Review Article | Open Access
Mechanism and Role of Tumor Microenvironment in the Initiation and Progression of Bladder Cancer
Na Cui1,Yajun Shi1,Yi Ding2,Yanhua Wang2
1Department of Pharmacology, Shaanxi University of Traditional Chinese medicine, Xianyang 712046, China.
2Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi'an 710032, China.
Correspondence: Yanhua Wang (Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Changle West Road 169, Xi'an, Shaanxi Province, P.R. China, 710000; Email: wangyanhua870101@163.com); Yajun Shi (Department of Pharmacology, Shaanxi University of Traditional Chinese medicine, Xianyang 712046, China, 710000; Email: 328573751@qq.com); Yi Ding (Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi’an 710032, China; Email: dingyi.007@163.com).
Annals of Urologic Oncology 2023, 6(3): 89-95. https://doi.org/10.32948/auo.2023.09.08
Received: 25 Jun 2023 | Accepted: 03 Sep 2023 | Published online: 09 Sep 2023
Tumor microenvironment (TME) is a huge network, composed by tumor cells, tumor associated stromal cells, immune cells, cytokines and chemokines secreted by these cells, in which various cells communicate with each other. Bladder cancer is characterized of tendency of relapse, progression, metastasis because of the role of TME. With the application and development of new technologies recently, such as tumor bulk RNA-sequencing and singlecell transcriptome sequencing, the composition of TME for bladder cancer is increasingly clear and the complex cell-to-cell communication network is fully duged, which provides a new vision for the therapy of bladder cancer. This paper reviewed and further analysed the research hotspots of cellular components and extracellular matrix components of bladder cancer on the basis of the latest research progress.
Key words bladder cancer, tumor microenvironment, tumor-associated macrophage, tumorassociated fibroblast, lymphocyte, anoxic microenvironment, exosome
In recent years, advances in gene sequencing technology have provided new horizons for cancer research, in which the tumor microenvironment (TME) is believed to play an important role in the occurrence and development of BC. TME is a complex system composed of tumor cells, immune and inflammatory cells within the tumor, tumor-associated fibroblasts, nearby interstitial tissue, microvessels, and various cytokines and chemokines. The formation and evolution of TME are closely related to the biological behavior of tumor and the prognosis of disease. In this paper, we will systematically review the important effects of TME on BC from the aspects of cell components and extracellular matrix components, and point out the direction for further research in this field.
Macrophages are a major component of immune infiltration in cancer and can influence tumor behavior. It is differentiated from mononuclear macrophage lineage and recruited to the tumor area under the action of various cytokines, chemokines and extracellular matrix released by tumor cells. In general, macrophages can polarize into two phenotypes due to different factors in the tumor microenvironment [2]. Liu et al. [3] found that Polyporus polysaccharide can regulate toll like receptor 2(TLR2)/nuclear factor-kappa B (nuclear factor-kappa B, NF-κB)/NLRP3 pathway to promote tumor-associated macrophages (tumor-associated macrophages, TAMs) Polarize to M1, secrete pro-inflammatory factors to improve the tumor microenvironment; bladder tumor cell-derived exosomes induce macrophage M2 polarization by down-regulating phosphatase and tensin homolog(PTEN) and activating AKT/STAT3/6 signaling pathway [4]. Among them, M1 type has pro-inflammatory and anti-tumor effects, M2 type has pro-tumor and immunosuppressive effects. In the bladder cancer tumor microenvironment, TAMs are influenced by tumor cells and other stromal cells, and most of them are polarized into M2-like phenotypes. Multiple studies have shown that M2-type TAMs increase infiltration in bladder tumors, shorten the survival of patients, and have a poor prognosis [5-7]. TAMs promote the development of bladder cancer through multiple mechanisms.
TAMs maintain the characteristics of tumor stem cells and promote tumor growth by secreting cytokines and activating signaling pathways. TAMs promotes the proliferation of BC cells by promoting the phosphorylation of protein kinase B (protein kinase B, AKT), activating the AKT signaling cascade amplification pathway, and promoting the cell cycle transition. And activate the pro-survival integrin α2β1/PI3K/AKT signaling pathway to maintain the characteristics of tumor stem cells and promote tumor growth [8]. At the same time, TAMs can secrete type I collagen and activate the pro-survival integrin α2β1/PI3K/AKT signaling pathway to maintain the characteristics of tumor stem cells and promote tumor growth [9]. In addition, the chemokine CXCL1 produced by TAMs can help the adhesion and interaction between cancer cells and stromal cells, and can enhance the invasion ability of cancer cells, thus promoting the progress of BC. By establishing a nude mouse model of bladder cancer, Miyake et al. [10] injected CXCL1-expressing TAMs into the skin of nude mice, and found that the subcutaneous tumor size was significantly different from that of the control group.
TAMs can promote invasion and metastasis of bladder cancer by epithelial-mesenchymal transition (EMT), extracellular matrix remodeling, formation of blood vessels and lymphatics. Wu et al. [11] found that with the infiltration of TAMs in TME, CXCL8 levels were significantly increased, which further promoted the secretion of vascular endothelial growth factor (VEGF) by bladder cancer cells and induced the formation of blood vessels in tumor tissues. In addition, abnormal activation of the NF-κB signaling pathway promotes EMT, distant metastasis, and tumor stem cell generation. Zhang et al. [12] found through the co-culture of TAMs and BC cells, accompanied by the expression of EMT and metastasis-related markers Snail, NF-κB, vimentin, and VEGF increased, and tumor cells showed enhanced migration and invasion abilities. Lymph node metastasis is one of the early metastasis pathways of BC, and its metastasis determines the treatment plan and the long-term survival of patients. Lymph node metastasis-associated transcript 1 in tumor cells can enhance the transcription of chemokine CCL2, promote the infiltration of TAMs, and produce higher levels of VEGF-C, thereby promoting the formation of lymphatic vessels and lymphatic metastasis of tumor cells. Chen et al. [13] analyzed the correlation between the expression level of CCL2 in tumor tissue and the status of lymph nodes in a nude mouse lymph node metastasis model and found that the expression level of CCL2 in the positive lymph node metastasis group was higher than that in the negative group, which indirectly proved that the density of TAMs was positively correlated with lymph node metastasis. However, another study found that the lymph node metastasis rate (58%) of the group with weak M2 macrophage infiltration in bladder cancer was greater than that of the strong group (36%), showing a negative correlation between macrophage infiltration and lymph node metastasis [14]. The possible reason is that the ratio of macrophages to tumor cells is different in the two experiments because when the number of macrophages exceeds a certain level, its tumor suppressive effect will be weakened.
TAMs can promote tumor progression by inhibiting immune cell function. Xu et al. [15] found that the infiltration of signal regulatory protein α+TAMs can induce CD8+ T cell dysfunction and enhance the expression of immune checkpoints. M2 TAMs can enhance the expression of immune-related genes such as STAT1, CACYBP, and CALR in bladder cancer cells by secreting transforming growth factor-β1 (transforming growth factor-β1, TGF-β1) and affect the formation of BC immune microenvironment, thereby promoting the recurrence and progression of BC [16]. Xu et al. [17] found that interleukin-10 (interleukin-10, IL-10) TAMs exhibited an immunosuppressive M2-like phenotype, and TAMs were closely associated with exhausted CD8+ T cells through single-cell transcriptome sequencing technology. The above studies show that M2 TAMs can inhibit the cell-killing function of CD8+ T cells, thereby forming an immunosuppressive microenvironment. Nevertheless, the specific molecular mechanism is still unclear, and further experimental exploration is needed.
In summary, TAMs are differentiated into two subtypes under the influence of TME, and play a role in inhibiting or promoting the growth, invasion and metastasis of tumor cells. Therefore, inducing TAMs to differentiate in the direction of cancer inhibition or inhibiting the cancer-promoting effect of M2-type macrophages is the breakthrough point to study the treatment of bladder cancer.
Tumor-associated fibroblasts
Cancer-associated fibroblasts (CAFs) are mainly derived from normal fibroblasts and are an important part of the tumor microenvironment. CAFs are closely related to tumor cells. Tumor cells initiate and maintain the activation of CAFs, while CAFs promote the proliferation and metastasis of tumor cells [18].
CAFs promote BC cell growth by secreting a variety of cytokines and increasing energy supply. Autophagy is a kind of behavior that realizes the metabolic needs of cells and the renewal of organelles.. Dong et al. [19] enhanced autophagy in CAFs with rapamycin, and then co-cultured with bladder cancer cells, observed that enhanced autophagy in CAFs induced increased expression of matrix metalloproteinase-9 (MMP9) in cancer cells. The expression levels of proteases MCT1, MCT4, HK2 and SLC2A1 related to energy metabolism pathway such as glycolysis were increased, and lactic acid levels were increased in tumor microenvironment. The study showed that cancer cells are able to induce the Weinberg effect in neighboring fibroblasts, so that aerobic metabolism is affected, and the lactic acid and pyruvate secreted by these fibroblasts are absorbed by cancer cells for energy supply, promoting tumor growth. By analyzing the single-cell transcriptome RNA sequencing data of 8 BC patients, Chen et al. [20] found that inflammatory tumor-associated fibroblasts could promote tumor cell proliferation. When it was co-cultured with bladder cancer cells, the tumor cells showed a higher proliferation capacity. They also found that inflammatory tumor-associated fibroblasts produce VEGF, including VEGFA and VEGFB, while tumor cells themselves express high levels of VEGFA, which binds to receptors on endothelial cells to promote angiogenesis and increase the supply of nutrients to tumor areas, leading to tumor cell proliferation and metastasis.
High infiltration of CAFs is also associated with tumor progression and poor prognosis. Cafs-related transcription factor RUNX2 is overexpressed in bladder cancer, promoting EMT and inducing tumor cells to express MMP, destroying basement membrane and type IV collagen, and remodeling extracellular matrix to promote the migration and invasion of cancer cells [21]. In addition, CAFs can also help regulate immune infiltration in tumor areas. In the study of single-cell transcriptome RNA sequencing, Chen et al. [20] found that inflammatory tumor-associated fibroblasts had more interactions with other cells and expressed CXCL12 at a higher level through the construction of cell-cell interaction networks, and its receptors CXCR4 and CXCR3 were widely expressed in immune cells, indicating that they were related to the immune infiltrating state of BC.
In conclusion, CAFs can promote the progression of bladder cancer through multiple approaches. Compared with other types of stromal cells in the tumor microenvironment, CAFs exhibit more close and complex interactions with tumor cells, so clarifying the interaction network between CAFs and cancer cells is of great significance for exploring new therapeutic strategies.
Lymphocytes
T lymphocytes are an important component of the microenvironment of bladder cancer. There are many subtypes of T lymphocytes, which can promote or inhibit tumor growth. Among them, CD8+ T cells are restricted by MHC class I molecules and differentiate into cytotoxic T cells after activation, which plays an important role in anti-tumor immunity. It recognizes tumor-associated antigens and exerts cytotoxic effects through two killing mechanisms: it directly targets and kills tumor cells by secreting cytotoxic molecules such as granase and perforin; The apoptosis of target cells is induced by the expression of FasL or the secretion of TNF-α, which binds to Fas or TNF receptors on the surface of target cells, respectively [22]. CD4+ T cells are classified into helper T cells (Th), cytotoxic T cells and regulatory T cells (TreGs) according to their functional characteristics. The initial CD4+ T cells unstimulated by antigen were Th0, differentiated into Th1 under the induction of tumor antigen, and secreted gamma interferon to enhance the function of CD8+ T cells and induce macrophages to M1 type polarization [23]. CD8+ T cells and CD4+ Th cells synergistically play an anti-tumor role. Studies have shown that patients with tumor tissue enrichment of CD8+ T cells and CD4 + T cells have a better prognosis [24, 25]. In addition, cytotoxic CD4+ T cells secrete killer cytokines that effectively kill tumor cells. Oh et al. [26] conducted single-cell transcriptome sequencing and paired T-cell receptor sequencing for myoinvasive bladder cancer, and found that cytotoxic CD4+ T cells were significantly enriched in bladder cancer compared with non-malignant tissues, while CD8+ T cells showed no significant difference between malignant and non-malignant tissues. These cells can recognize bladder tumor antigens and express cytolytic proteins to lyse tumor cells by means of MHC-class II.
However, the above antitumor T cell activity is easily inhibited by the tumor microenvironment. Tumor cells can express programmed cell death 1 ligand 1(PDL1), bind to PD-1 on the surface of T cells, and initiate the programmed cell death [27]. In the immunotherapy of bladder cancer, anti-PD-1 /PD-L1 antibodies enhance the cytotoxic activity of CD8+ T cells in bladder cancer patients precisely by blocking the binding of the two [28]. In addition, Treg cells also play a role in inhibiting the activity of anti-tumor T cells.
Tregs produced by CD4+ T cell differentiation are currently a hot topic in the field of bladder cancer. Also known as CD4+CD25+Foxp3+ T cells, they negatively regulate the immune response in two ways. (1) Direct contact inhibited target cell activation; (2) Secreting cytokines such as TGF-β and IL-10 to inhibit immune response. According to cell origin, Tregs can be classified into natural regulatory T cells and induced regulatory T cells. Among them, inducing the expansion of regulatory T cells under the action of tumor antigens and cytokines (such as TGF-β or IL-10) is the main reason for suppressing tumor immune response and tumor escape [29]. Horn et al. [30] found that patients with a high density of Tregs infiltration in bladder cancer had a shorter overall survival time, which might be due to the negative regulation of Tregs on tumor immunity. Tregs in bladder tumors maintain their stability and enhance their immunosuppressive function by up-regulating the expression of transcription factors FOXO1 and C-MAF through chemokine receptor CCR8 [31].
Tregs have long been known to inhibit the immune response of tumors, however, studies have shown that they can inhibit tumor inflammation and positively influence the prognosis of bladder cancer. Winerdal et al. [32] found that Tregs down-regulated MMP2 expression in M2 macrophages and invasive bladder cancer cell lines by interfering with the extracellular signal regulated kinase (ERK) and NF-κB pathways. Inhibition of tumor inflammatory response, thereby reducing the aggressiveness of tumor cells. In addition, in colon and breast cancer, Tregs protect the body from tissue damage and inhibit tumor development by producing adenosine or prostaglandin E2 to down-regulate the inflammatory response [33].
At present, there are few studies on the mechanism of inhibiting tumor inflammatory response by Tregs. In addition, further experiments are needed to clarify how Tregs affect tumor immune and inflammatory responses at various stages of tumor occurrence and development. By constructing the cell interaction network of Tregs, we can understand the influence of microenvironment on the function of TREgs and explore its role in bladder tumors.
Figure 1 shows that the cellular composition of TME.
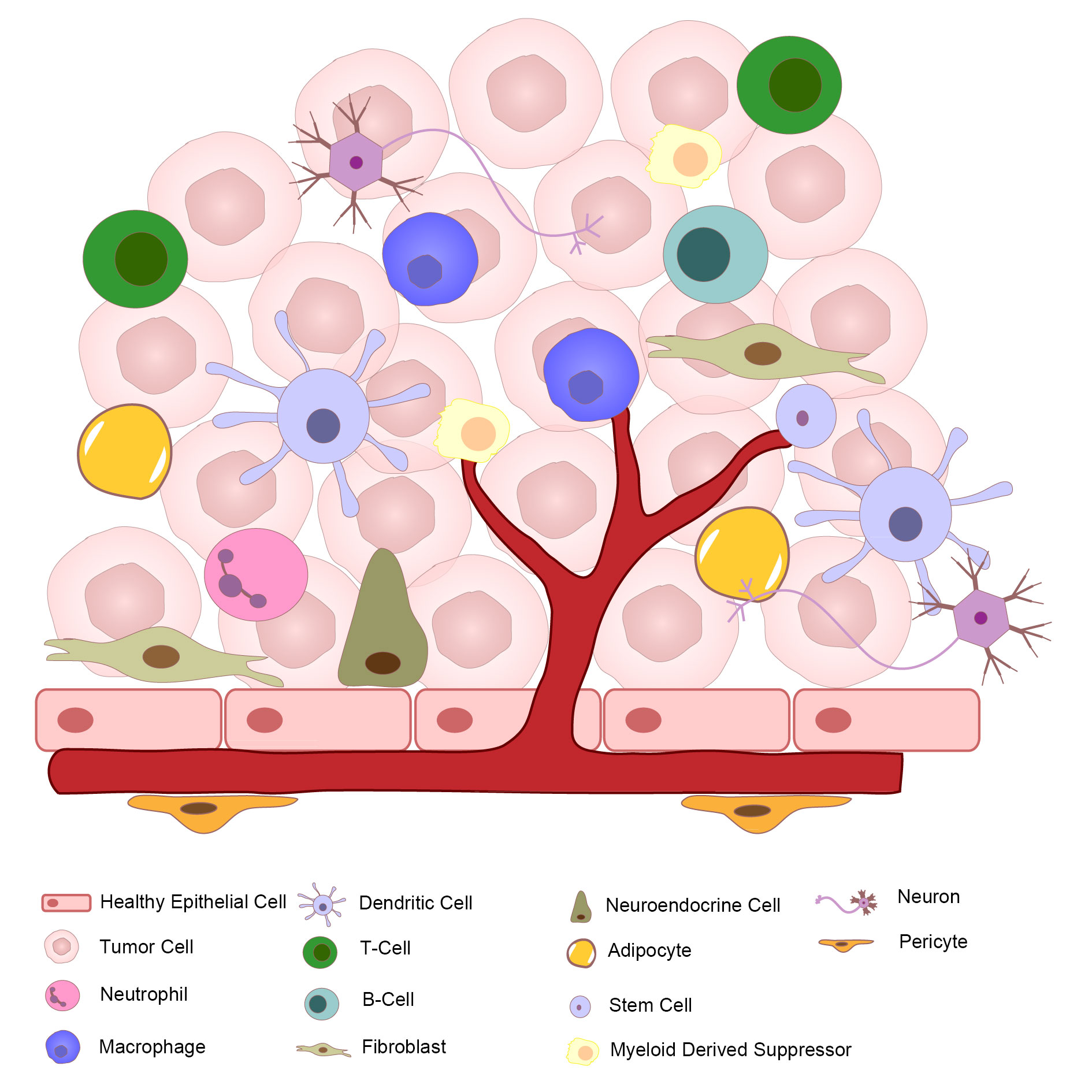 Figure 1. Diagram for tumor microenvironment.
Figure 1. Diagram for tumor microenvironment.
Hypoxia is an important feature of solid tumors, which can activate related genes of tumor cells to adapt to the microenvironment. Cellular response to the hypoxic microenvironment occurs mainly through the hypoxiainducible factor (HIF) family. The HIF1, HIF2, and HIF3 families have been discovered so far. Among them, HIF-1α/β can promote the expression of metabolism-related genes, and HIF-2α/β can affect the expression of genes related to the occurrence, migration and invasion of EMT, and also affect the expression of stem cell-related genes [34]. Wang et al. [35] found that HIF-1α can enhance the transcription and expression of pyruvate kinase isoenzyme M2 gene directly or indirectly through Aly/REF output factors, thereby promoting the glycolysis and proliferation of bladder cancer cells. Guan et al. [36] exposed BC cells transfected with plasmids overexpressing hypoxia-associated factor (HAF) to hypoxia environment and found that HAF drives HIF-1α to HIF-2α by activating the NFκB pathway. It also leads to the enhancement of malignant ability of tumor cells and the maintenance of stem cell markers. Therefore, inhibiting the production of HIF can not only block the energy supply of tumor cells, but also reduce their proliferation and invasion ability. In addition, the expression of urothelial carcinoma-associated 1(UCA1) is up-regulated in bladder cancer cells under hypoxia, which triggers EMT and leads to tumor growth and progression [37]. At the same time, overexpression of UCA1 makes PD-L1 not inhibited by miRNA, which contributes to immune escape of tumor cells [38].
Exosomes
Exosomes, a type of extracellular vesicle, are rich in biomolecules such as nucleic acids, proteins, and lipids, which are involved in intercellular communication and play a key role in tumorigenesis. In bladder cancer, exosomes play an important role in the communication between stromal cells, cancer cells and immune cells, and can play a role in promoting and suppressing cancer.
Cancer-promoting effect
LncRNA and miRNA, as components of bladder cancer exosomes, promote the invasion and metastasis of cancer cells by inducing EMT. Huang et al. [39] found that lnc00960 and lnc02470 derived from exosomes of high-grade bladder cancer cells can inhibit the expression of e-cadherin and enhance the expression of n-cadherin and vimentin by up-regulating the β-catenin signaling pathway, Notch signaling pathway and Smad2/3 signaling pathway, thus promoting EMT. Enhance the migration and invasion ability of low-grade bladder cancer cells. Yin et al. [40] found that the level of circulating exosome miR663b was significantly increased in patients with bladder cancer, which promoted cell proliferation and EMT by targeting the transcription factor Ets2 inhibitor. It is worth noting that tumor cells can secrete angiogenic-related factors through exosomes to promote angiogenesis in target organs. Yoshida et al. [41] found that receptor tyrosine kinase, together with its key regulatory factor CT10 kinase regulator, is transferred to the endothelial cells of the target organ through exosomes, and promotes vascular proliferation and leakage by inducing the activation of FAK and PI3K/AKT signals, creating conditions for distant metastasis.
Anticancer effect
There are also down-regulated expressions of some lncrnas and mirnas in patients with bladder cancer, and up-regulation of these genes can inhibit the proliferation, invasion and migration of cancer cells. Zheng et al. [42] found that the transfer of exosomes containing lncRNA PTENP1 secreted by normal cells to bladder cancer cells can increase the apoptosis of tumor cells and reduce the ability of cell invasion and migration. Li et al. [43] found that down-regulated miRNA375-3p in tumor cells can inhibit the expression of Frizzled-8 gene, block the Wnt/β-catenin pathway, down-regulate the downstream molecules cyclin D1 and C-MYC, thus inhibiting the proliferation of tumor cells and increasing the expression of apoptotic protein.
Exosomes are communication tools between various types of cells in the tumor microenvironment, and their contents determine the function of exosomes. We can use this property to identify exosomes of tumor cell origin for the diagnosis of bladder cancer and load relevant molecules into exosomes for the treatment of bladder cancer.
Figure 2 shows that the dynamic action of different cell types creating the mature tumor microenvironment (TME) in bladder cancer.
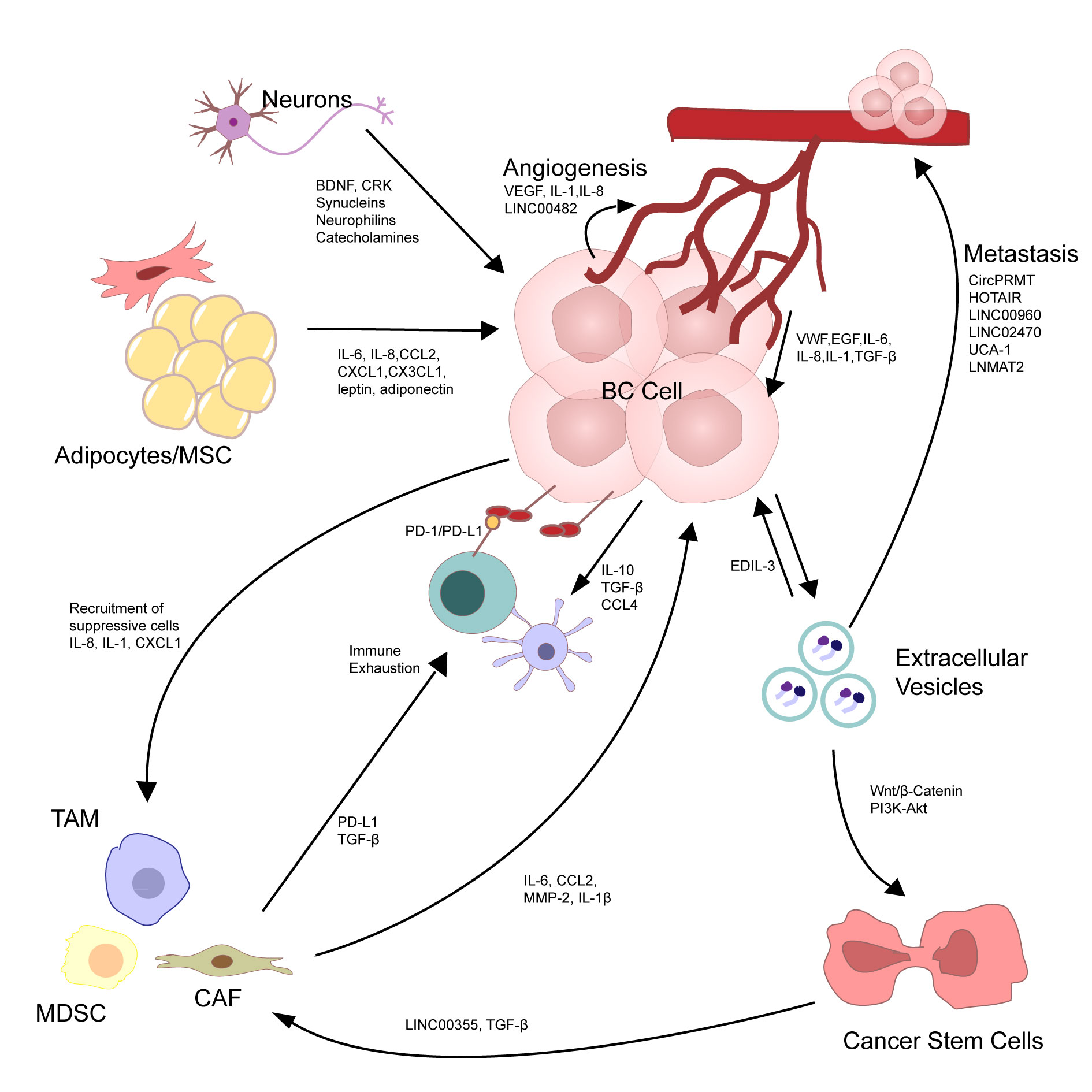 Figure 2. The dynamic action of different cell types creating the mature tumor microenvironment (TME) in bladder cancer. The diverse cells in the TME interact with each other, resulting in tumor growth and prognosis. Bladder cancer (BC) cells metastasis is through autocrine signaling pathways, regarding the production of tumor-promoting and pro-inflammatory cytokines such as IL-6, TNF, and IL-1. BC cells secrete extracellular vescicles (EVs), which can further activate tumor cells in a positive loop and induce tumor cell metastasis, especially through the delivery of non-coding RNAs. Tumor-derived EVs also fuel an immunosuppressive environment via recruiting and activating tumor-infiltrating fibroblasts. These cancer-associated fibroblasts (CAFs) and pro-tumorigenic immune cells, such as Myeloid-derived suppressor cells (MDSCs) and tumor-associated Macrophages (TAMs), are recruited to the TME by the cytokines and chemokines secreted by the BC cells. Once in the TME, these cells provide feedback to the tumor cells by secreting soluble molecules and also inactivate other immune cells, such as T-cells, by increasing the levels of exhaustion markers. BC cells also recruit and activate endothelial cells (angiogenesis) through the secretion of soluble factors such as IL-1, IL-8, and vascular endothelial growth factor (VEGF), as well as long intergenic non-coding RNAs such as LINC00482. These activated endothelial cells form disorganised networks of blood vessels, which are leaky and supply the tumor cells with life-sustaining nutrients. Endothelial cells, adipocytes, and neurons, in turn, influence the tumor cells by secreting inflammatory cytokines, chemokines, growth factors, hormones, and other signaling peptides.
Figure 2. The dynamic action of different cell types creating the mature tumor microenvironment (TME) in bladder cancer. The diverse cells in the TME interact with each other, resulting in tumor growth and prognosis. Bladder cancer (BC) cells metastasis is through autocrine signaling pathways, regarding the production of tumor-promoting and pro-inflammatory cytokines such as IL-6, TNF, and IL-1. BC cells secrete extracellular vescicles (EVs), which can further activate tumor cells in a positive loop and induce tumor cell metastasis, especially through the delivery of non-coding RNAs. Tumor-derived EVs also fuel an immunosuppressive environment via recruiting and activating tumor-infiltrating fibroblasts. These cancer-associated fibroblasts (CAFs) and pro-tumorigenic immune cells, such as Myeloid-derived suppressor cells (MDSCs) and tumor-associated Macrophages (TAMs), are recruited to the TME by the cytokines and chemokines secreted by the BC cells. Once in the TME, these cells provide feedback to the tumor cells by secreting soluble molecules and also inactivate other immune cells, such as T-cells, by increasing the levels of exhaustion markers. BC cells also recruit and activate endothelial cells (angiogenesis) through the secretion of soluble factors such as IL-1, IL-8, and vascular endothelial growth factor (VEGF), as well as long intergenic non-coding RNAs such as LINC00482. These activated endothelial cells form disorganised networks of blood vessels, which are leaky and supply the tumor cells with life-sustaining nutrients. Endothelial cells, adipocytes, and neurons, in turn, influence the tumor cells by secreting inflammatory cytokines, chemokines, growth factors, hormones, and other signaling peptides.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
NC and YW: Conception, design of study and manuscript preparation; YS: Data collection and analysis; YD: IHC images and manuscript editing.
Competing interests
The authors have no competing interest.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 82274313, 82204761 and 81901869).
- Miyazaki J, Nishiyama H: Epidemiology of urothelial carcinoma. Int J Urol 2017, 24(10): 730-734.
- Rubio C, Munera-Maravilla E, Lodewijk I, Suarez-Cabrera C, Karaivanova V, Ruiz-Palomares R, Paramio JM, Dueñas M: Macrophage polarization as a novel weapon in conditioning tumor microenvironment for bladder cancer: can we turn demons into gods? Clin Transl Oncol 2019, 21(4): 391-403.
- Liu C, He D, Zhang S, Chen H, Zhao J, Li X, Zeng X: Homogeneous Polyporus Polysaccharide Inhibit Bladder Cancer by Resetting Tumor-Associated Macrophages Toward M1 Through NF-κB/NLRP3 Signaling. Front Immunol 2022, 13: 839460.
- Jiang Z, Zhang Y, Zhang Y, Jia Z, Zhang Z, Yang J: Cancer derived exosomes induce macrophages immunosuppressive polarization to promote bladder cancer progression. Cell Commun Signal 2021, 19(1): 93.
- Qu G, Liu Z, Yang G, Xu Y, Xiang M, Tang C: Development of a prognostic index and screening of prognosis related genes based on an immunogenomic landscape analysis of bladder cancer. Aging (Albany NY) 2021, 13(8): 12099-12112.
- Lyu X, Wang P, Qiao Q, Jiang Y: Genomic stratification based on microenvironment immune types and PD-L1 for tailoring therapeutic strategies in bladder cancer. BMC Cancer 2021, 21(1): 646.
- Yan Y, Huang Z, Cai J, Tang P, Zhang F, Tan M, Shen B: Identification of a novel immune microenvironment signature predicting survival and therapeutic options for bladder cancer. Aging (Albany NY) 2020, 13(2): 2780-2802.
- Hori S, Miyake M, Onishi S, Morizawa Y, Nakai Y, Tatsumi Y, Onishi K, Iida K, Gotoh D, Itami Y et al: Evaluation of pro‑ and anti‑tumor effects induced by three colony‑stimulating factors, G‑CSF, GM‑CSF and M‑CSF, in bladder cancer cells: Is G‑CSF a friend of bladder cancer cells? Int J Oncol 2019, 54(6): 2237-2249.
- Qiu S, Deng L, Liao X, Nie L, Qi F, Jin K, Tu X, Zheng X, Li J, Liu L et al: Tumor-associated macrophages promote bladder tumor growth through PI3K/AKT signal induced by collagen. Cancer Sci 2019, 110(7): 2110-2118.
- Miyake M, Hori S, Morizawa Y, Tatsumi Y, Nakai Y, Anai S, Torimoto K, Aoki K, Tanaka N, Shimada K et al: CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia 2016, 18(10): 636-646.
- Wu H, Zhang X, Han D, Cao J, Tian J: Tumour-associated macrophages mediate the invasion and metastasis of bladder cancer cells through CXCL8. PeerJ 2020, 8: e8721.
- Zhang Q, Mao Z, Sun J: NF-κB inhibitor, BAY11-7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/Snail signaling in bladder cancer cells. Gene 2019, 710: 91-97.
- Chen C, He W, Huang J, Wang B, Li H, Cai Q, Su F, Bi J, Liu H, Zhang B et al: LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun 2018, 9(1): 3826.
- Aljabery F, Olsson H, Gimm O, Jahnson S, Shabo I: M2-macrophage infiltration and macrophage traits of tumor cells in urinary bladder cancer. Urol Oncol 2018, 36(4): 159.e119-159.e126.
- Xu Z, Zeng H, Liu Z, Jin K, Chang Y, Wang Y, Liu L, Zhu Y, Xu L, Wang Z et al: Poor clinical outcomes and immunoevasive contexture in SIRPα(+) tumor-associated macrophages enriched muscle-invasive bladder cancer patients. Urol Oncol 2022, 40(3): 109.e111-109.e120.
- Shen C, Wang L, Yang X, Liu J, Yang Q, Ding X, Niu H, Wang Y: Construction of a immune-associated genes based prognostic signature in bladder cancer. Artif Cells Nanomed Biotechnol 2021, 49(1): 108-119.
- Xu Y, Zeng H, Jin K, Liu Z, Zhu Y, Xu L, Wang Z, Chang Y, Xu J: Immunosuppressive tumor-associated macrophages expressing interlukin-10 conferred poor prognosis and therapeutic vulnerability in patients with muscle-invasive bladder cancer. J Immunother Cancer 2022, 10(3).
- Kuzet SE, Gaggioli C: Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res 2016, 365(3): 607-619.
- Dong D, Yao Y, Song J, Sun L, Zhang G: Cancer-Associated Fibroblasts Regulate Bladder Cancer Invasion and Metabolic Phenotypes through Autophagy. Dis Markers 2021, 2021: 6645220.
- Chen Z, Zhou L, Liu L, Hou Y, Xiong M, Yang Y, Hu J, Chen K: Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat Commun 2020, 11(1): 5077.
- Liu B, Pan S, Liu J, Kong C: Cancer-associated fibroblasts and the related Runt-related transcription factor 2 (RUNX2) promote bladder cancer progression. Gene 2021, 775: 145451.
- Xia W, Zhang S, Duan H, Wang C, Qian S, Shen H: The combination therapy of Everolimus and anti-PD-1 improves the antitumor effect by regulating CD8(+) T cells in bladder cancer. Med Oncol 2022, 39(3): 37.
- Wu J, Abraham SN: The Roles of T cells in Bladder Pathologies. Trends Immunol 2021, 42(3): 248-260.
- Bohner P, Chevalier MF, Cesson V, Rodrigues-Dias SC, Dartiguenave F, Burruni R, Tawadros T, Valerio M, Lucca I, Nardelli-Haefliger D et al: Double Positive CD4(+)CD8(+) T Cells Are Enriched in Urological Cancers and Favor T Helper-2 Polarization. Front Immunol 2019, 10: 622.
- Jin K, Yu Y, Zeng H, Liu Z, You R, Zhang H, Liu C, Su X, Yan S, Chang Y et al: CD103(+)CD8(+) tissue-resident memory T cell infiltration predicts clinical outcome and adjuvant therapeutic benefit in muscle-invasive bladder cancer. Br J Cancer 2022, 126(11): 1581-1588.
- Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, Aran D, Ilano A, Pai CS, Rancan C et al: Intratumoral CD4(+) T Cells Mediate Anti-tumor Cytotoxicity in Human Bladder Cancer. Cell 2020, 181(7): 1612-1625.e1613.
- Boegemann M, Aydin AM, Bagrodia A, Krabbe LM: Prospects and progress of immunotherapy for bladder cancer. Expert Opin Biol Ther 2017, 17(11): 1417-1431.
- Mathew Thomas V, Tripathi N, Agarwal N, Swami U: Current and emerging role of sacituzumab govitecan in the management of urothelial carcinoma. Expert Rev Anticancer Ther 2022, 22(4): 335-341.
- Whiteside TL: Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol Immunother 2014, 63(1): 67-72.
- Horn T, Laus J, Seitz AK, Maurer T, Schmid SC, Wolf P, Haller B, Winkler M, Retz M, Nawroth R et al: The prognostic effect of tumour-infiltrating lymphocytic subpopulations in bladder cancer. World J Urol 2016, 34(2): 181-187.
- Wang T, Zhou Q, Zeng H, Zhang H, Liu Z, Shao J, Wang Z, Xiong Y, Wang J, Bai Q et al: CCR8 blockade primes anti-tumor immunity through intratumoral regulatory T cells destabilization in muscle-invasive bladder cancer. Cancer Immunol Immunother 2020, 69(9): 1855-1867.
- Winerdal ME, Krantz D, Hartana CA, Zirakzadeh AA, Linton L, Bergman EA, Rosenblatt R, Vasko J, Alamdari F, Hansson J et al: Urinary Bladder Cancer Tregs Suppress MMP2 and Potentially Regulate Invasiveness. Cancer Immunol Res 2018, 6(5): 528-538.
- Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, Johnson JT, Gorelik E, Lang S, Whiteside TL: Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem 2010, 285(36): 27571-27580.
- Jiang H, Zhu Y, Xu H, Sun Y, Li Q: Activation of hypoxia-inducible factor-1alpha via nuclear factor-kappa B in rats with chronic obstructive pulmonary disease. Acta Biochim Biophys Sin (Shanghai) 2010, 42(7): 483-488.
- Wang JZ, Zhu W, Han J, Yang X, Zhou R, Lu HC, Yu H, Yuan WB, Li PC, Tao J et al: The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun (Lond) 2021, 41(7): 560-575.
- Guan Z, Ding C, Du Y, Zhang K, Zhu JN, Zhang T, He D, Xu S, Wang X, Fan J: HAF drives the switch of HIF-1α to HIF-2α by activating the NF-κB pathway, leading to malignant behavior of T24 bladder cancer cells. Int J Oncol 2014, 44(2): 393-402.
- Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, Pang H, An H, Wang X, Hou H et al: Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer 2017, 16(1): 143.
- Wang CJ, Zhu CC, Xu J, Wang M, Zhao WY, Liu Q, Zhao G, Zhang ZZ: The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol Cancer 2019, 18(1): 115.
- Huang CS, Ho JY, Chiang JH, Yu CP, Yu DS: Exosome-Derived LINC00960 and LINC02470 Promote the Epithelial-Mesenchymal Transition and Aggressiveness of Bladder Cancer Cells. Cells 2020, 9(6).
- Yin X, Zheng X, Liu M, Wang D, Sun H, Qiu Y, Chen J, Shi B: Exosomal miR-663b targets Ets2-repressor factor to promote proliferation and the epithelial-mesenchymal transition of bladder cancer cells. Cell Biol Int 2020, 44(4): 958-965.
- Yoshida K, Tsuda M, Matsumoto R, Semba S, Wang L, Sugino H, Tanino M, Kondo T, Tanabe K, Tanaka S: Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci 2019, 110(7): 2119-2132.
- Zheng R, Du M, Wang X, Xu W, Liang J, Wang W, Lv Q, Qin C, Chu H, Wang M et al: Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer 2018, 17(1): 143.
- Li Q, Huyan T, Cai S, Huang Q, Zhang M, Peng H, Zhang Y, Liu N, Zhang W: The role of exosomal miR-375-3p: A potential suppressor in bladder cancer via the Wnt/β-catenin pathway. Faseb j 2020, 34(9): 12177-12196.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript