Case Report | Open Access
Pseudoepitheliomatous Hyperplasia: Harbinger of Underlying Squamous Cell Carcinoma - Lessons Learnt
Sriram Krishnamoorthy1, Hariharasudhan Sekar1, Leena Dennis Joseph2, J. Sathish Kumar3
1Department of Urology & Renal Transplantation, Sri Ramachandra Institute of Higher Education & Research (SRIHER), Chennai, India.
2Department of Pathology, SRIHER, Chennai, India.
3Department of Plastic Surgery, SRIHER, Chennai, India.
Correspondence: Sriram Krishnamoorthy (Department of Urology & Renal Transplantation, Sri Ramachandra Institute of Higher Education & Research, Chennai, 600116, India; Email: sriramuro@gmail.com).
Annals of Urologic Oncology 2022; 5(1): 52-56. https://doi.org/10.32948/auo.2022.12.22
Received: 18 Dec 2022 | Accepted: 22 Dec 2022 | Published online: 25 Dec 2022
Non-healing perineal lesions are not uncommon. Most of the lesions turn out to be Squamous Cell Carcinoma. But, if the histopathological picture suggests Pseudoepitheliomatous Hyperplasia, it is vital to consider the limitations of the biopsy, and a solid clinicopathological correlation is required to look aggressively for underlying Squamous Cell Carcinoma. Due to the benign nature of PEH, most cases are treated via excision biopsy, while grafts or flaps are occasionally required to restore severe tissue defects.
It is therefore crucial to rule out and distinguish this condition from other benign and malignant conditions, as the treatment and prognosis differ widely. It is of utmost importance to sample the base of the lesion, analyze multiple sections, and consider clinical data to ensure an accurate diagnosis.
Key words squamous cell carcinoma, pseudoepitheliomatous hyperplasia, perineum, ulcer
On follow-up after three weeks, the size of the ulcer increased to 5x5 cm with foul-smelling purulent discharge. He was taken up for wound debridement. Edge-wedge biopsy was taken to rule out squamous cell carcinoma. Histopathological examination revealed hyperplastic squamous epithelium with marked hyperkeratosis, parakeratosis, and ulceration with a dense acute inflammatory granulation tissue suggestive of PEH with no evidence of malignancy even in deeper sections (Figure 1).
In view of the benign nature of the lesion, he was advised regular wound care at a nearby hospital. After two weeks, he presented with rapidly progressive ulcero-proliferative growth and the repeat edge wedge biopsy was again suggestive of PEH. With a clinical suspicion of Squamous Cell Carcinoma, a wide local excision of the growth was performed. Fasciocutaneous flap reconstruction was done to cover the wide defect (Figure 2).
Histopathology suggested squamous cell carcinoma with verrucous background, invading the reticular dermis, with no lymphovascular and perineural invasion (Figure 3). Patient received adjuvant radiotherapy.
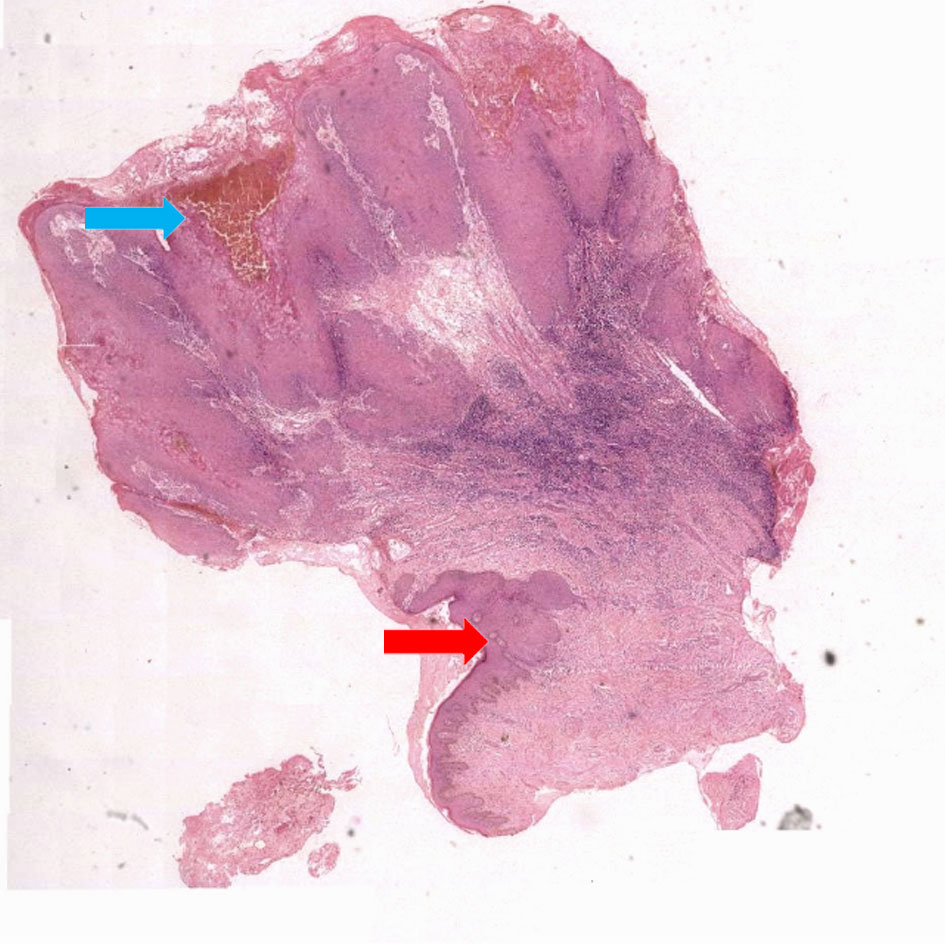 Figure 1. Low power view microphotograph. Microphotographs showing normal squamous epithelium (red arrow) and marked pseudoepitheliomatous hyperplasia (blue arrow) (H&E X 40).
Figure 1. Low power view microphotograph. Microphotographs showing normal squamous epithelium (red arrow) and marked pseudoepitheliomatous hyperplasia (blue arrow) (H&E X 40).
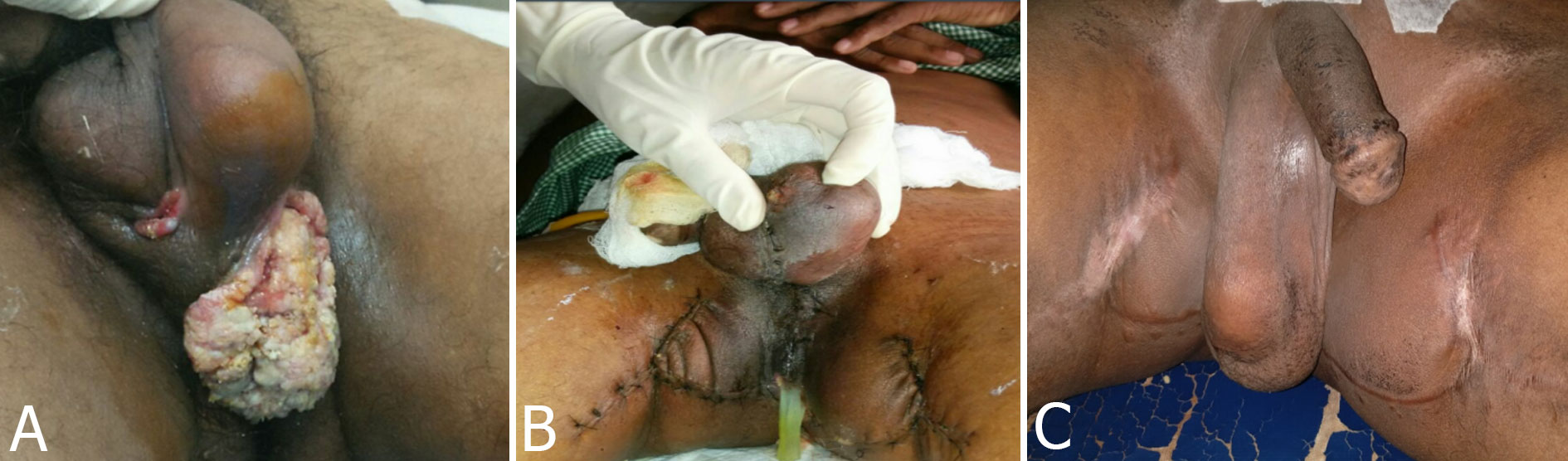 Figure 2. Clinical images of the lesion. Pre operative (Fig. 2a), fascio-cutaneous flaps (Fig. 2b) and post operative (Fig. 2c) images, illustrating the perineal lesion, flaps taken and post-operative healed wound respectively.
Figure 2. Clinical images of the lesion. Pre operative (Fig. 2a), fascio-cutaneous flaps (Fig. 2b) and post operative (Fig. 2c) images, illustrating the perineal lesion, flaps taken and post-operative healed wound respectively.
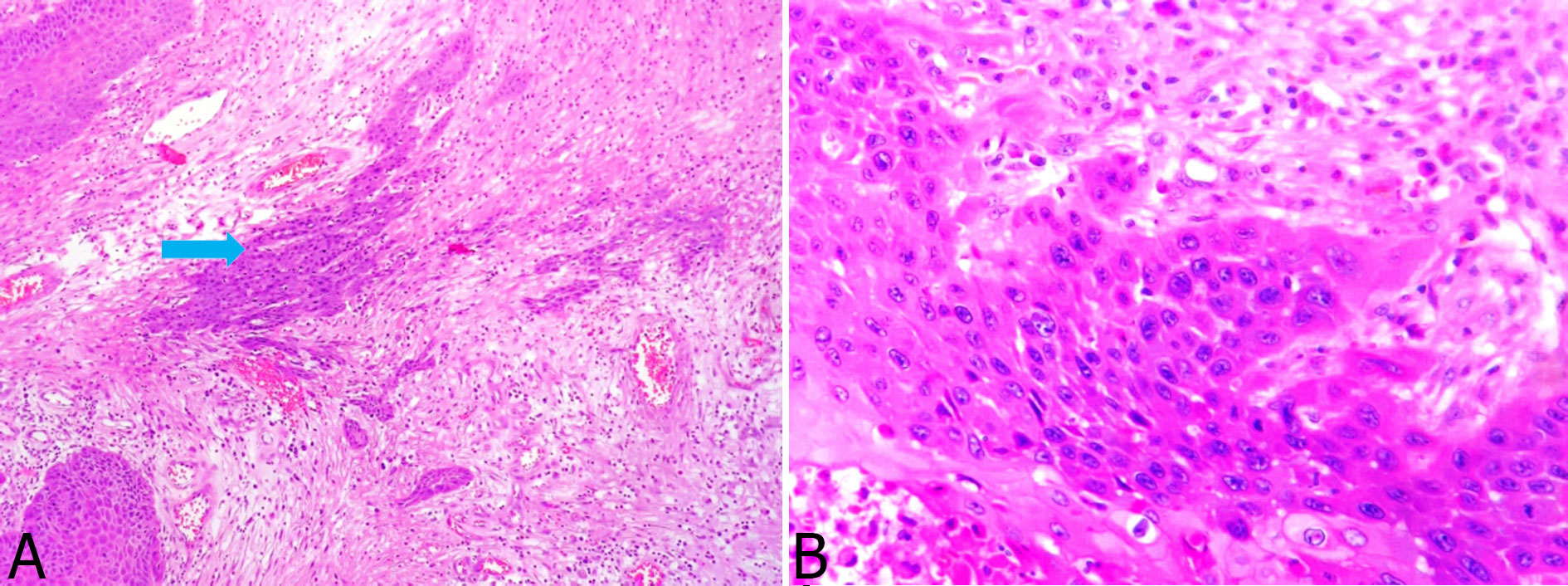 Figure 3. High-power view microphotograph of malignant cells. Islands of malignant squamous cells (H&E X 100) infiltrating into the sub epithelium (blue arrow) (Fig. 3a) and Higher power view (H&E X 200) of the malignant cells, with abundant eosinophilic cytoplasm and pleomorphic nuclei (Fig. 3b).
Figure 3. High-power view microphotograph of malignant cells. Islands of malignant squamous cells (H&E X 100) infiltrating into the sub epithelium (blue arrow) (Fig. 3a) and Higher power view (H&E X 200) of the malignant cells, with abundant eosinophilic cytoplasm and pleomorphic nuclei (Fig. 3b).
An array of morphological features may help differentiate invasive SCC from PEH on routine haematoxylin-eosin-stained tissue sections. Histologically, PEH has an irregular invasion of the dermis by uneven, jagged, pointed epidermal cell masses and strands with keratin pearl formation [6]. Squamous cell carcinoma usually demonstrates some degree of cytologic atypia, including nuclear pleomorphism, maturational atypia, and mitoses. Furthermore, invasion of epithelial proliferation by leucocytes and disintegration of some of the epidermal cells are classical of PEH, a finding that is absent in SCC. Other features that provide clues for distinguishing these conditions are the width of the strands and the degree of keratinocytic atypia. SCCs have broad strands and a greater degree of atypia compared to PEH. The presence of inflammatory infiltrate does not preclude the diagnosis of SCC.
The role of immunohistochemistry is not well documented. The expression of the p53 protein has been utilised to differentiate PEH from SCC. However, as it is an indicator of the proliferative capacity of the cells rather than one of malignancy, it has been observed in a variety of skin lesions, including in situ and invasive SCC, Pseudoepitheliomatous hyperplasia, and keratoacanthoma [7]. The number of Langerhans cells in both the conditions was similar. Immunohistochemistry using CD1a for the quantification of Langerhans cells has no added value in differentiating SCC and PEH. However, the density of Langerhans cells was decreased in SCC compared to that of PEH. This finding was correlated with decreased expression of E-Cadherin in squamous cell carcinoma [8]. Matrix metalloproteinase (MMP)-1, or interstitial collagenase, are expressed in low or absent levels in benign mucosal tissue [9, 10]. In SCCs, the epithelial expression of MMP-7, MMP-13, and MMP-12 is raised and provides a diagnostic clue while the expression of MMP-1 along with MMP-3, MMP-8, MMP-9, and MMP-10 is decreased or absent [11]. Loss of MMP-19 and p16 from epithelial cells might be an important indicator of malignancy arising in the setting of chronic wounds that are exhibiting PEH. Features like keratinocyte necrosis, vascular and perineural invasion are absent in PEH. Zarovnaya et al. in their study on distinguishing Pseudoepitheliomatous Hyperplasia from Squamous Cell Carcinoma in Mucosal Biopsy Specimens from the Head and Neck evaluated the role of p53, E-cadherin, collagen IV and MMP-1 and concluded that properly oriented hematoxylin-eosin-stained sections remain ideal and use of these markers (p53, E-cadherin, MMP-1 and Collagen IV) may have a limited adjunct role due to the small specimen size of the edge wedge biopsy [12]. PEH has also been reported following tattooing in a three-case series and following Mohs micrographic surgery [13, 14]. Table 1 illustrates the list of various authors who have reported their experiences on PEH.
Management of PEH differs greatly from SCC. As PEH is a benign condition, it can be managed by surgical excision, although grafts or flaps are occasionally needed to reconstruct major tissue defects. However, a flawed diagnosis of malignancy will lead to radical surgery and surgery-related morbidity, but the converse may delay early management [20].
PEH may develop in response to various triggers. Bacterial, viral, fungal infection or underlying malignancy are the major causes. Though PEH resembles SCC histologically, management differs between the two clinically distinct conditions. Hence, to achieve an accurate diagnosis and prompt treatment, it is critical to recognize the pitfalls in diagnosis. Adequate sampling of the base of the lesion, analyzing multiple and deeper sections and proper evaluation of the clinical data are the basic pre-requisites for making an early recognition and appropriate treatment.
Nil.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
SK, HS and JSK were involved in performing this surgery;
SK was instrumental in writing this manuscript, with microphotographs and histopathological inputs from LDJ.
Competing interests
The authors declare no conflict of interest.
Funding
None.
- Chakrabarti S, Chakrabarti PR, Agrawal D, Somanath S: Pseudoepitheliomatous hyperplasia: a clinical entity mistaken for squamous cell carcinoma. J Cutan Aesthet Surg 2014, 7(4): 232-234.
- Scott G, Chen KT, Rosai J: Pseudoepitheliomatous hyperplasia in Spitz nevi. A possible source of confusion with squamous cell carcinoma. Arch Pathol Lab Med 1989, 113(1): 61-63.
- Gacek MR, Gacek RR, Gantz B, McKenna M, Goodman M: Pseudoepitheliomatous hyperplasia versus squamous cell carcinoma of the external auditory canal. Laryngoscope 1998, 108(4 Pt 1): 620-623.
- Galan A, Ko CJ: Langerhans cells in squamous cell carcinoma vs. pseudoepitheliomatous hyperplasia of the skin. J Cutan Pathol 2007, 34(12): 950-952.
- Nayak VN, Uma K, Girish HC, Murgod S, Shyamala K, Naik RB: Pseudoepitheliomatous Hyperplasia in Oral Lesions: A Review. J Int Oral Health 2005, 7(9): 148-152.
- Bouquot JE, Nikai H: Lesions of oral cavity. In: Gnepp DR, editor. Diagnostic Surgical Pathology of the Head and Neck. Philadelphia, PA: WB Saunders 2001, 205-207.
- Lee YS, Teh M: p53 expression in pseudoepitheliomatous hyperplasia, keratoacanthoma, and squamous cell carcinoma of skin. Cancer 1994, 73(9): 2317-2323.
- Gupta A, Sharma S, Batra M, Abidullah M, Bhuvinder S, Katragadda P: Role of E-cadherin in Progression of Oral Squamous Cell Carcinoma: A Retrospective Immunohistochemical Study. J Contemp Dent Pract 2018, 19(9): 1105-1110.
- Gray ST, Wilkins RJ, Yun K: Interstitial collagenase gene expression in oral squamous cell carcinoma. Am J Pathol 1992, 141(2): 301-306.
- Muller D, Wolf C, Abecassis J, Millon R, Engelmann A, Bronner G, Rouyer N, Rio MC, Eber M, Methlin G: Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res 1993, 53(1): 165-169.
- Impola U, Jeskanen L, Ravanti L, Syrjänen S, Baldursson B, Kähäri VM, Saarialho-Kere U: Expression of matrix metalloproteinase (MMP)-7 and MMP-13 and loss of MMP-19 and p16 are associated with malignant progression in chronic wounds. Br J Dermatol 2005, 152(4): 720-726.
- Zarovnaya E, Black C: Distinguishing pseudoepitheliomatous hyperplasia from squamous cell carcinoma in mucosal biopsy specimens from the head and neck. Arch Pathol Lab Med 2005, 129(8): 1032-1036.
- Kluger N, Durand L, Minier-Thoumin C, Plantier F, Cotten H, Berteloot E, Blatière V, Dereure O: Pseudoepitheliomatous epidermal hyperplasia in tattoos: report of three cases. Am J Clin Dermatol 2008, 9(5): 337-340.
- Weber PJ, Johnson BL, Dzubow LM: Pseudoepitheliomatous hyperplasia following Mohs micrographic surgery. J Dermatol Surg Oncol 1989, 15(5): 557-560.
- Grauwin MY, Mane I, Cartel JL: Pseudoepitheliomatous hyperplasia in tropic ulcers in leprosy patients. A 28-case study. Lepr Rev 1996, 67: 203-207.
- Lynch, Jane: Understanding Pseudoepitheliomatous Hyperplasia. Pathology Case Reviews 2004, 9: 36-45.
- Zayour M, Lazova R: Pseudoepitheliomatous hyperplasia: A review. Am J Dermatopathol 2011, 33(2): 112-122.
- Sarangarajan R, Vedam VK, Sivadas G, Krishnaraj R, Sarangarajan A, Shanmugam KT: Pseudoepitheliomatous Hyperplasia: Relevance in Oral Pathology. J Int Oral Health 2015, 7(7): 132-136.
- Johnson DM, White LJ, Cruciani B, Gordon SV: Pseudoepitheliomatous Hyperplasia: A Word of Caution. JSM Clin Case Rep 2018, 6(4): 1156.
- Calhoun KH, Wagner RF Jr, Kumar D, Hokanson JA: Pseudoepitheliomatous hyperplasia mistaken for cancer after delayed reconstruction. South Med J 1995, 188(4): 454-457.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript