Review Article | Open Access
Research Progress on Ferroptosis as a Therapeutic Strategy in Renal Cell Carcinoma
Minna Liu1, Min Bai2, Na Cui2, Yi Ding2, Peng Zhang3
1Department of Nephrology, The 940th Hospital Joint Logistics Support Forces of PLA, Xi'an, Shaanxi, P.R. China.
2Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi'an, Shaanxi, P.R. China.
3Faculty of Medicine, Wuhan Polytechnic University, Wuhan, Hubei, P.R. China.
Correspondence: Ying Ding (Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Changle West Road 169, Xi'an, Shaanxi Province, P.R. China, 710000; Email: dingyi.007@163.com) and Peng Zhang (Faculty of Medicine, Wuhan Polytechnic University, Wuhan City, Hubei province, P.R. China, 430000; Email: monkeyzhangpeng@163.com).
Annals of Urologic Oncology 2022; 5(2): 75-80. https://doi.org/10.32948/auo.2022.12.09
Received: 30 Nov 2022 | Accepted: 08 Dec 2022 | Published online: 16 Dec 2022
Key words renal cell carcinoma, ferroptosis, iron metabolism, lipid peroxidation
Ferroptosis is a regulatable cell death method caused by the accumulation of lipid peroxidation products. The core mechanics is cell damage caused by iron overload and lipid peroxidation [7]. Iron overload can lead to the abnormal activation of the mitochondrial oxidative phosphorylation and the oxidative stress produces high levels of ROS during ATP production. High oxidative stress develops an unbalance between ROS production and the antioxidant system oxidizing unsaturated fatty acids on the cell membrane, forming lipid peroxides, and directly or indirectly altering cellular function and damaging cell structure referred as ferroptosis, a process of cell death. Ferroptosis is tightly related to GSH metabolism, iron metabolism, and lipid peroxidation. Some functional proteins such as transferrin receptor 1 (TFR1), ferritin, cystine/glutamic acid reverse transporter (system Xc-), glutathione peroxidase 4 (GPX4), and lipoxygenase (LOX) are involved in the occurrence of ferroptosis [8].
In fact, tumor cells are more sensitive to ferroptosis than normal cells, especially renal tumor cells [9]. Therefore, it is possible that ferroptosis may be developed as a new strategy for the treatment of advanced RCC. This review presents the role of ferroptosis in RCC progression and its treatment, providing novel strategies to advance RCC therapeutics.
Iron is a transitional metal abundantly present and indispensable trace element in the human body, which participates in numerous important physiological and biochemical functions, including cell division, DNA repair and death [10]. Iron overload is the main source of active oxygen, a key participation factor for ferroptosis, and emerging studies have shown that iron overload is closely related to RCC occurrence, especially clear cell renal cell carcinoma (ccRCC). These functional proteins participate in iron metabolism, including ferritin light chains (FTL), ferritin heavy chain (FTH1), ferritin transfer protein (FPN), transferrin receptor 1 (TFR1) during iron intake, and iron regulating proteins 1, 2 (IRP1/2 ) [11-13]. Schnetz et al. discovered that iron metabolism-related genes were significantly increased in RCC especially in the ccRCC tumors [14]. The expression of FTL, FTH1, TFR1, and IRP1/2 protein in the ccRCC association was higher, while FPN protein expression decreased as a result of the accumulation of iron in cancer cells. However, iron overload did not cause ferroptosis, but promoted RCC development. FTH1 has the activity of ferrous oxidase, which can be converted to Fe2+ into Fe3+. The latter combined with FTL can effectively reduce the toxicity of Fe2+ in the cell which produces hydroxyl radicals that can damage cellular DNA, lipids, and proteins causing ferroptosis [15]. Nuclear Auxiliary Activation Factor 4 (NCOA4) is an autophagy component involved in the ferritinophagy [16, 17]. The TCGA database analysis show that the NCOA4 -related gene is closely related to the malignant grade of renal cancer and TNM staging. Research show that NCOA4 is the receptor of autophagy-related gene autophagy-related protein 5 (ATG5) and ATG7. NCOA4 , ATG5 and ATG7 can promote ferritinophagy, thereby reducing the intracellular iron protein content, raising the intracellular ferrous iron concentration, promoting ferroptosis of cancer cell, indicating that ATG5-OTG7-NCOA4 autophagy pathway may be a new target for the treatment of kidney cancer [18, 19].
Figure 1 shows the molecular basis of ferroptosis and the strategies for therapeutic induction of ferroptosis.
Lipid oxidation
Lipid peroxidation is one of the main characteristics that drives cell ferroptosis [20]. However, even if ccRCC cells are rich in fat, but it did not cause ferroptosis. The proportion of lipid components in lipid droplets is different, and polyunsaturated fatty acids (PUFAs), especially pentanoic acid, and 22 carbonic acid in the process of ferroptosis [21, 22]. In the ccRCC tissue, the hypoxic induction factor 2α (HIF-2α) can selectively enrich PUFAs through the hypoxia-induced, lipid droplet-associated protein (HILPDA). ccRCC is not only more sensitive to ferroptosis than normal renal cells, but the PUFAs content of ccRCC cells increases with higher cancer stages [23]. It can be seen that the HIF-2α- HILPDA shaft is expected to be a new way to treat advanced renal cancer. However, in some ccRCC cells (FR1 and FR2 cells in the 786-O series), even in cells with high PUFAs content, it does not show increased sensitivity to ferroptosis [23]. Studies by Zou et al. [23] found that PUFA-ePLs can promote ferroptosis evasion. PUFA-ePLs synthesis is related with alkylglycerone phosphate synthase (AGPS) jn peroxysome, fatty acyl-CoA reductase 1 (FAR1), glyceronephosphate oacyltransferase (GNPAT), 1-acylglycerol-3- phosphate O-acyltransferase 3 (AGPAT 3) in endoplamic reticulum, plasmanylethanolamine desaturase 1 (PEDS1) in the plasma. FR1 and FR2 cells can reduce the level of PUFA-ePLS through spontaneous downregulation of AGPS, thereby reducing the sensitivity of cancer cells to ferroptosis, and promoting proliferation and metastasis of cancer cells. Therefore, it can be used as a treatment strategy for the cancer cells non -sensitive to ferroptosis by regulating AGPS expression. Nuclear factor erythroid2-related factor 2 (Nrf2) is Solute Carrier Family 7 Member 11(SLC7A11) closely related to ferroptosis. The key regulatory factors of oxidation reactions can prevent cells from being damaged by lipid peroxidation products of 4-hydroxyl-2-ethylene and Cyrum aldehyde, to inhibit the occurrence of ferroptosis. Studies shows that the expression of Nrf2 and its pathway is related to renal cancer grading, staging, and resistance to targeting drugs and poor prognosis [24-27]. Therefore, Nrf2 can be used as a potential target for future treatment of advanced renal cancer. However, the current researches on Nrf2 inhibitors for kidney cancer is particularly lacking. Currently discovered Nrf2 inhibitors include clobetasol propionate in the study of lung cancer and trigonelline in head-neck cancer research. These two compounds can enhance the sensitivity of tumor cells to ferroptosis [28]. Clobetasol propionate or trigonelline used in renal cancer has similar effects, however it still needs to be further discussed.
System Xc--GSH-GPX4
System Xc- can transfer the extracellular cystine into intra-cell and the intracellular glutamate outside, in the progress to synthesize glutathione (GSH). GPX4 can neutralize lipid peroxidation by reactive oxygen with the help of GSH to inhibit cell ferroptosis. Xu et al. [29] found that SLC7A11 is expressed at higher levels in the kidney cancer tissue compared with normal kidney tissue. It also inhibit ferroptosis, promote kidney cancer cell proliferation, migration, and invasion by promoting GPX4 expression, indicating that SLC7A11 is a putative target in the prevention of kidney cancer to metastatic stage. As an important antioxidant in the cell, GSH can neutralize lipid peroxide products. The γ-glutamyltransferase (GGT1) can catalyze the extracellular GSH lysis, providing cysteine for GSH generated in the cell, and is a part of the GSH salvage pathway [30]. Bansal et al. [31] demonstrate that GGT1 levels in the ccRCC cell line (786-O and RCC10) increased significantly, and GGT1 could prevent tumor cell from lipid peroxidation to ferroptosis by promoting GSH synthesis facilitating tumor cell proliferation and metastasis. Studies shows that there were less adverse reactions and good curative effects in GGT1 inhibitors viz. OU749 which can be used as a new potential method for the treatment of ccRCC [32]. Kruppel-like Factor 2 (KLF2) is one of the Kruppel-like transcription factor members, characterized by DNA binding domains containing zinc fingers. Recent research reveals that KLF2 participates in the development of various tumors such as liver cancer, kidney cancer, prostate cancer etc. [33-35]. There is a significant correlation between downregulation of KLF2 expression levels and ccRCCs TNM staging. It was significantly shortened that the overall survival and metastasis-free survival of ccRCC patients with low KLF2 expression. There was smaller lung metastases and fewer quantities with KLF2. Further studies suggest that KLF2 can be combined with GPX4 promoter in ccRCC, to downregulate the expression of GPX4, which prevent ccRCC cells from ferroptosis, and promote tumor metastasis [36]. Therefore, the System Xc--GSH-GPX4 shaft plays an important role in the development of kidney cancer. It is a new strategy to utilize ferroptosis to treat kidney cancer including drug-resistant kidney cancer in the future.
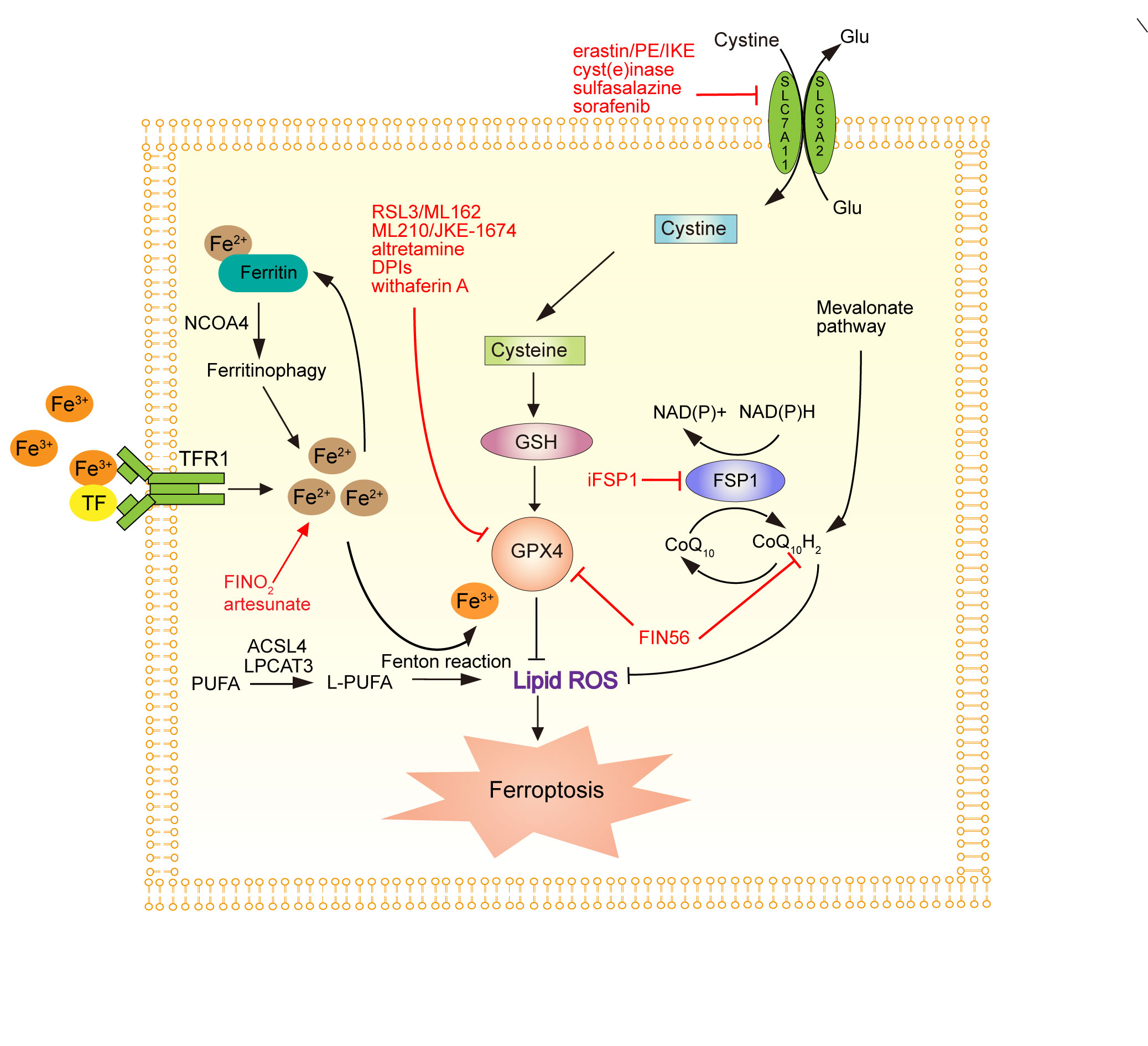 Figure 1. The molecular basis of ferroptosis and the strategies for therapeutic induction of ferroptosis. System Xc- inhibitors: PE, piperazineerastin; IKE, Imidazole ketone erastin; erastin; cyst(e)inase; sulfasalazine; sorafenib. GPX4 inhibitors: RSL3/ML 162, ML210/JKE-1674, altretamine, DPIs, withaferin A. Other ferroptosis inducers: FINO2, Artesunate, FIN56. TFR1, transferrin receptor 1; NCOA4, Nuclear Auxiliary Activation Factor 4; PUFAS, polyunsaturated fatty acids; GSH, glutathione; GPX4, glutathione peroxidase 4; TFR1, transferrin receptor 1; TF: transferrin.
Figure 1. The molecular basis of ferroptosis and the strategies for therapeutic induction of ferroptosis. System Xc- inhibitors: PE, piperazineerastin; IKE, Imidazole ketone erastin; erastin; cyst(e)inase; sulfasalazine; sorafenib. GPX4 inhibitors: RSL3/ML 162, ML210/JKE-1674, altretamine, DPIs, withaferin A. Other ferroptosis inducers: FINO2, Artesunate, FIN56. TFR1, transferrin receptor 1; NCOA4, Nuclear Auxiliary Activation Factor 4; PUFAS, polyunsaturated fatty acids; GSH, glutathione; GPX4, glutathione peroxidase 4; TFR1, transferrin receptor 1; TF: transferrin.
Ferroptosis is an emerging cancer inhibitory strategy while how to rapidly screen RCC patients for their sensitivity to ferroptosis remains a challenge. 18 F-TRX-PET can be used to predict the sensitivity of tumors for iron targeted therapy, but its cost in clinical diagnosis and treatment is high, limiting its clinical application [37]. In situ detection technology can detect the sensitivity of tumor tissue to ferroptosis. This technology uses high-power laser to induce cells or tissue samples to lipid oxidation in local acyl group of PUFAs, and displayed the sensitivity of cells or tissue to ferroptosis induction in situ. [38]. Therefore, in-situ detection is a low cost and convenient operation technology. It is expected to perform the rapid grading of ferroptosis sensitivity in cancer patients and accelerate the development of targeting anticancer treatment under ferroptosis.
Immunotherapy for ferroptosis and ccRCC
As the mechanism of ferroptosis is continuing to emerge, more research suggests that ferroptosis is closely related to tumor microenvironment. NCOA4 is an autophagy component participating in ferritin FTH1 and FTL autophagy. It can degrade ferritin, release ferrous, and promote cell ferroptosis. However, NCOA4 is generally low levels in the ccRCC tissue. Hu et al. [39] found that iron metabolic-related protein FTH1 and FTL expression levels were elevated in most of solid tumor tissues, and was also related to the regulated T cells (TREG) and tumor-related macrophages (TAMS), especially M2 macroscopic cells thru the analysis of the TCGA database. Studies shows that the iron derived from M2 macrophages among TAMS can be output through FPN and secrete an iron transfer protein lipocalin-2 (LCN-2) as an iron carrier to transport iron into ccRCC cells to promote ccRCC cell proliferation. This is positively related to poor prognosis [14, 40, 41]. Treg cells are the main factor for creating immunosurable tumor microenvironment. Treg cells infiltration in tumor is related to higher pathological stages and poor prognosis in ccRCC [42]. Therefore, NCOA4 is the key element of ferroptosis and related immunotherapy. Researches shows that ferroptosis-related gene CARS is a potential immunohistic-related ferroptosis regulator. High CARS expression is associated with poor prognosis and also positive correlation with PD-L1 expression in ccRCC, which suggests that CARS is a treatment target [43]. The above researches show that the iron metabolism and related genes in ferroptosis may become one of breakthrough points of ccRCC immunotherapy.
Ferroptosis and targeted therapy of ccRCC
Targeted therapy as a first-line treatment for ccRCC demonstrate optimal therapeutic effects the in clinic. However, along with the continuous application of targeted drugs, some patients experience drug resistance. As a newly discovered regulatory cell death, ferroptosis plays an important role in the progress of kidney cancer. In the past, more researches reminded ferroptosis and iron metabolism disorders, but recent studies show that there are similar effects of zinc compared to iron during ferroptosis [44]. The transfer between organelles and cytoplasm is through the SLC39 family (ZIP) or the transfer protein in the SLC30 (ZNT) family, of which ZIP7 and zinc participated in cell ferroptosis; decreased expression of ZIP7 in RCC4 cells can avoid the ferroptosis induced by ERASTIN, but complementary ZnCL 2 is to eliminate this protective effect. It is known that ZIP7 can also be used as a potential treatment target for ccRCC [44]. There are many sub-cell lines in the ccRCC lump with different sensitivity to ferroptosis. It is still a problem to be solved that how to improve the sensitivity of various cell lines to ferroptosis, which is possible to improve the efficacy of ferroptosis induction agents. Studies show that ferroptosis is much affected by cell density and fusion. Among them, the cell density affects the sensitivity of cells through the HIPPO pathway. Further studies suggest that there are two mutually compensated downstream molecular paths that YES related protein (YAP)-S-stage kinase-related protein 2 (SKP2) and containing WW domains transcriptional regulatory factor 1 (TAZ)-epithelial membrane protein 1 (EMP1) -Tiamide gonadotropinic phosphate 4 ( NOX4), while TAZ is mainly paths in kidney cancer cells [44, 45]. NOX4 can produce and accumulate hypoxides and hydrogen peroxide, thereby promoting lipid peroxidation and ferroptosis. Moisture SKP2 can promote the expression of sulinic acid kinase and rotor protein receptor mRNA, which helps ferroptosis [46]. The HIPPO pathway mainly involved in regulating ferroptosis through SKP2 and NOX4 provides new idea for ccRCC treatment.
Ferroptosis and nano-treatment of ccRCC
With the development of materials science, more and more studies suggest that nanoparticles can induce ferroptosis including lung cancer, breast cancer, liver cancer and other tumor cells [47, 48]. Nanoparticles in combination with small molecular ferroptosis induction agents have the advantages of solubility, targeted enhancement, low systemic toxicity, controllable drug control, and synergy advantage in emerging combination therapies [49]. A recent study found that 1 H-general fluorinetane (1 H-PFP) nanomoter (GBP @ FE3 O 4) in 786-O cells can trigger the heat-guided ferroptosis, under the laser of 808 nm, the local medium heat (45 °C) triggered the liquid-pFP liquid-gaseous transformation, resulting in the rapid release of the Fe3O4-nanometer particles. In the tumor microenvironment, a large amount of active oxygen is generated through Fanton's reaction. At the same time, thermal stress reduces the synthesis of GSH, inhibits the antioxidant response of tumor cells, and further aggravates the damage caused by active oxygen. Tumor cells undergo lipid metabolic reprogramming and produce large amounts of lipid peroxides, which eventually lead to tumor-specific ferroptosis [50, 51]. It is speculated that the nanotechnology will be a very important potential method to treat ccRCC in the future, even if it is still in the basic research stage. The related clinical research is less and their safety needs to be further verified.
Acknowledgements
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
ML, PZ and YD conceptualised, designed the study and was responsible for the writing of the original draft. ML, MB, NC, PZ and YD reviewed, edited, and approved the final manuscript.
Competing interests
The authors declare no conflict of interest.
Funding
This study was supported by the National Natural Science Foundation of China (82204746) and Natural Science Foundation of Gansu Province (20JR5RA591).
- Bukavina L, Bensalah K, Bray F, Carlo M, Challacombe B, Karam JA, Kassouf W, Mitchell T, Montironi R, O'Brien T et al: Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur Urol 2022, 82(5): 529-542.
- Capitanio U, Montorsi F: Renal cancer. Lancet 2016, 387(10021): 894-906.
- Jonasch E, Gao J, Rathmell WK: Renal cell carcinoma. BMJ 2014, 349: g4797.
- Bex A, Gore M, Mulders P, Sternberg CN: Recent advances in the treatment of advanced renal cell carcinoma: towards multidisciplinary personalized care. BJU Int 2012, 110(9): 1289-1300.
- Atkins MB, Tannir NM: Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev 2018, 70: 127-137.
- Iacovelli R, Ciccarese C, Bria E, Bimbatti D, Fantinel E, Mosillo C, Bisogno I, Brunelli M, Tortora G, Porta C: Immunotherapy versus standard of care in metastatic renal cell carcinoma. A systematic review and meta-analysis. Cancer Treat Rev 2018, 70: 112-117.
- Chen X, Li J, Kang R, Klionsky DJ, Tang D: Ferroptosis: machinery and regulation. Autophagy 2021, 17(9): 2054-2081.
- Xie B, Guo Y: Molecular mechanism of cell ferroptosis and research progress in regulation of ferroptosis by noncoding RNAs in tumor cells. Cell Death Discov 2021, 7(1): 101.
- Tong L, Yin ZF, Peng L, Li YT, Liu R, Cai JR, Kang L: A Ferroptosis-Related Gene Signature for Predicting Survival and Immunotherapy Effect in Renal Cancer. Comput Math Methods Med 2022, 2022: 3317624.
- Ni S, Yuan Y, Song S, Li X: A double-edged sword with a therapeutic target: iron and ferroptosis in immune regulation. Nutr Rev 2022, https://doi.org/10.1093/nutrit/nuac071. Epub ahead of print.
- McCullough K, Bolisetty S: Ferritins in Kidney Disease. Semin Nephrol 2020, 40(2):160-172.
- Gao M, Monian P, Jiang X: Metabolism and iron signaling in ferroptotic cell death. Oncotarget 2015, 6(34): 35145-35146.
- Shen L, Zhou Y, He H, Chen W, Lenahan C, Li X, Deng Y, Shao A, Huang J: Crosstalk between Macrophages, T Cells, and Iron Metabolism in Tumor Microenvironment. Oxid Med Cell Longev 2021, 2021: 8865791.
- Schnetz M, Meier JK, Rehwald C, Mertens C, Urbschat A, Tomat E, Akam EA, Baer P, Roos FC, Brune B et al: The Disturbed Iron Phenotype of Tumor Cells and Macrophages in Renal Cell Carcinoma Influences Tumor Growth. Cancers (Basel) 2020, 12(3).
- Torti SV, Torti FM: Iron and cancer: more ore to be mined. Nat Rev Cancer 2013, 13(5): 342-355.
- Mou Y, Wu J, Zhang Y, Abdihamid O, Duan C, Li B: Low expression of ferritinophagy-related NCOA4 gene in relation to unfavorable outcome and defective immune cells infiltration in clear cell renal carcinoma. BMC Cancer 2021, 21(1): 18.
- Zhao GJ, Wu Z, Ge L, Yang F, Hong K, Zhang S, Ma L: Ferroptosis-Related Gene-Based Prognostic Model and Immune Infiltration in Clear Cell Renal Cell Carcinoma. Front Genet 2021, 12: 650416.
- Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, 3rd, Kang R, Tang D: Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 2016, 12(8): 1425-1428.
- Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D: Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol 2020, 66: 89-100.
- Tan SK, Mahmud I, Fontanesi F, Puchowicz M, Neumann CKA, Griswold AJ, Patel R, Dispagna M, Ahmed HH, Gonzalgo ML et al: Obesity-Dependent Adipokine Chemerin Suppresses Fatty Acid Oxidation to Confer Ferroptosis Resistance. Cancer Discov 2021, 11(8): 2072-2093.
- Hassannia B, Vandenabeele P, Vanden Berghe T: Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35(6): 830-849.
- Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR: Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 2016, 113(34): E4966-4975.
- Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, Paradkar S, Boehnke N, Deik AA, Reinhardt F et al: Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585(7826): 603-608.
- Deng Y, Wu Y, Zhao P, Weng W, Ye M, Sun H, Xu M, Wang C: The Nrf2/HO-1 axis can be a prognostic factor in clear cell renal cell carcinoma. Cancer Manag Res 2019, 11: 1221-1230.
- Fabrizio FP, Costantini M, Copetti M, la Torre A, Sparaneo A, Fontana A, Poeta L, Gallucci M, Sentinelli S, Graziano P et al: Keap1/Nrf2 pathway in kidney cancer: frequent methylation of KEAP1 gene promoter in clear renal cell carcinoma. Oncotarget 2017, 8(7): 11187-11198.
- Ji S, Xiong Y, Zhao X, Liu Y, Yu LQ: Effect of the Nrf2-ARE signaling pathway on biological characteristics and sensitivity to sunitinib in renal cell carcinoma. Oncol Lett 2019, 17(6): 5175-5186.
- Clerici S, Boletta A: Role of the KEAP1-NRF2 Axis in Renal Cell Carcinoma. Cancers (Basel) 2020, 12(11).
- Robledinos-Anton N, Fernandez-Gines R, Manda G, Cuadrado A: Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid Med Cell Longev 2019, 2019: 9372182.
- Xu F, Guan Y, Xue L, Zhang P, Li M, Gao M, Chong T: The roles of ferroptosis regulatory gene SLC7A11 in renal cell carcinoma: A multi-omics study. Cancer Med 2021, 10(24): 9078-9096.
- Zhang L, Hobeika CS, Khabibullin D, Yu D, Filippakis H, Alchoueiry M, Tang Y, Lam HC, Tsvetkov P, Georgiou G et al: Hypersensitivity to ferroptosis in chromophobe RCC is mediated by a glutathione metabolic dependency and cystine import via solute carrier family 7 member 11. Proc Natl Acad Sci U S A 2022, 119(28): e2122840119.
- Bansal A, Sanchez DJ, Nimgaonkar V, Sanchez D, Riscal R, Skuli N, Simon MC: Gamma-Glutamyltransferase 1 Promotes Clear Cell Renal Cell Carcinoma Initiation and Progression. Mol Cancer Res 2019, 17(9): 1881-1892.
- Takemura K, Board PG, Koga F: A Systematic Review of Serum gamma-Glutamyltransferase as a Prognostic Biomarker in Patients with Genitourinary Cancer. Antioxidants (Basel) 2021, 10(4).
- Wang B, Liu M, Song Y, Li C, Zhang S, Ma L: KLF2 Inhibits the Migration and Invasion of Prostate Cancer Cells by Downregulating MMP2. Am J Mens Health 2019, 13(1): 1557988318816907.
- Li Y, Tu S, Zeng Y, Zhang C, Deng T, Luo W, Lian L, Chen L, Xiong X, Yan X: KLF2 inhibits TGF-beta-mediated cancer cell motility in hepatocellular carcinoma. Acta Biochim Biophys Sin (Shanghai) 2020, 52(5): 485-494.
- Li M, Zhang M, Chen M, Xiao J, Mu X, Peng J, Fan J: KLF2-induced circZKSCAN1 potentiates the tumorigenic properties of clear cell renal cell carcinoma by targeting the miR-1294/PIM1 axis. Cell Cycle 2022, 21(13): 1376-1390.
- Lu Y, Qin H, Jiang B, Lu W, Hao J, Cao W, Du L, Chen W, Zhao X, Guo H: KLF2 inhibits cancer cell migration and invasion by regulating ferroptosis through GPX4 in clear cell renal cell carcinoma. Cancer Lett 2021, 522: 1-13.
- Zhao N, Huang Y, Wang YH, Muir RK, Chen YC, Wei J, Hooshdaran N, Viswanath P, Seo Y, Ruggero D et al: Ferronostics: Measuring Tumoral Ferrous Iron with PET to Predict Sensitivity to Iron-Targeted Cancer Therapies. J Nucl Med 2021, 62(7): 949-955.
- Wang F, Graham ET, Naowarojna N, Shi Z, Wang Y, Xie G, Zhou L, Salmon W, Jia JM, Wang X et al: PALP: A rapid imaging technique for stratifying ferroptosis sensitivity in normal and tumor tissues in situ. Cell Chem Biol 2022, 29(1): 157-170 e156.
- Hu ZW, Chen L, Ma RQ, Wei FQ, Wen YH, Zeng XL, Sun W, Wen WP: Comprehensive analysis of ferritin subunits expression and positive correlations with tumor-associated macrophages and T regulatory cells infiltration in most solid tumors. Aging (Albany NY) 2021, 13(8): 11491-11506.
- Rehwald C, Schnetz M, Urbschat A, Mertens C, Meier JK, Bauer R, Baer P, Winslow S, Roos FC, Zwicker K et al: The iron load of lipocalin-2 (LCN-2) defines its pro-tumour function in clear-cell renal cell carcinoma. Br J Cancer 2020, 122(3): 421-433.
- Ito J, Omiya S, Rusu MC, Ueda H, Murakawa T, Tanada Y, Abe H, Nakahara K, Asahi M, Taneike M et al: Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice. Elife 2021, 10.
- Zhang Y, Chen X, Fu Q, Wang F, Zhou X, Xiang J, He N, Hu Z, Jin X: Comprehensive analysis of pyroptosis regulators and tumor immune microenvironment in clear cell renal cell carcinoma. Cancer Cell Int 2021, 21(1): 667.
- Wang S, Chen S, Ying Y, Ma X, Shen H, Li J, Wang X, Lin Y, Liu B, Zheng X et al: Comprehensive Analysis of Ferroptosis Regulators With Regard to PD-L1 and Immune Infiltration in Clear Cell Renal Cell Carcinoma. Front Cell Dev Biol 2021, 9: 676142.
- Chen PH, Wu J, Xu Y, Ding CC, Mestre AA, Lin CC, Yang WH, Chi JT: Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis 2021, 12(2): 198.
- Yang WH, Ding CC, Sun T, Rupprecht G, Lin CC, Hsu D, Chi JT: The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep 2019, 28(10): 2501-2508 e2504.
- Yang WH, Lin CC, Wu J, Chao PY, Chen K, Chen PH, Chi JT: The Hippo Pathway Effector YAP Promotes Ferroptosis via the E3 Ligase SKP2. Mol Cancer Res 2021, 19(6): 1005-1014.
- Zaffaroni N, Beretta GL: Nanoparticles for Ferroptosis Therapy in Cancer. Pharmaceutics 2021, 13(11).
- Bae C, Kim H, Kook YM, Lee C, Kim C, Yang C, Park MH, Piao Y, Koh WG, Lee K: Induction of ferroptosis using functionalized iron-based nanoparticles for anti-cancer therapy. Mater Today Bio 2022, 17: 100457.
- Xu Y, Qin Z, Ma J, Cao W, Zhang P: Recent progress in nanotechnology based ferroptotic therapies for clinical applications. Eur J Pharmacol 2020, 880: 173198.
- Xie S, Sun W, Zhang C, Dong B, Yang J, Hou M, Xiong L, Cai B, Liu X, Xue W: Metabolic Control by Heat Stress Determining Cell Fate to Ferroptosis for Effective Cancer Therapy. ACS Nano 2021, 15(4): 7179-7194.
- Wang L, Chen X, Yan C: Ferroptosis: An emerging therapeutic opportunity for cancer. Genes Dis 2022, 9(2): 334-346.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript