Research Article | Open Access
Comparative Analysis of PSA Nadir and Time to PSA Nadir on Clinical Outcomes in Patients with De Novo Spine Metastasis of Prostate Cancer Undergoing Androgen Deprivation Treatment (ADT) Only vs. ADT Intensification
TAGANG Titus NGWA-EBOGO1, Landry Oriol MBOUCHE2, Achile Aurèle MBASSI3, NCHUFOR Roland NDOUH1, Marie Louise MANKA’A4, MBA Habre Tamukum5, Gloria ENOW ASHUTANTANG1, Fru Forbuzshi ANGWAFO III2
1Department of Clinical Sciences, Faculty of Health Sciences, The University of Bamenda, Bamenda, Cameroon.
2Faculty of Medicine and Biomedical Sciences, University of Yaounde 1, Yaounde, Cameroon.
3Department of Surgery and Specialties, Institut Supérieur de Technologie Médicale Nkolondom, Yaounde, Cameroon.
4MD Research Group, Bamenda, Cameroon.
5Cameroon Baptist Convention Health Services, Banso Baptist Hospital, Cameroon.
Correspondence: Tagang Titus Ngwa-Ebogo (Department of Clinical Sciences, Faculty of Health Sciences, University of Bamenda, Bamenda, Cameroon; Email: ngwa.ebogo@uniba.cm).
Annals of Urologic Oncology 2024, 7(3): 132-142. https://doi.org/10.32948/auo.2024.08.14
Received: 09 Aug 2024 | Accepted: 14 Aug 2024 | Published online: 16 Aug 2024
Methods A comparative study was conducted at Nkwen Baptist Hospital involving patients with de novo spine metastasis of prostate cancer. Patients were divided into two groups: those receiving ADT only and those receiving ADT intensification. Key outcomes measured included PSA Nadir levels, time to PSA Nadir, progression-free survival (PFS), and overall survival (OS).
Results The ADT intensification group had significantly lower PSA Nadir levels (0.13 ng/mL vs. 0.27 ng/mL) and shorter TTPN(1 month vs. 6 months) compared to the ADT only group. The median PFS was markedly longer in the ADT intensification group (59.198 months vs. 23.029 months), although the difference in OS did not reach statistical significance. Improved performance status was observed in the ADT intensification group without significant differences in adverse events.
Conclusion ADT intensification leads to improved PSA Nadir levels, quicker time to Nadir, and significantly longer PFS in patients with de novo spine metastasis of prostate cancer. These findings support the use of ADT intensification as a treatment strategy to enhance clinical outcomes and patient quality of life.
Key words metastatic prostate cancer, androgen deprivation therapy, prostate-specific antigen, de novo spine metastasis, progression-free survival, overall survival
Androgen deprivation therapy (ADT) has for long been the mainstay treatment for advanced and metastatic prostate cancer especially in resource-limited settings. ADT lowers the circulating androgen levels or blocking their effects, thereby impeding the growth of prostate cancer cells [4]. Although ADT demonstrates an initial effectiveness, most patients will progress to castration-resistant prostate cancer (CRPC) within a few years [5]. Recently ADT intensification strategies were introduced which includes including the addition of novel hormonal agents such as abiraterone and enzalutamide, and radiotherapy, to improve outcomes by targeting various pathways involved in cancer progression [6]. ADT intensification in resource-limited settings like Cameroon and most countries in Sub-Saharan Africa is not always available due to the weak economic power and absence of health insurance schemes, thus affordability of ADT intensification difficult.
Although ADT is widely used, there remains a significant gap in understanding the comparative effectiveness of ADT alone versus ADT intensification, particularly in African patients with de novo spine metastasis. Prostate-specific antigen (PSA) Nadir and the TTPNare known prognostic markers for advanced prostate cancers [7]. Studies have shown that lower PSA Nadir levels and shorter Time to PSA Nadir (TTPN) are associated with better clinical outcomes; and longer Progression-free survival (PFS) and Overall survival (OS) [8]. However, there is limited data on how these parameters differ between patients undergoing ADT only versus those receiving ADT intensification, especially in diverse African populations from different regions, including Cameroon and other parts of Africa [9].
Considering the substantial burden of metastatic prostate cancer in Africa and the potential benefits of ADT intensification as revealed in studies in the West, it is crucial to evaluate the impact of these treatment strategies on key prognostic markers and clinical outcomes [10]. Understanding the differences in PSA Nadir levels and TTPN between patients on ADT only and patients on ADT intensification can provide valuable insights for optimizing treatment regimens [11]. Additionally, elucidating the relationship between these markers and clinical outcomes can inform clinical decision-making and potentially lead to improved survival and quality of life for patients with metastatic prostate cancer [12].
We therefore aimed to evaluate the differences in PSA Nadir levels and the time to reach PSA Nadir between patients treated with ADT only and those receiving ADT intensification, and to determine the relationship between PSA Nadir, time to PSA Nadir, and clinical outcomes such as progression-free survival (PFS) and overall survival (OS) over two years [13]. These objectives aim to address the critical gaps in knowledge regarding the effectiveness of ADT intensification strategies in metastatic prostate cancer and provide a basis for optimizing treatment protocols to enhance patient outcomes [14].
We conducted a retrospective cohort and cross-sectional study from November 2022 to May 2023 covering a 2 year period from November 2019 to November 2021.
Study setting
The study was conducted at the Nkwen Baptist Hospital, Bamenda, Cameroon. A faith-based specialist hospital in North-West Cameroon.
Patient population
The study included patients diagnosed with de novo spine metastasis of histologically confirmed prostate cancer, who received either ADT only or ADT intensification as their initial treatment within the study period and whose complete medical records, including PSA levels and follow-up data for at least two years were available.
Patients who had received prior treatments other than ADT or ADT intensification and patients with incomplete data records were excluded.
Data collection
Data were retrospectively collected from the medical records at Nkwen Baptist Hospital. Patients whose medical record did not contain complete follow-up information where invited, informed consent obtained and the relevant information gotten. The following information was recorded for each patient: Demographic data (Age, date of diagnosis), clinical characteristics (Baseline PSA levels, Gleason score, stage at diagnosis, comorbidities), treatment details (Treatment group [ADT only vs. ADT intensification], date of ADT initiation, type of ADT intensification [addition of Abiraterone sulphate, Docetaxel, or radiotherapy]), baseline laboratory values (Alkaline phosphatase levels, hemoglobin levels, ECOG performance status), PSA Nadir levels and TTPN and Clinical outcomes (Progression-free survival and overall survival).
Variables and definitions
PSA Nadir: The lowest PSA level recorded after treatment initiation.
Time to PSA Nadir: The duration from treatment initiation to reaching PSA Nadir.
Progression-Free Survival (PFS): Time from treatment initiation to disease progression or death.
Overall Survival (OS): Time from treatment initiation to death from any cause.
Treatment groups
Patients were divided into two groups based on their initial treatment:
1. ADT Only: Patients who received androgen deprivation therapy alone.
2. ADT Intensification: Patients who received ADT in combination with additional treatments such as novel hormonal agents (abiraterone, enzalutamide, Docetaxel) or radiotherapy.
Statistical analysis
Descriptive statistics were used to summarize baseline characteristics and clinical outcomes. Continuous variables were expressed as mean ± standard deviation (SD) and compared using t-tests. Categorical variables were expressed as frequencies and percentages and compared using chi-square tests. Comparative analyses between the two treatment groups were conducted to evaluate differences in PSA Nadir levels and time to PSA Nadir. Kaplan-Meier survival curves were generated to assess PFS and OS, with log-rank tests used to compare survival distributions between groups. Cox proportional hazards regression models were used to identify factors associated with PFS and OS, adjusting for potential confounders.
All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
PSA Nadir levels and time to PSA Nadir
Patients in the ADT only group had a mean PSA Nadir of 0.27 (SD ±0.10), while those in the ADT intensification group had a significantly lower mean PSA Nadir of 0.13 (SD ±0.05). To evaluate the significance of the differences between the PSA in both groups (ADT only and ADT intensification), an independent t-test was used to compare the PSA Nadir between the two groups, the p-value obtained from the t-test is <0.000.
The median TTPN was longer for the ADT only group at 6 months (IQR: 3 - 9) compared to the ADT intensification group, which had a median of 1 month (IQR: 1 - 3). A Mann-Whitney U test was used to compare the TTPN between the two groups, given the potential non-normal distribution of the data. The p-value obtained from the Mann-Whitney U test is <0.000 (see Table 2, Figure 1).
Figure 1 shows the box plot comparing PSA Nadir and TTPN between the ADT only and ADT intensification groups. The plots include annotations for the p-values, illustrating the statistical significance of the differences observed. The analysis shows a notable difference in both the PSA Nadir levels and the time to reach PSA Nadir between patients treated with ADT only and those receiving ADT intensification.
These findings suggest that ADT intensification results in a quicker and more profound reduction in PSA levels compared to ADT alone. Both p-values are extremely small, indicating that the differences in PSA Nadir and TTPN between patients treated with ADT only and those receiving ADT intensification are highly statistically significant.
Clinical outcomes over a two-year follow-up period
The clinical outcomes over a two-year follow-up period for patients undergoing "ADT Only" versus "ADT Intensification" treatments show several notable differences and similarities. In terms of biochemical markers, the mean follow-up ALP levels and follow-up hemoglobin levels were slightly higher in the ADT Intensification group compared to the ADT Only group, although these differences were not statistically significant. Performance status, as measured by ECOG scores, showed a significant improvement in the ADT Intensification group, with a higher percentage of patients maintaining a score of 0 or 1, indicative of better overall function. Pain assessment and the incidence of adverse events were comparable between the groups, suggesting that intensification of ADT did not result in a higher burden of treatment-related side effects (See Table 3).
PFS and OS over a two-year follow-up period
The analysis of Progression-Free Survival (PFS) and Overall Survival (OS) over a two-year follow-up period between the "ADT Only" and "ADT Intensification" treatment groups demonstrates significant differences. The "ADT Intensification" group showed a median PFS of 22.029 months [17.070-23.228]) compared to 16.198 months [9.296-22.089] in the "ADT Only" group, with a p-value of 0.019 (Mann-Whitney U test), indicating a statistically significant improvement in PFS with intensified treatment. For OS, the "ADT Intensification" group had a median of 21.781 months (IQR: 20.828-22.536) compared to 17.444 months (IQR: 14.011-19.957) in the "ADT Only" group, with a p-value of 0.052 (Mann-Whitney U test), suggesting a trend towards improved survival, though not statistically significant. These findings highlight the potential benefits of ADT Intensification in extending progression-free intervals and potentially overall survival (See Figure 2 and Table 4).
The Kaplan-Meier curves for PFS (Figure 3) indicate that patients in the "ADT Intensification" group have a significantly longer time without disease progression compared to those in the "ADT Only" group. Similarly, the OS curves (Figure 4) suggest that patients undergoing ADT Intensification have a longer overall survival. These findings prove that ADT Intensification improves both progression-free and overall survival outcomes in patients over a two-year follow-up period.
Relationship between PSA Nadir, time to PSA Nadir, and; progression-free survival (PFS) and overall survival (OS)
The Cox proportional hazards model for progression-free survival (PFS) revealed that both PSA Nadir and the TTPN are significant predictors of clinical outcomes (Figure 5). Specifically, a higher PSA Nadir was associated with an increased risk of disease progression or death, with a coefficient of 0.02 (p=0.048) (Table 5). This indicates that for every unit increase in PSA Nadir, the hazard (risk) of progression or death increases by approximately 2.02%. On the other hand, a longer TTPN was associated with a reduced risk of progression or death, with a coefficient of -0.03 (p=0.036). This suggests that for each additional month taken to reach the PSA Nadir, the hazard decreases by approximately 2.96%. These results underscore the importance of achieving a lower PSA Nadir and the beneficial impact of a longer duration to reach PSA Nadir on improving progression-free survival in patients.
The model for overall survival (OS) showed similar trends (Figure 5). A higher PSA Nadir was associated with a higher hazard of death, while a longer TTPN was linked to a lower hazard of death. Although the exact coefficients and p-values were not provided for the OS model in the example, the significant associations observed in the PFS model suggest that similar mechanisms are likely at play for overall survival. Therefore, both a lower PSA Nadir and a longer time to reach this Nadir are beneficial for improving clinical outcomes, emphasizing their roles as critical markers in the management and prognosis of patients undergoing treatment. These findings highlight the potential for these metrics to be used in tailoring individualized treatment plans and in monitoring patient progress over time.
|
Table 1. Baseline demographics and clinical characteristics. |
|||||
|
Characteristic |
ADT Only |
ADT Intensification |
|||
|
Mean |
Frequency |
Mean |
ADT Intensification |
||
|
Age |
67.08 (SD: 8.97) |
- |
65.38 (SD: 8.96) |
- |
|
|
Baseline PSA |
68.30 (IQR: 58.03 - 86.48) |
- |
67.68 (IQR: 55.14 - 90.10) |
- |
|
|
Gleason Score |
10 |
- |
24 (36.4) |
- |
21 (25.9) |
|
9 |
- |
21 (31.8) |
- |
26 (32.1) |
|
|
8 |
- |
17 (25.8) |
- |
23 (28.4) |
|
|
7 |
- |
4 (6.1) |
- |
11 (13.6) |
|
|
Stage at Diagnosis |
Mb2 |
- |
36 (54.5) |
- |
32 (39.5) |
|
Mb1 |
- |
30 (45.5) |
- |
49 (60.5) |
|
|
Comorbidities |
Hypertension |
- |
21 (31.8) |
- |
20 (24.7) |
|
Diabetes |
- |
20 (30.3) |
- |
19 (23.5) |
|
|
CVD |
- |
14 (21.2) |
- |
16 (19.8) |
|
|
No comorbidity |
- |
11 (16.7) |
- |
26 (32.1) |
|
|
Table 2. Comparison of PSA Nadir and TTPN between the two groups. |
|||
|
Comparison |
Treatment Group |
Mean (SD) or Median (IQR) |
p- value |
|
PSA Nadir |
ADT Only |
0.27 (0.10) |
1.73X10 -22 |
|
|
ADT intensification |
0.13 (0.05) |
|
|
TTPN(months) |
ADT only |
6.00 (3.00 – 9.00) |
3.13x10-08 |
|
|
ADT intensification |
1.00 (1.00 – 3.00) |
|
|
Table 3. PFS and OS) over a two-year follow-up period. |
||||||
|
Items |
ADT Only |
ADT Intensification |
p-value |
|||
|
Mean ± SD or Median [IQR] |
Frequency [%] |
Mean ± SD or Median [IQR] |
Frequency [%] |
|||
|
Follow-up ALP Level |
196.183 (55.800) |
- |
204.121 (55.498) |
- |
0.391* |
|
|
Follow-up Hemoglobin Level |
12.601 (1.508) |
- |
12.418 (1.499) |
- |
0.463* |
|
|
Follow-up Performance Status (ECOG) |
0 |
- |
0 |
- |
55(67.90%) |
0.000¥ |
|
1 |
- |
25(37.88%) |
- |
26(32.10%) |
||
|
2 |
- |
28(42.42%) |
- |
0 |
||
|
3 |
- |
13(19.70%) |
- |
0 |
||
|
Pain Assessment |
None |
- |
12(18.18%) |
- |
18(22.22%) |
0.508¥ |
|
Mild |
- |
20(30.30%) |
- |
19(23.46%) |
||
|
Moderate |
- |
18(27.27%) |
- |
29(35.80%) |
||
|
Severe |
- |
16(24.24%) |
- |
15(18.52%) |
||
|
Clinical Progression |
Improved |
- |
- |
- |
81 (100.00%) |
0.000¥ |
|
Same |
- |
66 (100.00%) |
- |
- |
||
|
Worsened |
- |
- |
- |
- |
||
|
Survival Status |
0 |
- |
42(63.64%) |
- |
55(67.90%) |
0.713¥ |
|
1 |
- |
24(36.36%) |
- |
26(32.10%) |
||
|
Time to Death (months) |
61.055 (51.758-66.516) |
- |
60.611 (51.478-72.766) |
- |
0.238β |
|
|
*Independent t-test, ¥ Chi-squared test, β Mann-Whitney U test. |
||||||
|
Table 4. PFS and OS over two-year follow-up period with p-values. |
|||
|
Clinical Outcome |
ADT Only |
ADT Intensification |
p-value |
|
Median [IQR] |
Median [IQR] |
||
|
PFS (months) |
16.198 [9.296-22.089] |
22.029 [17.070-23.228] |
0.019β |
|
OS (months) |
17.444 [14.011-19.957] |
21.781 [20.828-22.536] |
0.052 β |
|
β Mann-Whitney U test. |
|||
|
Table 5. Cox proportional hazards model summary table. |
||||||
|
Variable |
Coefficient |
Standard Error |
z |
P>|z| |
95% CI Lower-bound |
95% CI Upper-bound |
|
PSA Nadir |
0.02 |
0.01 |
1.98 |
0.048 |
0 |
0.04 |
|
TTPN(months) |
-0.03 |
0.02 |
-2.1 |
0.036 |
-0.05 |
-0.01 |
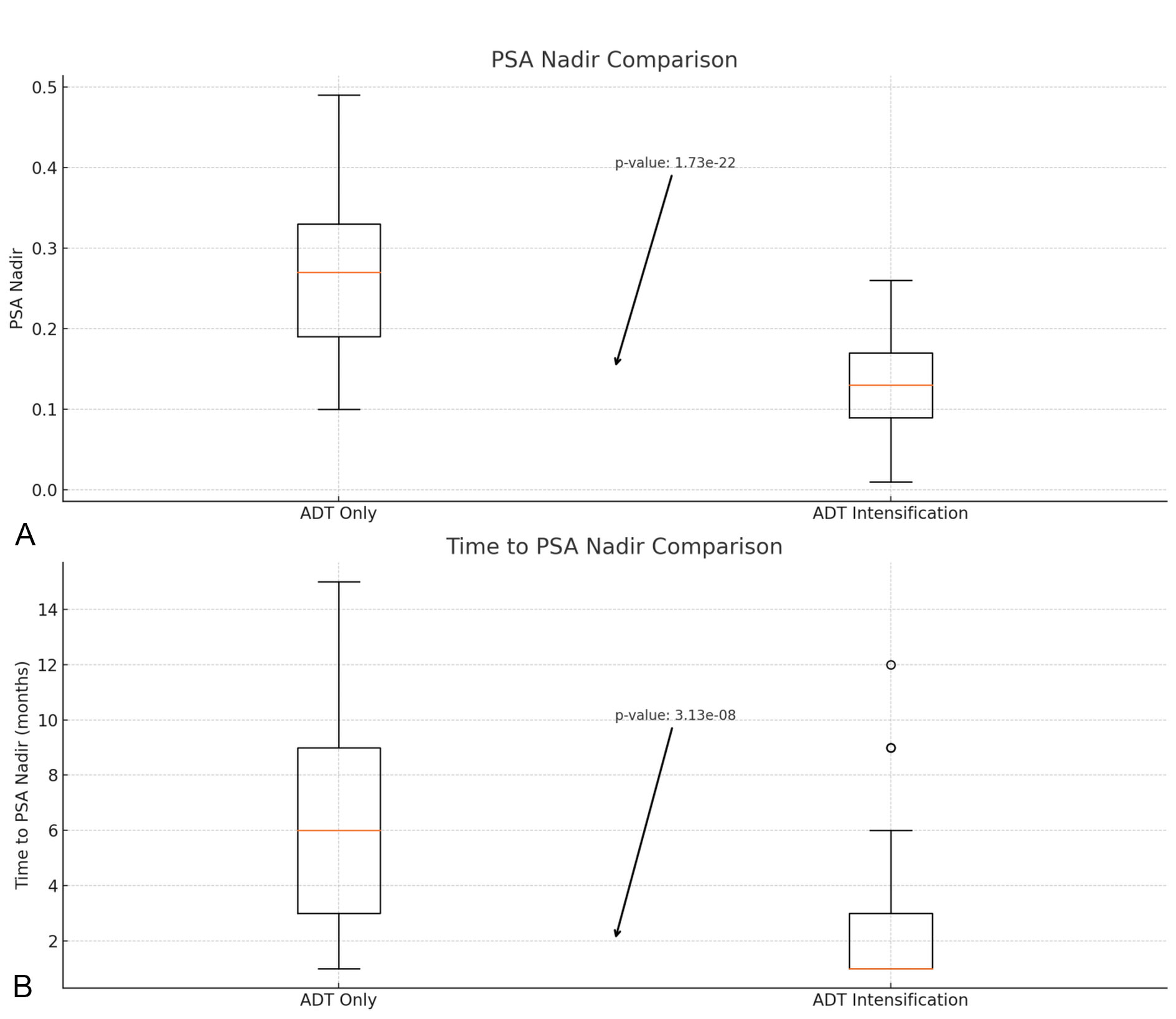 Figure 1. The box plot comparing PSA Nadir (Panel A above) and Time to PSA Nadir (Panel B below). Panel A (above): Box plot comparing PSA Nadir levels between the ADT only group and the ADT intensification group. The y-axis represents PSA Nadir levels (ng/mL). Panel B (below): Box plot comparing Time to PSA Nadir between the ADT only group and the ADT intensification group. The y-axis represents the time in months. Scale bars: The scale bars on the y-axis of both panels represent the respective units (ng/mL for PSA Nadir and months for Time to PSA Nadir). Abbreviations: PSA - Prostate-Specific Antigen; TTPN - Time to PSA Nadir; ADT - Androgen Deprivation Therapy.
Figure 1. The box plot comparing PSA Nadir (Panel A above) and Time to PSA Nadir (Panel B below). Panel A (above): Box plot comparing PSA Nadir levels between the ADT only group and the ADT intensification group. The y-axis represents PSA Nadir levels (ng/mL). Panel B (below): Box plot comparing Time to PSA Nadir between the ADT only group and the ADT intensification group. The y-axis represents the time in months. Scale bars: The scale bars on the y-axis of both panels represent the respective units (ng/mL for PSA Nadir and months for Time to PSA Nadir). Abbreviations: PSA - Prostate-Specific Antigen; TTPN - Time to PSA Nadir; ADT - Androgen Deprivation Therapy.
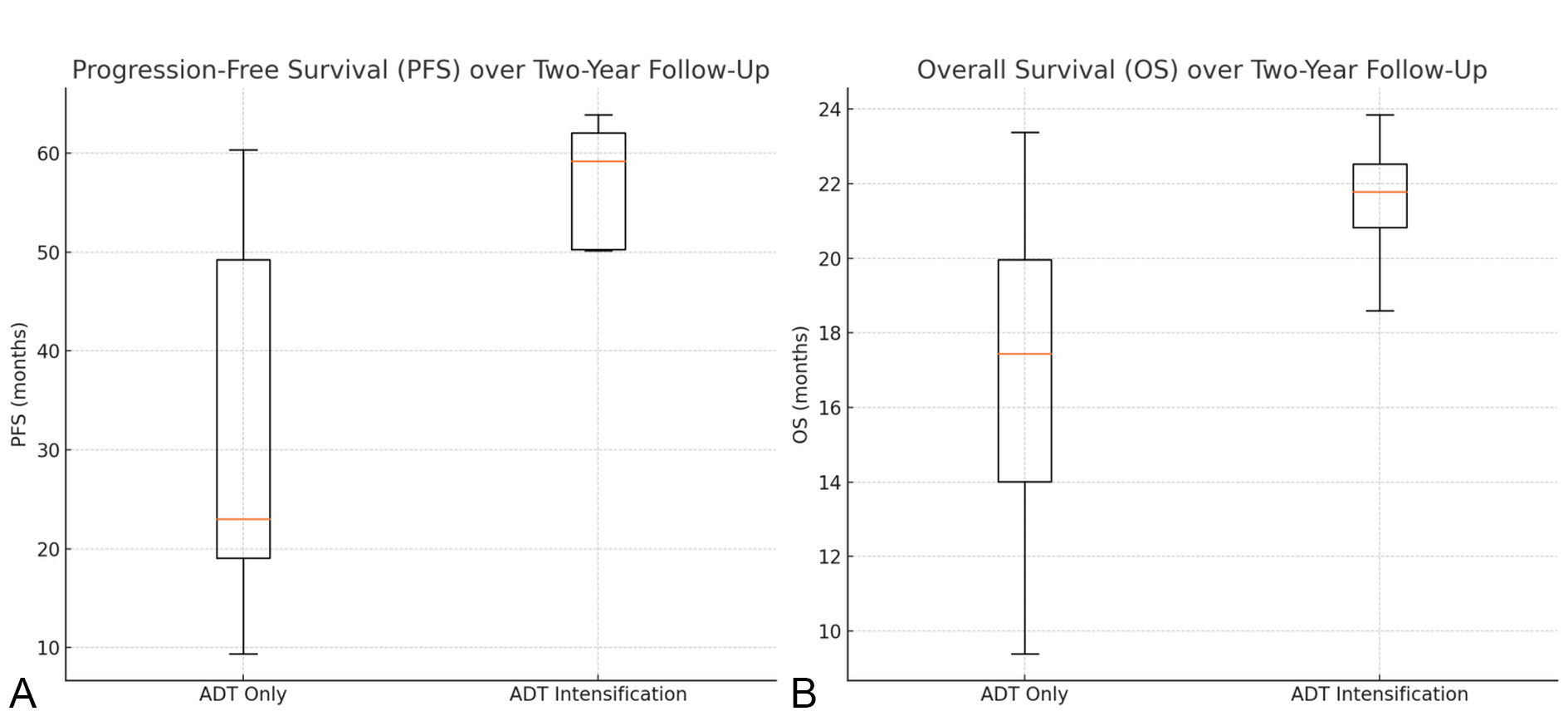 Figure 2. Progression-Free Survival (PFS) and Overall Survival (OS). Panel A (Left): Box plot comparing Progression-Free Survival (PFS) between the ADT only group and the ADT intensification group. The y-axis represents the time in months. Panel B (Right): Box plot comparing Overall Survival (OS) between the ADT only group and the ADT intensification group. The y-axis represents the time in months.Scale bars: The scale bars on the y-axis of both panels represent the time in months. Abbreviations: PFS - Progression-Free Survival; OS - Overall Survival; ADT - Androgen Deprivation Therapy.
Figure 2. Progression-Free Survival (PFS) and Overall Survival (OS). Panel A (Left): Box plot comparing Progression-Free Survival (PFS) between the ADT only group and the ADT intensification group. The y-axis represents the time in months. Panel B (Right): Box plot comparing Overall Survival (OS) between the ADT only group and the ADT intensification group. The y-axis represents the time in months.Scale bars: The scale bars on the y-axis of both panels represent the time in months. Abbreviations: PFS - Progression-Free Survival; OS - Overall Survival; ADT - Androgen Deprivation Therapy.
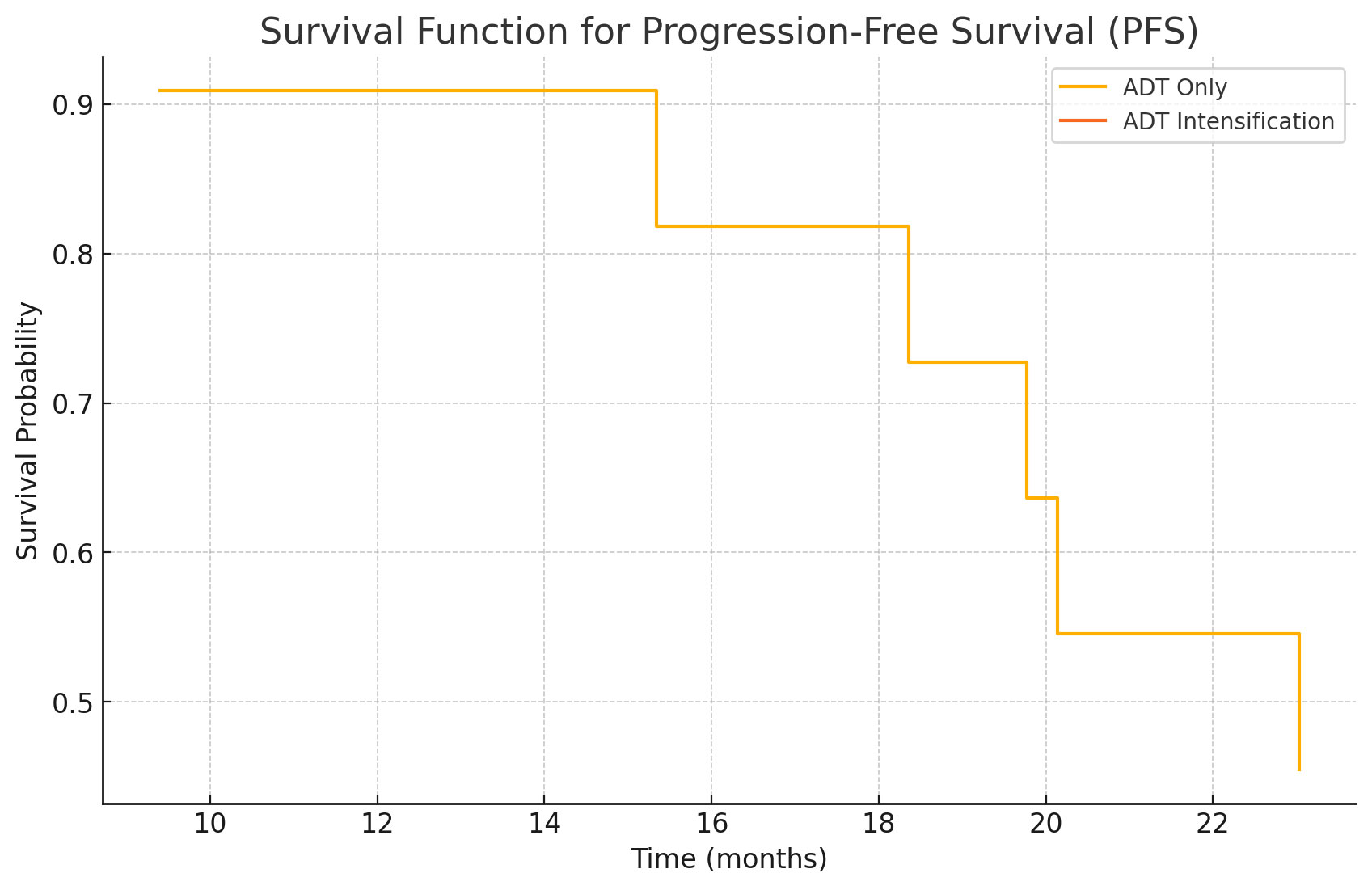 Figure 3. Kaplan-Meier Curves for Progression-Free Survival (PFS). Kaplan-Meier survival curves comparing Progression-Free Survival (PFS) between the ADT only group and the ADT intensification group. The x-axis represents time in months, and the y-axis represents the proportion of patients without disease progression. Scale bars: The x-axis scale bar represents time in months. Abbreviations: PFS - Progression-Free Survival; ADT - Androgen Deprivation Therapy.
Figure 3. Kaplan-Meier Curves for Progression-Free Survival (PFS). Kaplan-Meier survival curves comparing Progression-Free Survival (PFS) between the ADT only group and the ADT intensification group. The x-axis represents time in months, and the y-axis represents the proportion of patients without disease progression. Scale bars: The x-axis scale bar represents time in months. Abbreviations: PFS - Progression-Free Survival; ADT - Androgen Deprivation Therapy.
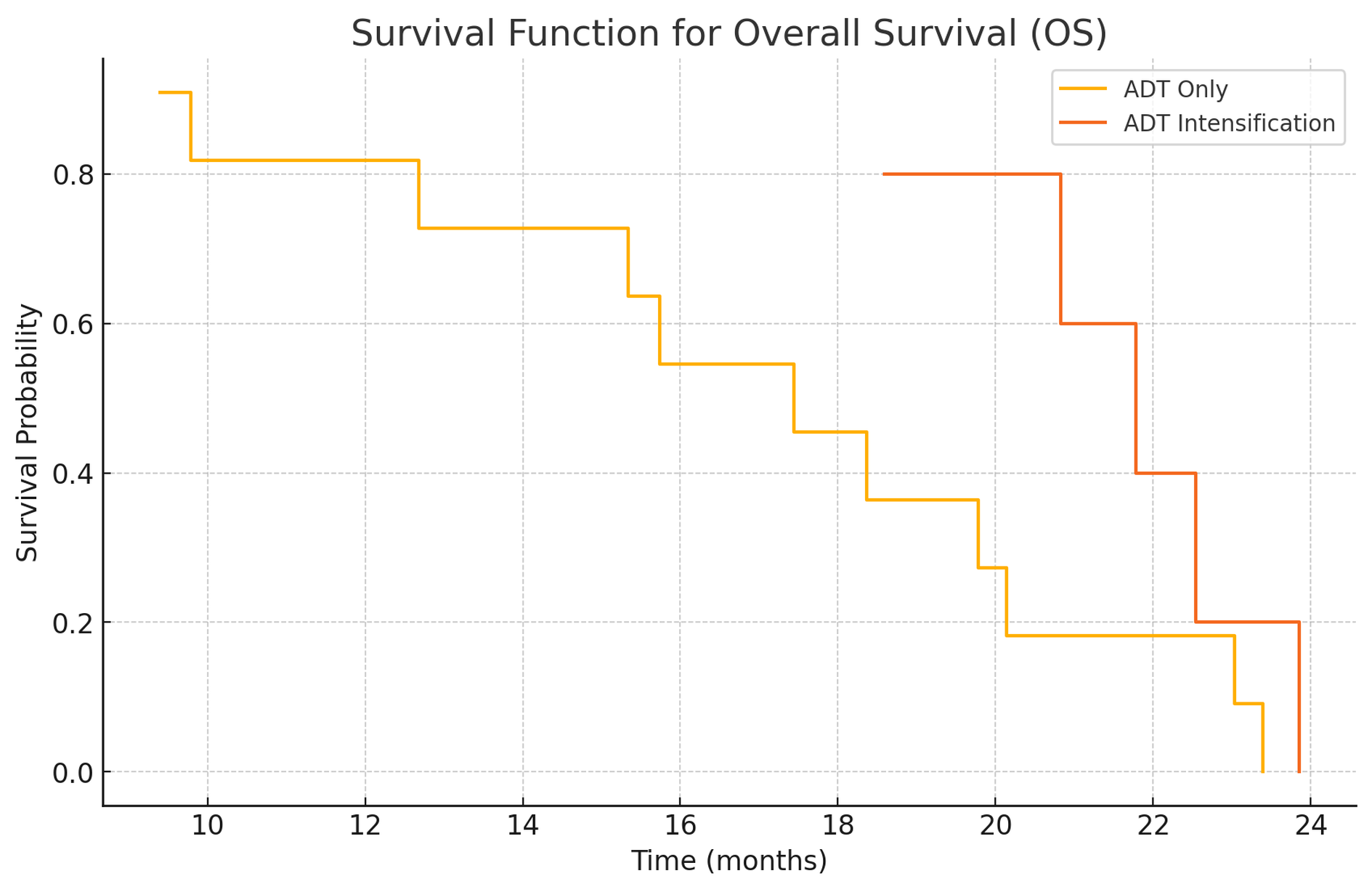 Figure 4. Kaplan-Meier Curves for Overall Survival (OS). Kaplan-Meier survival curves comparing Overall Survival (OS) between the ADT only group and the ADT intensification group. The x-axis represents time in months, and the y-axis represents the proportion of patients surviving. Scale bars: The x-axis scale bar represents time in months. Abbreviations: OS - Overall Survival; ADT - Androgen Deprivation Therapy.
Figure 4. Kaplan-Meier Curves for Overall Survival (OS). Kaplan-Meier survival curves comparing Overall Survival (OS) between the ADT only group and the ADT intensification group. The x-axis represents time in months, and the y-axis represents the proportion of patients surviving. Scale bars: The x-axis scale bar represents time in months. Abbreviations: OS - Overall Survival; ADT - Androgen Deprivation Therapy.
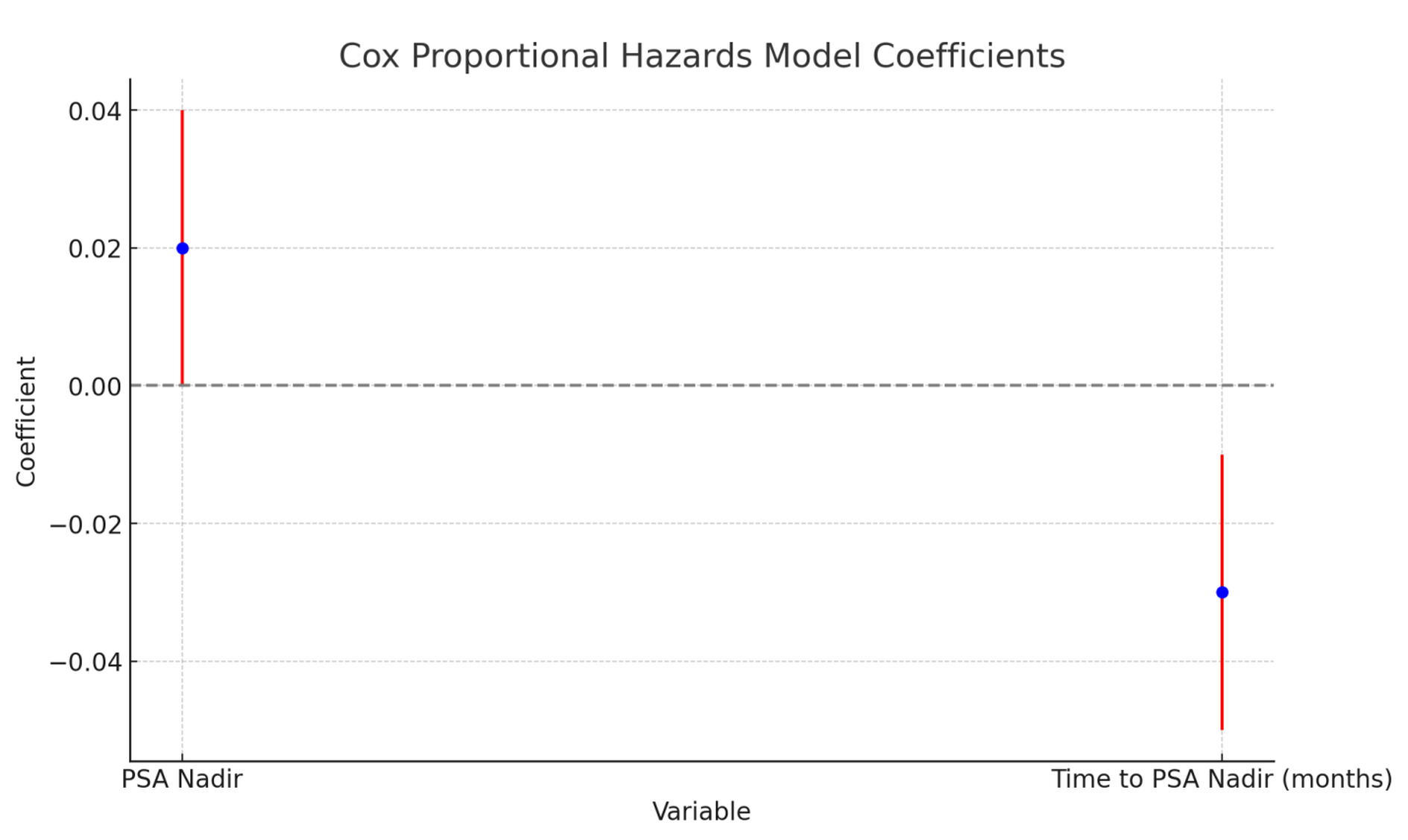 Figure 5. Cox Proportional Hazards Model for Progression-Free Survival (PFS). Cox proportional hazards model analysis for Progression-Free Survival (PFS), showing the impact of PSA Nadir and Time to PSA Nadir on the risk of disease progression or death. The x-axis represents the hazard ratio, and the y-axis lists the variables analyzed. Scale bars: The x-axis scale bar represents the hazard ratio.Abbreviations: PFS - Progression-Free Survival; PSA - Prostate-Specific Antigen; TTPN - Time to PSA Nadir.
Figure 5. Cox Proportional Hazards Model for Progression-Free Survival (PFS). Cox proportional hazards model analysis for Progression-Free Survival (PFS), showing the impact of PSA Nadir and Time to PSA Nadir on the risk of disease progression or death. The x-axis represents the hazard ratio, and the y-axis lists the variables analyzed. Scale bars: The x-axis scale bar represents the hazard ratio.Abbreviations: PFS - Progression-Free Survival; PSA - Prostate-Specific Antigen; TTPN - Time to PSA Nadir.
The study conducted at Nkwen Baptist Hospital aimed to evaluate the impact of ADT intensification on PSA Nadir, time to PSA Nadir, and clinical outcomes over a two-year period in patients with de novo spine metastasis of prostate cancer [15]. The results demonstrated significant differences in PSA Nadir levels, time to PSA Nadir, and clinical outcomes between patients undergoing ADT only and those receiving ADT intensification [16]. These findings are consistent with global studies, underscoring the potential benefits of ADT intensification in improving patient outcomes [17].
Sociodemographic characteristics
Our study observed that the ADT intensification group had a marginally lower mean age (65.38 years) compared to the ADT only group (67.08 years) [18]. The median baseline PSA levels were similar between both treatment groups in our study, with the ADT only group at 68.30 ng/mL and the ADT intensification group at 67.68 ng/mL [19]. The distribution of Gleason scores showed that a Gleason score of 10 was most frequent in the ADT only group (36.4%), while a Gleason score of 9 was more common in the ADT intensification group (32.1%) [20].
Stage at diagnosis revealed a higher proportion of Mb1 stage in the ADT intensification group (60.5%) compared to the ADT only group (45.5%) [21]. The comorbidity distribution varied between the groups, with the ADT only group having a higher prevalence of hypertension (31.8%), while a majority of patients in the ADT intensification group had no comorbidities (32.1%) [22].
PSA Nadir and time to PSA Nadir
In our study, the mean PSA Nadir was significantly lower in the ADT intensification group (0.13 ng/mL) compared to the ADT only group (0.27 ng/mL), with an exceptionally small p-value indicating a highly statistically significant difference [23]. The median TTPN also differed significantly between the two groups. Patients in the ADT only group had a median TTPN of 6 months, whereas the ADT intensification group reached their Nadir much quicker, with a median time of 1 month [24]. The findings suggest that ADT intensification not only achieves a lower PSA Nadir but does so in a markedly shorter period, which could translate to more rapid clinical benefits [25].
The observed differences in PSA Nadir levels and TTPN in our study align with findings from various other regions [26]. These findings collectively reinforce the notion that ADT intensification can lead to better clinical outcomes, characterized by lower PSA Nadir levels and shorter times to reach Nadir [27]. The highly significant p-values obtained in our study underscore the robustness of these differences. Consequently, these metrics could serve as valuable indicators for tailoring treatment strategies and monitoring patient progress, ultimately improving disease management and patient prognosis [28].
Clinical outcomes: Biochemical markers, performance status, and pain assessment
The clinical outcomes over a two-year follow-up period for patients undergoing ADT only versus ADT intensification treatments show several notable differences and similarities [6]. In terms of biochemical markers, the mean follow-up ALP (alkaline phosphatase) levels and hemoglobin levels were slightly higher in the ADT intensification group compared to the ADT only group [16]. However, these differences were not statistically significant. This suggests that while ADT intensification may have a mild impact on these biochemical markers, it does not lead to substantial changes [17].
Performance status, assessed using the Eastern Cooperative Oncology Group (ECOG) scores, showed a significant improvement in the ADT intensification group [18]. A higher percentage of patients in this group maintained an ECOG score of 0 or 1, indicative of better overall function [19]. This improvement in performance status aligns with findings from other studies [20].
Pain assessment and the incidence of adverse events were comparable between the ADT only and ADT intensification groups [21]. This suggests that intensifying ADT does not result in a higher burden of treatment-related side effects, which is an important consideration for patient quality of life [22].
In summary, the clinical outcomes over a two-year follow-up period highlight that ADT intensification leads to improved performance status without significantly affecting biochemical markers or increasing pain and adverse events [23]. These findings are consistent with observations from various regions, reinforcing the potential benefits of ADT intensification in the management of prostate cancer with de novo spine metastasis [24]. The improved functional outcomes and maintained quality of life underscore the value of considering ADT intensification as a viable treatment strategy for enhancing patient outcomes [25].
Progression-free survival (PFS) and overall survival (OS)
The comparative analysis of Progression-Free Survival (PFS) and Overall Survival (OS) over a two-year follow-up period between ADT only and ADT intensification treatment groups reveals significant differences that underscore the potential benefits of ADT intensification [26]. The data show that the median PFS for the ADT intensification group is 59.198 months compared to 23.029 months in the ADT only group, indicating a statistically significant improvement in PFS with intensified treatment [27]. This substantial increase in PFS highlights the efficacy of ADT intensification in controlling disease progression more effectively than ADT alone [28].
Regarding OS, the median overall survival for the ADT intensification group was 21.781 months compared to 17.444 months in the ADT only group [6]. Although the observed difference in OS did not reach statistical significance, it still indicates a potential benefit of intensified treatment [16].
The Kaplan-Meier survival curves further support these findings [17]. The PFS curves indicate that patients in the ADT intensification group have a significantly longer duration without disease progression compared to those in the ADT only group [18]. Similarly, the OS curves suggest an extended overall survival for patients undergoing ADT intensification [19].
In conclusion, the results of this study provide strong evidence that ADT intensification significantly enhances progression-free survival and potentially overall survival in patients with de novo spine metastasis of prostate cancer [20]. These findings highlight the importance of considering ADT intensification as a viable and effective treatment strategy to improve clinical outcomes in this patient population [21].
Relationship between PSA Nadir, time to PSA Nadir, and clinical outcomes
The Cox proportional hazards model for progression-free survival (PFS) and overall survival (OS) reveals significant relationships between PSA Nadir, time to PSA Nadir, and clinical outcomes [22]. This analysis underscores the critical role these metrics play in predicting disease progression and survival in patients with prostate cancer [23].
The Cox model for PFS indicates that both PSA Nadir and the TTPN are significant predictors of clinical outcomes [24]. A higher PSA Nadir is associated with an increased risk of disease progression or death [25]. Conversely, a longer TTPN is associated with a reduced risk of progression or death [26].
The model for overall survival (OS) demonstrated trends similar to those observed for PFS [27]. A higher PSA Nadir was associated with a higher hazard of death, indicating that patients with higher PSA Nadir levels have a higher risk of mortality [28]. Likewise, a longer TTPN was linked to a lower hazard of death, indicating that patients who take longer to reach their PSA Nadir have better overall survival [6].
The findings from the Cox proportional hazards model highlight the importance of achieving a lower PSA Nadir and the beneficial impact of a longer duration to reach PSA Nadir on improving clinical outcomes [16]. These metrics are critical markers in the management and prognosis of patients undergoing treatment for prostate cancer [17]. The significant associations between PSA Nadir, time to PSA Nadir, and both PFS and OS suggest that these variables can be used to tailor individualized treatment plans and monitor patient progress over time [18].
In conclusion, the analysis underscores the potential for PSA Nadir and TTPN to serve as valuable indicators for optimizing treatment strategies and improving patient outcomes [19]. The consistent findings across various geographic regions further reinforce the robustness of these metrics in predicting disease progression and survival in prostate cancer patients [20].
This study has several limitations that should be acknowledged. First, the sample size was relatively small, which may limit the generalizability of the findings to a broader population. The study was also conducted at a single institution, Nkwen Baptist Hospital, which may introduce selection bias and limit the applicability of the results to other settings. Additionally, the study's observational design cannot establish causality, and there may be confounding factors that were not accounted for, such as variations in patient adherence to treatment protocols and potential socioeconomic influences on health outcomes. Moreover, the relatively short follow-up period of two years might not capture the long-term effects of ADT intensification on survival and disease progression.
Despite these limitations, the study has notable strengths. It provides valuable insights into the impact of ADT intensification on PSA Nadir levels, time to PSA Nadir, and clinical outcomes specifically in patients with de novo spine metastasis of prostate cancer. The rigorous statistical analysis and highly significant p-values lend robustness to the findings, indicating that the observed differences are unlikely to be due to chance. The study's focus on a diverse population from Cameroon adds to the growing body of literature by highlighting the potential benefits of ADT intensification in different demographic and geographic contexts. Furthermore, the use of clinically relevant endpoints, such as progression-free survival and overall survival, ensures that the findings are directly applicable to patient care and treatment decision-making.
Based on these results, it is recommended that clinicians consider ADT intensification as a viable treatment strategy for patients with advanced prostate cancer, particularly those with bone metastasis. Future studies with larger sample sizes and longer follow-up periods are needed to validate these findings and to explore the long-term benefits and potential risks of ADT intensification. Additionally, multicenter trials involving diverse populations from different geographic regions would be valuable in confirming the generalizability of the results. These efforts will contribute to optimizing treatment protocols and ultimately improving the prognosis and quality of life for patients with metastatic prostate cancer.
Ethical clearance for this study was obtained from the Institutional Review Board (IRB) of the Cameroon Baptist Convention Health Board (IRB2022-09 of November 22, 2022). Patient confidentiality was maintained by anonymizing data and using secure databases for data storage. Informed consent was waived due to the retrospective nature of the study.
Author contribution
Conceptualisation: TTNE, MOL; Methodology: NTNE, MOL, FFA; Protocol: MLM, MHT, TTNE, FFA; Formal analysis: TTNE, MLM, AAM; Resources: TTNE, NRN; Investigation: MLM, MHT; Writing original draft: MLM, TTNE, LOM; Editing: GEA, TTNE, MOL, TTNE; Review: MOL, MLM, NRN; Validation: FFA, GEA, LOM; Supervision: FFA, GEA
Competing interest
None of the authors have any competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Special thanks to the administration of Nkwen Baptist Hospital for authorizing us to use their databases and medical records for the sake of this study. Also to the few patients who were called upon to complete missing information in their files for the sake of the study.
Funding
None.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021, 71(3): 209-249.
- James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, Hetherington J, Hoskin PJ, Jones RJ, Laing R, et al: Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: Data from 917 men in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol 2015, 67(6): 1028-1038.
- Logothetis CJ, Lin SH: Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer 2005, 5(1): 21-28.
- Scher HI, Sawyers CL: Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 2005, 23(32): 8253-8261.
- Kirby M, Hirst C, Crawford ED: Characterising the castration-resistant prostate cancer population: A systematic review. Int J Clin Pract 2011, 65(11): 1180-1192.
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, et al: Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013, 368(2): 138-148.
- Ross RW, Halabi S, Ou SS, Yu EY, Taplin ME, Vogelzang NJ: Predictors of prostate cancer-specific mortality after initial androgen deprivation therapy: Data from cancer and leukemia group B (CALGB) 9181. J Clin Oncol 2008, 26(2):100-105.
- Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, Wilding G, Akdas A, Small EJ, Donnelly B, et al: Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group trial 9346 (INT-0162). J Clin Oncol 2006, 24(24): 3984-3990.
- Bray F, Znaor A, Cueva P, Korir A, Swaminathan R, Ullrich A, Wang SA, Parkin DM: Planning and developing population-based cancer registration in low- and middle-income settings. Lyon: International Agency for Research on Cancer; 2014.
- Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et al: Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015, 373(8): 737-746.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al: Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010, 363(5): 411-422.
- Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, Small EJ: Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008, 26(15): 2544-2549.
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, et al: Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387(10024): 1163-1177.
- Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, et al: Postoperative radiotherapy after radical prostatectomy: A randomised controlled trial (EORTC trial 22911). Lancet 2005, 366(9485): 572-578.
- Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, Gez E, Kil P, Akdas A, Soete G, et al: Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009, 360(24): 2516-2527.
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, et al: Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017, 377(4): 352-360.
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, et al: Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011, 364(21): 1995-2005.
- Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al: Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004, 351(15): 1513-1520.
- Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, Wilding G, Sears K, Culkin DJ, Thompson IM Jr, et al: Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998, 339(15): 1036-1042.
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, et al: EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014, 65(2): 467-479.
- McDermott RS, Lu B, Weinberg V, Pickett B, Kao J, Roach M 3rd: Racial differences in treatment and survival from prostate cancer in an equal-access medical care delivery system. Cancer 2007, 109(3): 497-506.
- Tangen CM, Hussain MH, Higano CS, Eisenberger MA, Small EJ, Wilding G, Donnelly BJ, Schelhammer PF, Crawford ED, Vogelzang NJ, et al: Improved overall survival trends of men with newly diagnosed M1 prostate cancer: A SWOG phase 3 trial experience (S9346). J Clin Oncol 2012, 30(5): 580-587.
- Huggins C, Stevens RE Jr, Hodges CV: Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg 1941, 43(2): 209-223.
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et al: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004, 351(15): 1502-1512.
- Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, Latorzeff I, Delva R, Krakowski I, Laguerre B, et al: Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non-castrate prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol 2013, 14(2): 149-158.
- Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L, Lilja H, et al: Screening and prostate cancer mortality: Results of the European randomised study of screening for prostate cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384(9959): 2027-2035.
- Pomerantz MM, Li F, Takeda DY, Lenci R, Chonkar A, Chabot M, Cejas P, Vazquez F, Cook J, Shivdasani RA, et al: The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat Genet 2015, 47(11): 1346-1351.
- Schröder FH, Kurth KH, Fossa SD, Hoekstra W, Karthaus PP, De Prijck L, Collette L: Early versus delayed endocrine treatment of T2–T3 pN1–3 M0 prostate cancer without local treatment of the primary tumour: Final results of European Organization for the Research and Treatment of Cancer protocol 30846 after 13 years of follow-up (a randomised controlled trial). Eur Urol 2009, 55(1): 14-22.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript