant differences between the two groups in terms of overall survival (HR 1.04, 95% CI 0.82-1.32, P=0.75), time to tumor progression (HR 1.05, 95% CI 0.85-1.30), time to treatment failure (HR 0.89, 95% CI 0.72-1.10), and response rate (GC 49% vs. MVAC 46%). However, the GC regimen exhibited a higher completion rate of all six chemotherapy cycles, fewer dose adjustments, and reduced drug-related mortality (1% vs. 3% in MVAC). The drug-related mortality rate was 1% in the GC group compared to 3% in the MVAC group. Although the GC group had a higher incidence of Grade 3-4 anemia (27% vs. 18%) and thrombocytopenia (57% vs. 21%), the rates of neutropenia (71% vs. 82%), febrile neutropenia (2% vs. 14%), sepsis (1% vs. 12%), mucositis (1% vs. 22%), and alopecia (11% vs. 55%) were significantly lower. Despite similar quality-of-life outcomes between the two groups, the GC regimen showed better results in terms of weight, performance status, and fatigue [15]. Long-term survival follow-up results indicated that there was no significant difference in overall survival between the GC and MVAC groups (HR 1.09, 95% CI 0.88-1.34, P=0.66). The median survival for the GC and MVAC groups was 14 months and 15.2 months, respectively, with 5-year overall survival rates of 13.0% and 15.3% (p=0.53). The progression-free survival was 7.7 months for the GC group and 8.3 months for the MVAC group (HR 1.09), and the 5-year progression-free survival rates were 9.8% and 11.3% (p=0.63). Given its comparable efficacy to the MVAC regimen and fewer side effects, the GC regimen has become the preferred chemotherapy option for bladder cancer in clinical practice [16]. Dash et al. found no significant differences in the pathological response rates between the GC and MVAC regimens when used as neoadjuvant chemotherapy [18]. The similarity in efficacy coupled with the reduced toxicity profile of the GC regimen has led to its preference over MVAC in many treatment protocols. Notably, the GC regimen leads to fewer severe adverse effects like neutropenia and alopecia, offering a more manageable side effect profile that aligns with current clinical trends and guidelines. Galsky et al. [19] analyzed data from an international multicenter retrospective study, which reaffirmed these conclusions, showing a complete pathological response rate of 31% for the GC regimen, comparable to 29% for MVAC (OR 0.91, 95% CI 0.48-1.72, P=0.77). There was also no significant difference in survival rates between the two groups (HR 0.78, 95% CI 0.40-1.54, P=0.48). Additionally, research by Zargar et al. indicated that both the GC and MVAC regimens, when used as neoadjuvant chemotherapy, showed a lower rate of complete pathological response compared to previously reported figures. However, there was no significant difference between the two regimens (23.9% vs. 24.5%) [20]. Several reviews comparing the GC and MVAC regimens for neoadjuvant chemotherapy have corroborated these findings [21, 22], consistently showing that while both regimens yield similar pathological response rates and survival outcomes, the GC regimen's favorable toxicity profile often secures its preference in clinical settings. The consistent findings across multiple studies and reviews highlight the reliability of the GC regimen as a viable alternative to MVAC, reflecting its growing acceptance in contemporary treatment protocols for bladder cancer.
To shorten the interval between a definitive diagnosis and radical cystectomy, intensified chemotherapy can be considered as an option. In a preclinical trial involving 39 patients with muscle-invasive bladder cancer scheduled for radical cystectomy, MVAC chemotherapy was administered every two weeks for a total of four cycles. Postoperative results confirmed a pathological response rate (pT<2) of 49%, with 10% of patients experiencing grade 3-4 toxicities [23]. Another study by Plimack et al. found that patients receiving MVAC chemotherapy biweekly for three cycles resulted in a pathological downstaging rate to pT<2 of 53%, with 38% of patients achieving a pathological stage of pT0 after surgery [24]. Previous studies reported the patient with pelvic lymph node positivity (cN1). Choueiri et al. identified a rate of 43% for N1+ patients, while Plimack et al. reported 7% for N1+ patients. Choueiri et al. observed that 82% of patients preoperatively diagnosed with pelvic lymph node metastasis (cN1+) had pathological confirmation of negativity (pN0) after surgery, highlighting the significant therapeutic impact of preoperative systemic chemotherapy for lymph node-positive patients. However, due to severe cardiovascular adverse events in 7 out of 31 participants, the initial clinical trial using intensified GC regimen neoadjuvant chemotherapy was redesigned. The revised protocol found that increasing the chemotherapy density with the GC regimen resulted in a pathological response rate (< pT2) of 56.5%. Pouessel et al. also concluded that intensified chemotherapy did not significantly increase the rate of adverse effects [25]. These findings collectively suggest that intensified chemotherapy regimens, while potentially increasing the pathological response rates, can be managed with acceptable levels of toxicity, thus offering a promising approach to improve outcomes in muscle-invasive BCa patients. Systemic therapy drugs recommended for advanced bladder cancer are listed in Table 1.
Patients with muscle-invasive bladder cancer and non-suitable for cisplatin-based chemotherapy currently lack access to well-established chemotherapy regimens demonstrated to effectively reduce mortality. Therefore, these patients should either participate in clinical trials or proceed directly to radical cystectomy.
|
Table 1. Recommended systemic therapy drugs for advanced bladder cancer. |
|||||
|
Therapy schedule |
Chemotherapy drug 1 |
Chemotherapy drug 2 |
Chemotherapy drug 3 |
Immunotherapy drug |
Recommendation level |
|
First-line |
|
|
|
|
|
|
First-line 1 |
Fluorouracil |
Xeloda |
Oxaliplatin |
Trastuzumab |
ⅠA |
|
First-line 2 |
Fluorouracil |
Xeloda |
Cis-platinum |
Palizumab |
ⅠB |
|
Second-line |
|
|
|
|
|
|
Second-line 1 |
Fluorouracil |
Docetaxel |
Oxaliplatin |
Nivolumab |
ⅡA |
|
Second-line 2 |
Xeloda |
Cis-platinum |
Oxaliplatin |
|
ⅡA |
|
Other |
|
|
|
|
|
|
Other 1 |
Fluorouracil |
Irinotecan |
|
|
ⅡB |
|
Other 2 |
Paclitaxel |
Cis-platinum |
|
|
ⅡB |
There is no clear evidence from randomized controlled trials (RCTs) supporting the use of adjuvant chemotherapy [29]. However, some clinical observations and retrospective studies suggest that adjuvant chemotherapy might help prevent tumor recurrence and metastasis in patients who have undergone radical cystectomy without prior neoadjuvant chemotherapy [30]. Most clinical studies on adjuvant chemotherapy for MIBC are hampered by small sample sizes and often produce inconsistent findings [31-33]. A meta-analysis published in 2005 indicated that adjuvant chemotherapy could extend survival (HR 0.75, 95% CI 0.60-0.96), but this analysis included only 491 patients across 6 studies, which limits the robustness of the conclusion [34]. Subsequent large-scale randomized controlled trials have yielded conflicting results. An updated meta-analysis in 2014 pooled data from 9 clinical studies found that adjuvant chemotherapy could prolong survival (HR 0.77, 95% CI 0.59-0.99) [35]. Most of the patients were high-risk individuals, such as those with extravesical tumor invasion or positive lymph nodes. Evidence supporting the benefits of adjuvant chemotherapy exists, particularly for high-risk patients, yet the overall consensus is inconclusive due to the variability and limitations of the studies conducted.
The SOGUG 99/01 study enrolled 142 high-risk MIBC patients undergoing radical cystectomy. Following surgery, the patients were randomly divided into treatment and observation groups. The treatment group received four courses of adjuvant chemotherapy with paclitaxel, gemcitabine, and cisplatin (PGC regimen) after surgery. Subsequent follow-up revealed that the 5-year survival rate of patients receiving PGC adjuvant chemotherapy was significantly higher than that of the observation group (60% vs 31%, p<0.0001). The EORTC 30994 study represents the largest phase 3 clinical trial on adjuvant chemotherapy to date, originally targeting an enrollment of 660 patients, yet enrolled 284 patients, casting doubts on its reliability. High-risk MIBC patients with pT3-pT4 or lymph node-positive status undergoing radical cystectomy were randomly divided into two groups after surgery. The experimental group received four cycles of adjuvant chemotherapy after surgery, including GC, MVAC, or high-dose MVAC regimens. In contrast, the control group was followed up until tumor recurrence and then received delayed chemotherapy. The progression-free survival rate was significantly improved in the adjuvant chemotherapy group (HR 0.54, 95% CI 0.4-0.73, p<0.0001). The median progression-free survival time was 3.11 years (95% CI 1.84-7.77) in the adjuvant chemotherapy group, compared to 0.99 years (95% CI 0.63-1.49) in the delayed chemotherapy group. However, there was no significant difference in overall survival between the two groups (HR 0.78, 95% CI 0.56-1.08) [36]. Moreover, the study found that lymph node-negative patients benefited most from adjuvant chemotherapy, suggesting that four cycles of chemotherapy might be insufficient for lymph node-positive patients. Consequently, while adjuvant chemotherapy appears to enhance survival rates in high-risk MIBC patients, especially those who are lymph node-negative, the best chemotherapy regimen and duration are still unclear. Further research is needed to determine the most effective treatment strategies for these patients. Additionally, the low enrollment in the EORTC 30994 trial highlights the challenges of conducting large-scale clinical trials in this field and the need for continued efforts to improve patient recruitment and participation.
Galsky et al. presented an abstract at ASCO comparing the efficacy of adjuvant chemotherapy. They analyzed data from the NCDB database and compared two groups of patients with propensity score matching: those who received adjuvant chemotherapy after radical cystectomy versus those who underwent only radical cystectomy. The survival rate in the adjuvant chemotherapy group was significantly higher than that in the observation group (HR 0.72, 95% CI 0.71-0.86).
However, clinical randomized controlled trials on adjuvant chemotherapy may not be feasible in the foreseeable future. Given the risks and benefits of adjuvant chemotherapy, such as its side effects and the possibility of postoperative tumor recurrence, it is recommended that high-risk bladder cancer patients with pT3-pT4 or lymph node-positive status receive systemic chemotherapy prior to undergoing radical cystectomy. This recommendation takes into account the complex balance between potential therapeutic benefits and the risks associated with adjuvant chemotherapy. Addressing these factors before surgery might improve the overall treatment outcomes and potentially reduce the risk of disease recurrence, ultimately leading to better patient survival rates. Further research and clinical trials will be crucial in refining these recommendations and optimizing treatment strategies for high-risk bladder cancer patients.
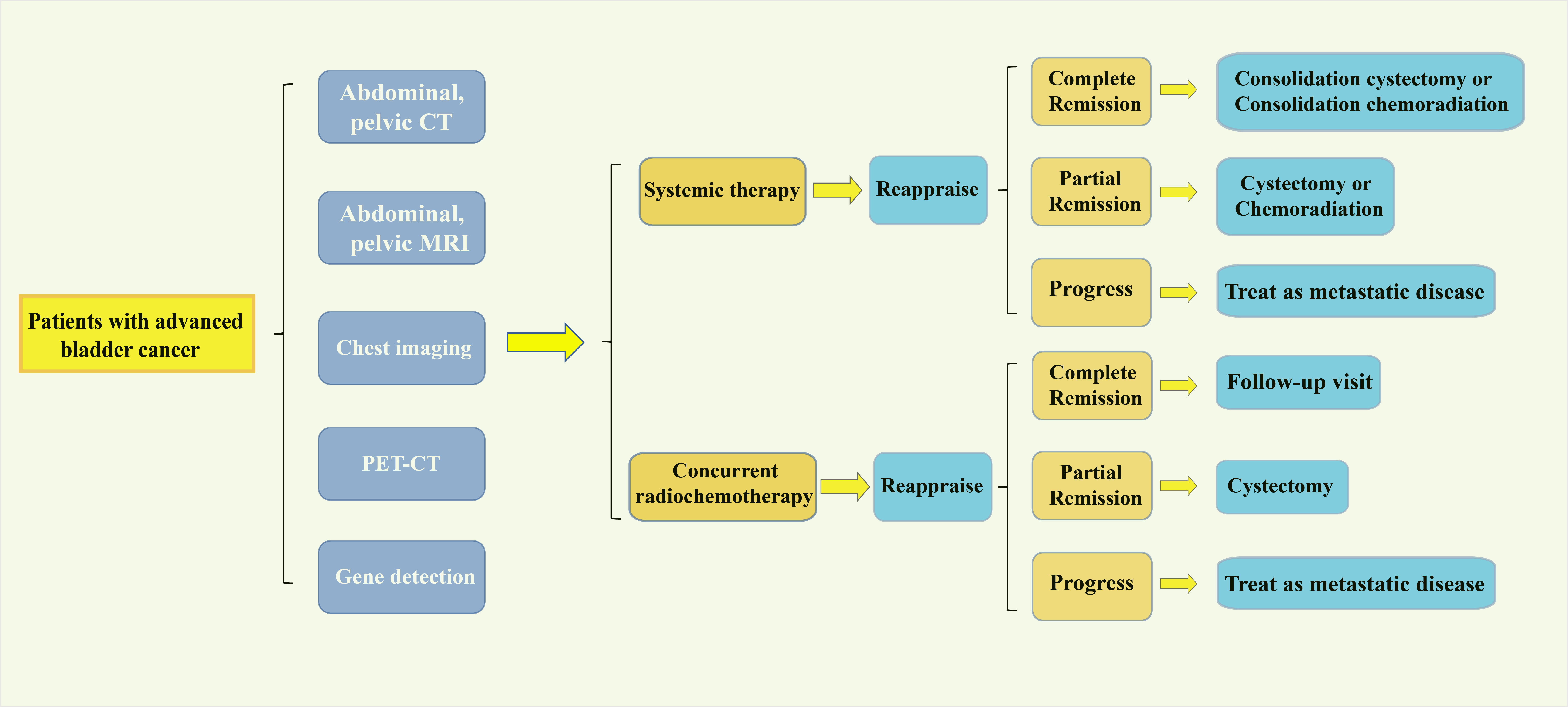 Figure 1. Possible mechanisms of obesity affecting prostate cancer.
Figure 1. Possible mechanisms of obesity affecting prostate cancer.
Moreover, 44% of bladder cancers exhibit gene mutations in the receptor tyrosine kinase (RTK)/RAS signaling pathway, including 9% of tumors with epidermal growth factor receptor (EGFR) amplification [46]. However, the targeted agents, gefitinib, erlotinib, and cetuximab, have not demonstrated significant drug activity, even without selective enrollment based on EGFR status [47-51]. TCGA research found that 7% of bladder cancers have ERBB2 copy number variations [52]. Techniques like immunohistochemistry and fluorescence in situ hybridization (FISH) have been employed to detect HER2 overexpression in advanced bladder cancer [53, 54]. A regimen combining trastuzumab with gemcitabine, carboplatin, and paclitaxel yielded a promising overall response rate of 70% and a median overall survival of 14.1 months. Nonetheless, the absence of a randomized trial design precludes definitive conclusions regarding trastuzumab's effectiveness [52]. Although the immunohistochemistry results of most bladder tumors were positive, only a small portion tested positive with FISH, suggesting ongoing uncertainty about the optimal diagnostic method for confirming HER2 overexpression.
Fibroblast growth factor receptors (FGFRs) have mutations in 70% of non-muscle-invasive bladder cancers (NMIBC) and 15% of muscle-invasive bladder cancers [55, 56]. Some targeted drugs for FGFR signaling pathways are currently under investigation. Additionally, the vascular endothelial growth factor (VEGF) signaling pathway has been widely studied. Sunitinib has shown limited improvement in survival outcomes for advanced bladder cancer patients who underwent other treatments, with progression-free survival (PFS) of 2 months and median overall survival (OS) of 6-7 months [57]. As a first-line treatment for those unfit for cisplatin, sunitinib had a PFS of 4.8 months and an OS of 8.1 months [58]. The efficacy of sorafenib is correspondingly lowered [59, 60]. Necchi et al. found that pazopanib had an overall response rate of 17% in previously treated bladder cancer patients, but 5% of patients developed a bladder fistula during treatment [61]. Several novel targeted therapeutics are currently under development. Considering the high frequency of variations in the RB and CDK signaling pathways in bladder cancer, a phase II clinical trial (NCT02334527) is exploring the efficacy of palbociclib in the treatment of metastatic bladder cancer. Furthermore, ongoing research is evaluating additional cytotoxic chemotherapy drugs. Notably, at the 2015 ASCO Annual Meeting, eribulin demonstrated a 35% response rate and an overall survival of 9.5 months in a phase II clinical trial for metastatic bladder cancer, showing improvement compared to previous chemotherapy regimens.
Tumor mutations can generate new antigens that are expressed on the surface of tumor cells and are specific to certain tumors, thereby inducing specific immune responses. In melanoma, early research suggests that a higher tumor mutation burden is associated with better responses to immune therapies targeting CTLA-4. Bladder cancer exhibits the highest tumor mutation burdens compared to normal tumors, underscoring the efficacy of immunotherapy in its treatment [62]. Recent studies have identified molecular subtypes of bladder cancer, classifying them into "basal" and "luminal" subtypes, similar to the classification of breast cancer. These subtypes exhibit distinctly different RNA expression profiles and prognoses. The basal subtype, in particular, is rich in immune infiltration, suggesting its enhanced responsiveness to immunotherapy [63]. This differential response underscores the potential for tailored immunotherapeutic approaches based on the specific molecular characteristics of bladder cancer subtypes, potentially leading to more effective treatment outcomes for patients [64, 65]. Further research into these subtypes and their interactions with immunotherapy will be crucial for optimizing treatment strategies and improving patient prognosis.
Recent advances in treatment for advanced bladder cancer have made significant progress. The use of neoadjuvant chemotherapy for muscle-invasive bladder cancer is increasingly attributed to its proven survival benefits. Adjuvant chemotherapy serves as a valuable supplement for patients not previously treated with neoadjuvant therapy. In bladder-preserving treatments, combining systemic therapy with radiation has been shown to improve patient outcomes. Advances in the molecular characterization of bladder cancer have propelled the development of targeted therapies. Clinical trials of immunotherapy have yielded promising results, and ongoing randomized controlled trials aim to further validate their effectiveness. To optimize the selection of patients for targeted or immunotherapy, research into predictive biomarkers must progress alongside the development of these therapies. As the understanding of bladder cancer's molecular landscape deepens, targeted and immunotherapeutic strategies are expected to become increasingly effective. Identifying reliable biomarkers for predicting treatment response will be crucial for personalizing therapy and improving overall patient outcomes. Continued research and clinical trials will play a key role in advancing these treatments and refining therapeutic approaches for bladder cancer.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
Haijun Hu and Xianghui Wu searched academic literature, wrote the draft manuscript, supervised the review writting progress and approved the final manuscript submission.
Competing interests
Authors report no conflict of interest.
Funding
None.
- Siegel RL, Miller KD, Jemal A: Cancer statistics, 2016. CA Cancer J Clin 2016, 66(1): 7-30.
- Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F: Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017, 71(1): 96-108.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J: Cancer statistics in China, 2015. CA Cancer J Clin 2016, 66(2): 115-32.
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE: The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016, 70(1): 106-119.
- Kaufman DS, Shipley WU, Feldman AS: Bladder cancer. Lancet 2009, 374(9685): 239-249.
- Li T, Xing Y, Liu SC, Han XM, Li WC, Chen M: Long-term versus short-term introvesical chemotherapy in patients with non-muscle-invasive bladder cancer: a systematic review and meta-analysis of the published results of randomized clinical trials. J Huazhong Univ Sci Technolog Med Sci 2014, 34(5): 706-715.
- Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D, et al: ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol 2013, 63(1): 58-66.
- Racioppi M, D'Agostino D, Totaro A, Pinto F, Sacco E, D'Addessi A, Marangi F, Palermo G, Bassi PF: Value of current chemotherapy and surgery in advanced and metastatic bladder cancer. Urol Int 2012, 88(3): 249-258.
- Alfred Witjes J, Lebret T, Compérat EM, Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J, Rouanne M, et al: Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017, 71(3): 462-475.
- Sagaster P, Flamm J, Flamm M, Mayer A, Donner G, Oberleitner S, Havelec L, Lepsinger L, Ludwig H: Neoadjuvant chemotherapy (MVAC) in locally invasive bladder cancer. Eur J Cancer 1996, 32(8): 1320-1334.
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP Jr, Raghavan D, et al: Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003, 349(9): 859-866.
- Rosenblatt R, Sherif A, Rintala E, Wahlqvist R, Ullén A, Nilsson S, Malmström PU; Nordic Urothelial Cancer Group: Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol 2012, 61(6): 1229-1238.
- Sonpavde G, Goldman BH, Speights VO, Lerner SP, Wood DP, Vogelzang NJ, Trump DL, Natale RB, Grossman HB, Crawford ED: Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 2009, 115(18): 4104-4109.
- No authors: Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet 1999, 354(9178): 533-540.
- International Collaboration of Trialists, Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group), European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, Australian Bladder Cancer Study Group, National Cancer Institute of Canada Clinical Trials Group, Finnbladder, Norwegian Bladder Cancer Study Group, Club Urologico Espanol de Tratamiento Oncologico Group, Griffiths G, Hall R, Sylvester R, Raghavan D, et al: International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011, 29(16): 2171-2177.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 2005, 48(2): 202-215.
- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000, 18(17): 3068-3077.
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M: Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005, 23(21): 4602-4608.
- Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, Yu EY, Powles T, Moshier EL, Ladoire S, et al: Retrospective International Study of Cancers of the Urothelial Tract (RISC) Investigators. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer 2015, 121(15): 2586-2593.
- Zargar H, Espiritu PN, Fairey AS, Mertens LS, Dinney CP, Mir MC, Krabbe LM, Cookson MS, Jacobsen NE, Gandhi NM, et al: Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2015, 67(2): 241-249.
- Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, Kaag M, Fransen van de Putte EE, Horenblas S, Drabick JJ: Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist 2016, 21(6): 708-715.
- Kaimakliotis HZ, Monn MF, Cho JS, Pedrosa JA, Hahn NM, Albany C, Gellhaus PT, Cary KC, Masterson TA, Foster RS, et al: Neoadjuvant chemotherapy in urothelial bladder cancer: impact of regimen and variant histology. Future Oncol 2016, 12(15): 1795-1804.
- Choueiri TK, Jacobus S, Bellmunt J, Qu A, Appleman LJ, Tretter C, Bubley GJ, Stack EC, Signoretti S, Walsh M, et al: Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 2014, 32(18): 1889-1894.
- Plimack ER, Hoffman-Censits JH, Viterbo R, Trabulsi EJ, Ross EA, Greenberg RE, Chen DY, Lallas CD, Wong YN, Lin J, et al: Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014, 32(18): 1895-1901.
- Pouessel D, Chevret S, Rolland F, Gravis G, Geoffrois L, Roubaud G, Terrisse S, Boyle H, Chevreau C, Dauba J, et al: Standard or accelerated methotrexate, vinblastine, doxorubicin and cisplatin as neoadjuvant chemotherapy for locally advanced urothelial bladder cancer: Does dose intensity matter? Eur J Cancer 2016, 54: 69-74.
- David KA, Milowsky MI, Ritchey J, Carroll PR, Nanus DM: Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol 2007, 178(2): 451-464.
- Zaid HB, Patel SG, Stimson CJ, Resnick MJ, Cookson MS, Barocas DA, Chang SS: Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology 2014, 83(1): 75-80.
- Reardon ZD, Patel SG, Zaid HB, Stimson CJ, Resnick MJ, Keegan KA, Barocas DA, Chang SS, Cookson MS: Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol 2015, 67(1): 165-170.
- Ruggeri EM, Fabbri MA, Nelli F: Should We Use Adjuvant Chemotherapy for Muscle-Invasive Bladder Cancer After Radical Cystectomy? J Clin Oncol 2016, 34(26): 3223-3234.
- Boström PJ, Mirtti T, van Rhijn B, Fleshner NE, Finelli A, Laato M, Jewett MA, Moore MJ, Sridhar S, Nurmi M, et al: Benefit of Adjuvant Chemotherapy and Pelvic Lymph Node Dissection in pT3 and Node Positive Bladder Cancer Patients Treated with Radical Cystectomy. Bladder Cancer 2016, 2(2): 263-272.
- Cognetti F, Ruggeri EM, Felici A, Gallucci M, Muto G, Pollera CF, Massidda B, Rubagotti A, Giannarelli D, Boccardo F: Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Ann Oncol 2012, 23(3): 695-700.
- Stöckle M, Meyenburg W, Wellek S, Voges G, Gertenbach U, Thüroff JW, Huber C, Hohenfellner R: Advanced bladder cancer (stages pT3b, pT4a, pN1 and pN2): improved survival after radical cystectomy and 3 adjuvant cycles of chemotherapy. Results of a controlled prospective study. J Urol 1992, 148(2Pt1): 302-306.
- Skinner DG, Daniels JR, Russell CA, Lieskovsky G, Boyd SD, Nichols P, Kern W, Sakamoto J, Krailo M, Groshen S: The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol 1991, 145(3): 459-464.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration: Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol 2005, 48(2): 189-199.
- Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL, Choueiri TK, et al: Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol 2014, 66(1): 42-54.
- Sternberg CN, Skoneczna I, Kerst JM, Albers P, Fossa SD, Agerbaek M, Dumez H, de Santis M, Théodore C, Leahy MG, et al: Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015, 16(1): 76-86.
- Zapatero A, Martin De Vidales C, Arellano R, Ibañez Y, Bocardo G, Perez M, Rabadan M, García Vicente F, Cruz Conde JA, Olivier C: Long-term results of two prospective bladder-sparing trimodality approaches for invasive bladder cancer: neoadjuvant chemotherapy and concurrent radio-chemotherapy. Urology 2012, 80(5): 1056-1062.
- Nowak-Sadzikowska J, Skóra T, Szyszka-Charewicz B, Jakubowicz J: Muscle-invasive bladder cancer treated with TURB followed by concomitant boost with small reduction of radiotherapy field with or without of chemotherapy. Rep Pract Oncol Radiother 2016, 21(1): 31-36.
- Shipley WU, Prout GR Jr, Einstein AB, Coombs LJ, Wajsman Z, Soloway MS, Englander L, Barton BA, Hafermann MD: Treatment of invasive bladder cancer by cisplatin and radiation in patients unsuited for surgery. JAMA 1987, 258(7): 931-935.
- Housset M, Maulard C, Chretien Y, Dufour B, Delanian S, Huart J, Colardelle F, Brunel P, Baillet F: Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J Clin Oncol 1993, 11(11): 2150-2157.
- Sauer R, Dunst J, Altendorf-Hofmann A, Fischer H, Bornhof C, Schrott KM: Radiotherapy with and without cisplatin in bladder cancer. Int J Radiat Oncol Biol Phys 1990, 19(3): 687-691.
- Shipley WU, Kaufman DS, Zehr E, Heney NM, Lane SC, Thakral HK, Althausen AF, Zietman AL: Selective bladder preservation by combined modality protocol treatment: long-term outcomes of 190 patients with invasive bladder cancer. Urology 2002, 60(1): 62-67.
- Weiss C, Engehausen DG, Krause FS, Papadopoulos T, Dunst J, Sauer R, Rödel C: Radiochemotherapy with cisplatin and 5-fluorouracil after transurethral surgery in patients with bladder cancer. Int J Radiat Oncol Biol Phys 2007, 68(4): 1072-1080.
- Chung PW, Bristow RG, Milosevic MF, Yi QL, Jewett MA, Warde PR, Catton CN, McLean M, Moore M, Tannock IF, et al: Long-term outcome of radiation-based conservation therapy for invasive bladder cancer. Urol Oncol 2007, 25(4): 303-309.
- Cancer Genome Atlas Research Network: Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507(7492): 315-522.
- Petrylak DP, Tangen CM, Van Veldhuizen PJ Jr, Goodwin JW, Twardowski PW, Atkins JN, Kakhil SR, Lange MK, Mansukhani M, Crawford ED: Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int 2010, 105(3): 317-321.
- Pruthi RS, Nielsen M, Heathcote S, Wallen EM, Rathmell WK, Godley P, Whang Y, Fielding J, Schultz H, Grigson G, et al: A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: clinical and pathological results. BJU Int 2010, 106(3): 349-354.
- Wülfing C, Machiels JP, Richel DJ, Grimm MO, Treiber U, De Groot MR, Beuzeboc P, Parikh R, Pétavy F, El-Hariry IA: A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 2009, 115(13): 2881-2890.
- Wong YN, Litwin S, Vaughn D, Cohen S, Plimack ER, Lee J, Song W, Dabrow M, Brody M, Tuttle H, et al: Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol 2012, ;30(28): 3545-3551.
- Hussain M, Daignault S, Agarwal N, Grivas PD, Siefker-Radtke AO, Puzanov I, MacVicar GR, Levine EG, Srinivas S, Twardowski P, et al: A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer 2014, 120(17): 2684-2693.
- Ko YJ, Canil CM, Mukherjee SD, Winquist E, Elser C, Eisen A, Reaume MN, Zhang L, Sridhar SS: Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol 2013, 14(8): 769-776.
- Junker K, van Oers JM, Zwarthoff EC, Kania I, Schubert J, Hartmann A: Fibroblast growth factor receptor 3 mutations in bladder tumors correlate with low frequency of chromosome alterations. Neoplasia 2008, 10(1): 1-7.
- Oh DY, Bang YJ: HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol 2020, 17(1): 33-48.
- Marchiò C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A: Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol 2021, 72: 123-135.
- Gallagher DJ, Milowsky MI, Gerst SR, Ishill N, Riches J, Regazzi A, Boyle MG, Trout A, Flaherty AM, Bajorin DF: Phase II study of sunitinib in patients with metastatic urothelial cancer. J Clin Oncol 2010, 28(8): 1373-1379.
- Bellmunt J, González-Larriba JL, Prior C, Maroto P, Carles J, Castellano D, Mellado B, Gallardo E, Perez-Gracia JL, Aguilar G, et al: Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol 2011, 22(12): 2646-2653.
- Sridhar SS, Winquist E, Eisen A, Hotte SJ, McWhirter E, Tannock IF, Mukherjee SD, Wang L, Blattler C, Wright JJ, et al: A phase II trial of sorafenib in first-line metastatic urothelial cancer: a study of the PMH Phase II Consortium. Invest New Drugs 2011, 29(5): 1045-1049.
- Dreicer R, Li H, Stein M, DiPaola R, Eleff M, Roth BJ, Wilding G: Phase 2 trial of sorafenib in patients with advanced urothelial cancer: a trial of the Eastern Cooperative Oncology Group. Cancer 2009, 115(18): 4090-4095.
- Necchi A, Mariani L, Zaffaroni N, Schwartz LH, Giannatempo P, Crippa F, Morosi C, Lanocita R, Sava T, Ortega C, et al: Pazopanib in advanced and platinum-resistant urothelial cancer: an open-label, single group, phase 2 trial. Lancet Oncol 2012, 13(8): 810-816.
- Milowsky MI, Iyer G, Regazzi AM, Al-Ahmadie H, Gerst SR, Ostrovnaya I, Gellert LL, Kaplan R, Garcia-Grossman IR, Pendse D, et al: Phase II study of everolimus in metastatic urothelial cancer. BJU Int 2013, 112(4): 462-470.
- Seront E, Rottey S, Sautois B, Kerger J, D'Hondt LA, Verschaeve V, Canon JL, Dopchie C, Vandenbulcke JM, Whenham N, et al: Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol 2012, 23(10): 2663-2670.
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al: Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014, 371(23): 2189-2199.
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, et al: Signatures of mutational processes in human cancer. Nature 2013, 500(7463): 415-421.
- Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, Yeh JJ, Milowsky MI, Iyer G, Parker JS, et al: Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A 2014, 111(8): 3110-3115.
- Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, et al: Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25(2): 152-165.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript