REVIEW | Open Access
Treatment Strategies for BCG Unresponsive Non-muscle Invasive Bladder Cancer
Anusha Gupta1, 2, Shiv Verma1, 3, Sanjay Gupta1, 3, 4, 5, 6, 7
1Department of Urology, Case Western Reserve University, School of Medicine, Cleveland, OH 44106, USA.
2Faculty of Science Program, University of Western Ontario, London N6A 5A5, Canada.
3The Urology Institute, University Hospitals Cleveland Medical Center, Cleveland, OH 44106, USA.
4Department of Pathology, Case Western Reserve University, School of Medicine, Cleveland, OH 44106, USA.
5Department of Pharmacology, Case Western Reserve University, School of Medicine, Cleveland, OH 44106, USA.
6Department of Nutrition, Case Western Reserve University, School of Medicine, Cleveland, OH 44106, USA.
7Division of General Medical Sciences, Case Comprehensive Cancer Center, Cleveland, OH 44106, USA.
Annals of Urologic Oncology 2024, 7(3): 97-109. https://doi.org/10.32948/auo.2024.08.25
Received: 13 Aug 2024 | Accepted: 29 Aug 2024 | Published online: 29 Aug 2024
Key words non-muscle invasive bladder cancer, BCG failure, immunotherapy, intravesical chemotherapy, gene therapy, clinical trials
For over 40 years, Bacillus Calmette-Guérin (BCG) has been a cornerstone in the treatment of bladder cancer. Originally developed from Mycobacterium bovis, which was isolated from an infected organism to create a tuberculosis vaccine, BCG has emerged as a promising option for both clinicians and patients with NMIBC [2]. BCG exerts cytotoxic effects on bladder cancer cells through various mechanisms, including immune system activation, apoptosis, necrosis, and oxidative stress [3]. Upon instillation into the bladder, BCG binds to cancer cells, triggering a strong immune response. This activation involves T-cells and cytokine production, which in turn stimulates natural killer (NK) cells, macrophages, and neutrophils. These immune cells work together to eliminate tumor cells in the bladder (Figure 1). BCG can also directly induce apoptosis and necrosis by activating death receptor-mediated signaling pathways and upregulating proteases. The treatment generates oxidative stress by releasing reactive oxygen species, which contribute to cell death. BCG therapy is considered successful if no tumor recurrence is observed for two years, at which point the patient is considered tumor-free. However, additional rounds of BCG may be necessary if the tumor recurs. Its effectiveness in reducing bladder cancer has been demonstrated through a meta-analysis of 24 randomized controlled trials [4]. The results showed that BCG treatment reduced the odds of bladder cancer progression by about 27% over 2.5 years. While BCG is effective in curing 35% of patients, 30% to 40% may experience BCG failure, which increases the risk of tumor recurrence and progression. Additionally, BCG treatment can lead to toxic side effects, including cystitis, severe flu-like symptoms, prostatitis, and other systemic infections. These issues highlight the need for alternative treatments for patients who do not respond to BCG therapy [5, 6]. Although radical cystectomy is the primary treatment option following BCG failure, it may not be suitable for all patients or could be declined due to its considerable impact on quality-of-life [7, 8]. This review explores the range of treatment options available for high-risk NMIBC patients who have experienced BCG failure and are either unable or unwilling to undergo radical cystectomy.
|
Table 1. High-Risk NMIBC characteristics defined by the American and European Urologic Association. |
||
|
Characteristic |
American Urologic Association |
European Urologic Association |
|
Carcinoma in situ |
Yes |
Yes |
|
High-grade T1 Tumor |
Yes |
Any high-grade tumor |
|
Any T1 tumor |
Indefinite |
Yes |
|
Recurrent or multifocal or large (>3cm) high-grade Ta tumors |
Yes |
Indefinite |
|
Any tumor following BCG failure |
Yes |
Indefinite |
|
Lymphovascular invasion or non-urothelial histology |
Yes |
Indefinite |
|
High-grade tumor involving prostatic urethra |
Yes |
Indefinite |
|
T1 HG with CIS |
Indefinite |
Highest risk |
|
Multiple, large, or recurrent T1 high-grade tumors |
Indefinite |
Highest risk |
|
T1 with CIS in prostatic urethra |
Indefinite |
Highest risk |
|
Some variant histology or lymphovascular invasion |
Indefinite |
Highest risk |
|
Table 2. Criteria of BCG-unresponsive non-muscle invasive bladder cancer and adequate BCG treatment. |
|
|
BCG-unresponsive non-muscle invasive bladder cancer |
Adequate BCG treatment |
|
Persistent or recurrent CIS alone or with Ta/T1 disease within 12 months of adequate BCG therapy |
At least five of six doses of the initial induction course and at least two of three doses of the maintenance treatment |
|
Recurrent high-grade Ta/T1 disease within 6 months of completion of adequate BCG therapy |
At least five of six doses of the initial induction course and at least two of six doses of the second induction course |
|
T1 high-grade disease on the first evaluation following an induction BCG course |
|
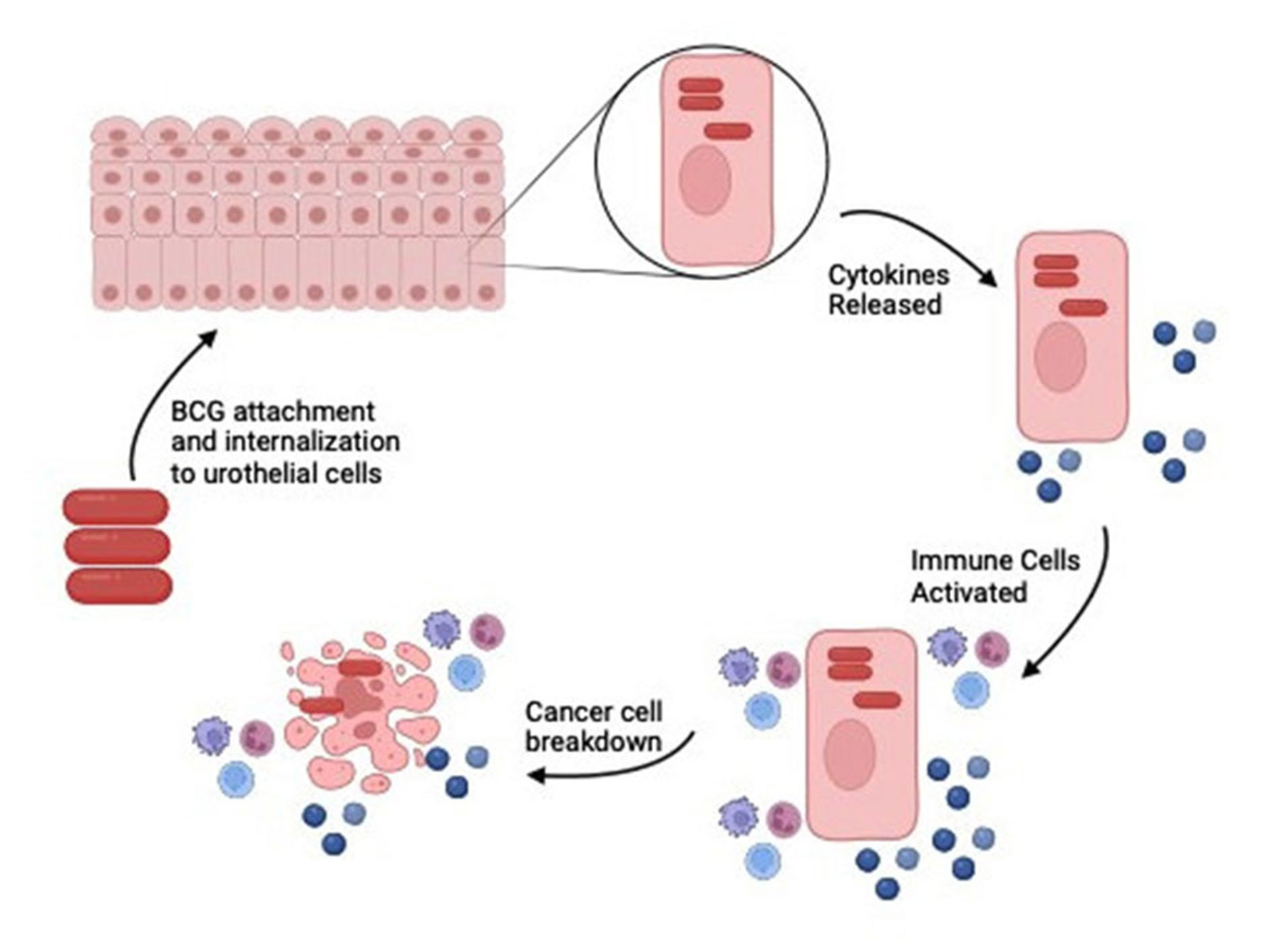 Figure 1. BCG induces an immune response by activating immune cells to treat NMIBC. Following treatment BCG is attached and internalized to the urothelial cells. The presence of BCG triggers the recruitment and activation of a variety of immune cells, including macrophages, dendritic cells, and neutrophils. These cells present antigens and secrete cytokines, which further activate T-cells and natural killer (NK) cells. This cascade of immune responses leads to the production of pro-inflammatory cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which play crucial roles in attacking and destroying cancer cells.
Figure 1. BCG induces an immune response by activating immune cells to treat NMIBC. Following treatment BCG is attached and internalized to the urothelial cells. The presence of BCG triggers the recruitment and activation of a variety of immune cells, including macrophages, dendritic cells, and neutrophils. These cells present antigens and secrete cytokines, which further activate T-cells and natural killer (NK) cells. This cascade of immune responses leads to the production of pro-inflammatory cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which play crucial roles in attacking and destroying cancer cells.
Intravesical chemotherapy
Intravesical chemotherapy involves administering cytotoxic drugs directly into the bladder to target cancer cells and prevent tumor recurrence. [29, 30] Drugs used for this treatment include valrubicin, gemcitabine, gemcitabine with mitomycin C, docetaxel, gemcitabine with docetaxel, and paclitaxel as combination (Figure 2).
Valrubicin is used in intravesical therapy for NMIBC. It integrates into the DNA and disrupts nucleotide incorporation, effectively halting the cell cycle during the G2 phase [31]. This FDA-approved drug inhibits the enzyme topoisomerase II, which is crucial for unwinding double-stranded DNA during replication. By suppressing this enzyme, valrubicin causes DNA damage, leading to the death of cancer cells. In a phase III study, 90 patients with multiple failed courses of intravesical therapy, including one round of BCG, were treated with six weekly instillations of 800 mg valrubicin. After 30 months, 21% of these patients achieved a complete response, with 7 remaining disease-free and 14 showing superficial Ta disease at the last evaluation [32]. The potential of valrubicin was further assessed in a retrospective study [33], involving 113 NMIBC patients who had experienced BCG failure and were not candidates for cystectomy. The study found event-free survival (EFS) rates of 51.6% at 3 months, 30.4% at 6 months, and 16.4% at 12 months following valrubicin instillations, with patients also reporting local bladder symptoms. Valrubicin is considered an effective short-term alternative to more invasive treatments like cystectomy and is associated with relatively mild side-effects, as demonstrated in numerous studies. However, due to its limited long-term effectiveness, there is currently no ongoing research focused on this treatment. Resistance to valrubicin often develops as cancer cells overexpress reductive enzymes, such as carbonyl reductases and aldo-keto reductases, which convert the drug into less cytotoxic metabolites [34]. These metabolites are then efficiently expelled from the cells by ABC transporters, reducing the drug's efficacy and contributing to resistance. This situation underscores the need for continued research into alternative chemotherapy drugs to improve long-term outcomes for patients who are unresponsive to BCG.
Gemcitabine is a widely used intravesical chemotherapy drug most commonly used for treating high-risk and BCG-unresponsive NMIBC patients. Once inside the cell, gemcitabine undergoes phosphorylation to form its active metabolites, including gemcitabine diphosphate and gemcitabine triphosphate [11]. Gemcitabine diphosphate inhibits ribonucleotide reductase, an enzyme essential for DNA synthesis, thereby disrupting DNA replication. Concurrently, gemcitabine triphosphate is incorporated into DNA, blocking the addition of new nucleotides and inhibiting DNA synthesis. The combined effects of ribonucleotide reductase inhibition and the interruption of DNA synthesis lead to cancer cell apoptosis. A comparative study [35] evaluated the effectiveness of intravesical gemcitabine versus BCG in 117 patients. After administering 1 g of gemcitabine, patients were randomly assigned to receive either BCG or intravesical gemcitabine weekly for 6 weeks. The study found that the efficacy of both treatments was relatively similar, but gemcitabine was associated with significantly fewer side effects compared to BCG (13.6% for gemcitabine versus 44.8% for BCG). In a controversial Phase II study [13] conducted by the Southwest Oncology Group, the effectiveness of gemcitabine was assessed. In this study, 47 patients received 2 g of gemcitabine weekly for 6 weeks, followed by monthly treatments for a year. Despite this regimen, the durable response rate remained below 30% even after 12 months. Gemcitabine presents a promising alternative for patients with BCG failure, especially for those seeking a less toxic treatment option. When administered intravesically, gemcitabine generally has fewer side-effects compared to valrubicin. Although both drugs demonstrate similar efficacy, gemcitabine is considered more versatile for NMIBC due to its broader mechanism of action. Exploring the combination of gemcitabine with other drugs has been on rise and could provide new opportunities for developing more effective and long-lasting treatments for NMIBC.
Docetaxel significantly affects bladder cancer by stabilizing microtubules, which are made up of tubulin units that help maintain cell structure and shape. This stabilization interferes with the normal dynamic process of microtubule assembly and disassembly, thereby stopping cell division and inhibiting cancer cell proliferation [36]. Additionally, docetaxel binds to the apoptosis-inhibiting protein B-cell leukemia 2 (Bcl2), promoting cancer cell death. In a study conducted by Shantharam et al. [12], 13 patients received docetaxel chemotherapy at a dose of 75 mg. The recurrence-free survival rates were 75% at 6 months, 50% at both 12 and 18 months, and 25% at 24 months. Additionally, a separate group of 54 patients who received weekly docetaxel treatments for 6 weeks had recurrence-free survival rates of 40% at one year and 25% at three years [37]. Docetaxel demonstrates a promising initial response in treatment, but the recurrence-free survival rate significantly declines over time as tumor cells develop resistance to the drug. Further research is necessary to clarify the mechanisms behind this resistance, particularly since docetaxel works by stabilizing microtubules. Understanding these resistance mechanisms could pave the way for developing combination therapies that leverage docetaxel’s initial effectiveness while addressing and overcoming resistance.
Combining gemcitabine and mitomycin C takes advantage of their complementary mechanisms to enhance cancer cell eradication. Gemcitabine incorporates into DNA, inhibiting DNA synthesis and blocking ribonucleotide reductase, while mitomycin C induces apoptosis by cross-linking DNA. This synergistic approach allows both drugs to work together more effectively against cancer cells [11, 38]. Breyer et al. [39] administered a combination of gemcitabine (1000 mg in 50 ml sterile water) followed by mitomycin C (40 mg in 20 ml sterile water) to 10 patients who were resistant to BCG. After a median follow-up of 26.5 months, the study reported that 6 patients (60%) remained recurrence-free, while 4 patients (40%) experienced tumor recurrence. Additionally, a multi-institutional analysis [40] involving 47 patients evaluated this combination therapy, with 1 g of gemcitabine administered for 90 minutes followed by 40 mg of mitomycin C for 90 minutes. The results showed that 30% of patients remained recurrence-free after a median follow-up of 26 months. Gemcitabine and mitomycin C are generally well-tolerated, with only mild urinary symptoms, such as rashes, which typically resolve with treatment. However, the recurrence-free rate for this combination therapy does not match the success rates of other NMIBC treatments. Although the combined approach was anticipated to reduce the likelihood of cancer cells developing resistance, its overall success rate remains modest. Due to its limited efficacy, there are currently no ongoing studies investigating this treatment.
Sequential intravesical gemcitabine and docetaxel, first described in 2015, has emerged as an effective and well-tolerated therapy for patients who experience BCG failure. This approach leverages the distinct mechanisms of both drugs, allowing them to complement each other in targeting and treating tumor cells. By combining gemcitabine's ability to inhibit DNA synthesis and docetaxel's stabilization of microtubules, this regimen enhances the overall therapeutic effect against bladder cancer. A retrospective study conducted by Steinberg et al. [15] assessed the sequential treatment of gemcitabine and docetaxel. In this study, 45 predominantly male patients with BCG failure received 6 weekly instillations of 1 g of gemcitabine in 50 ml of sterile water, followed immediately by 37.5 mg of docetaxel in 50 ml of saline. The treatment success rates were 66% at the first surveillance, 54% at one year, and 34% at two years. Another retrospective study [41] evaluated the efficacy and safety of sequential gemcitabine and docetaxel treatment in 60 patients with BCG failure over a 6-week period. The treatment success rates, defined as the absence of recurrence, progression, cystectomy, or death, were 83% at the first surveillance, 69% at one year, and 55% at two years. Although the success rate decreases over time, the combination of gemcitabine and docetaxel shows reasonable efficacy as an alternative treatment after BCG failure. The initial effectiveness of docetaxel, along with gemcitabine's dual mechanism of action, contributes to this outcome. However, the limitations of small sample sizes and short follow-up periods in both studies underscore the need for further research to confirm the treatment's efficacy and to explore its broader applicability.
Paclitaxel, similar to docetaxel, is a taxane drug that stabilizes microtubules, thereby disrupting cell division—a critical process for cancer cell survival and proliferation [42]. By inhibiting microtubule dynamics, paclitaxel induces apoptosis in cancer cells, effectively slowing or halting disease progression. A Phase II study assessed the impact of paclitaxel on 28 NMIBC patients who had experienced recurrence and failed at least one course of BCG [43]. After treatment with 500 mg/100 ml of paclitaxel, 36% of the patients achieved a complete response, providing promising prospects for managing NMIBC in patients with extensive prior treatments. The impact of paclitaxel was also evaluated in an additional 28 patients, resulting in a 35.7% complete response rate immediately after treatment [44]. Paclitaxel exhibited low toxicity, and the complete response rate remained favorable even after a one-year follow-up. While paclitaxel provides a viable treatment option by targeting microtubules similar to docetaxel, it is less potent, which accounts for the differences in efficacy between the two drugs. Current research is investigating the potential role of paclitaxel in treating NMIBC utilizing various carrier molecules. Exploring the combination of paclitaxel and docetaxel as a therapy could be a promising future direction, as their combined use may enhance microtubule stabilization and improve treatment outcomes.
In summary, valrubicin, gemcitabine, docetaxel, and paclitaxel each hold promise as treatments for NMIBC, though their efficacy and success rates differ. Despite initial successes, challenges such as drug resistance and long-term effectiveness remain. Exploring combination therapies, such as gemcitabine and docetaxel, could offer a promising alternative with potentially improved long-term outcomes compared to BCG. Additionally, investigating the combination of docetaxel and paclitaxel is a potential future direction, as this approach may enhance microtubule stabilization and provide a more durable impact on NMIBC patients.
Immunotherapy
For NMIBC, immunotherapy represents a promising treatment option due to the high mutation rate in tumor cells, which can trigger a strong immune response [45-47]. Immune checkpoint inhibitors such as pembrolizumab, atezolizumab, durvalumab, and nivolumab play a crucial role in enhancing this immune response. These inhibitors work by blocking specific proteins that tumors use to evade the immune system. For example, following BCG infection, the immune evasion protein programmed death-ligand 1 (PD-L1) is often upregulated on T cells. Immune checkpoint inhibitors target and block PD-L1, thereby enhancing the immune response against NMIBC.
Pembrolizumab administered intravenously represents a promising non-surgical alternative for treating NMIBC. Pembrolizumab is a humanized monoclonal antibody that targets PD-1, blocking its interaction with PD-L1 and thereby enhancing the immune response by activating T-cells. The PD-1 receptor on T-cells, when bound to its ligands, such as PD-L1, inhibits T-cell activity and allows tumor cells to evade anti-tumor immunity. By preventing this interaction, pembrolizumab helps to restore T-cell activity and promote a more effective immune response against cancer cells. [48] The KEYNOTE-057 study [17, 49], conducted across 54 sites in 14 countries, assessed the efficacy of pembrolizumab in NMIBC patients resistant to BCG. The study involved 101 eligible patients who received 200 mg of pembrolizumab intravenously every 3 weeks for up to 24 months or until disease recurrence. The results showed a 41% complete response rate after 3 months, with 46% of responders remaining disease-free for 12 months or longer. Grade 3 or 4 treatment-related adverse effects were observed in 13% of patients, while 8% experienced serious events. The trial is ongoing to further explore pembrolizumab's role in NMIBC treatment. A phase I trial conducted by Meghani et al. [50] investigated the effects of intravesical administration of pembrolizumab in 9 NMIBC patients, exploring an under-researched method of treatment. Although the trial was halted due to COVID-19, the results showed 67% recurrence-free rates at 6 months and 22% at 1 year. The treatment was associated with mild side-effects and one potentially treatment-related death. Pembrolizumab effectively blocks PD-1, inducing a durable immune response with good tolerability. It offers potential for positive long-term outcomes, addressing a common challenge in intravesical chemotherapy. Intravesically delivered pembrolizumab also shows safety and feasibility and can evoke a lasting immune response. Both delivery methods are currently under further investigation, but intravenous administration remains the primary focus, as the drug's FDA approval was based on intravenous clinical studies.
Nivolumab is a fully human monoclonal antibody that, like pembrolizumab, targets PD-1 to keep T-cells active and capable of destroying cancer cells [51]. The phase II CheckMate 9UT study evaluated the efficacy of nivolumab both as a monotherapy and in combination with BCG [52]. The study found that nivolumab offers promising survival rates with minimal side-effects, such as fatigue. While nivolumab has been approved for muscle-invasive bladder cancer, its role in treating NMIBC is still under investigation. Despite both drugs blocking the same receptor, pembrolizumab has more clinical evidence supporting its efficacy, which may be attributed to differences in their molecular structures. Future studies comparing nivolumab and pembrolizumab could provide valuable insights into the effectiveness of PD-1 immune checkpoint inhibitors.
Atezolizumab is a humanized monoclonal antibody that targets PD-L1, preventing it from binding to its receptor PD-1 [53]. By blocking this interaction, atezolizumab facilitates an immune response by allowing T-cells to remain active and uninhibited, thereby enhancing their ability to target and destroy cancer cells. A single-arm phase II trial evaluated patients receiving atezolizumab intravenously every 3 weeks for a year [54]. The study found a 27% complete response rate after 6 months and a 49% event-free survival rate at 18 months. However, 90.2% of patients experienced adverse events during the trial. Another study assessed the effects of atezolizumab alone versus in combination with BCG in patients who were unresponsive to BCG therapy [55]. The study found that 33% of patients in the atezolizumab-only group experienced severe side-effects, whereas no severe side-effects were reported in the combination group. Six months after treatment, the complete response rate was 33% for the atezolizumab-only group and 42% for the combination group. While atezolizumab is a promising therapy, it has shown moderate efficacy and is associated with serious side-effects, making it crucial to weigh its benefits against potential harms. The combination of atezolizumab with BCG demonstrated a higher success rate and fewer side-effects compared to atezolizumab alone. This finding underscores the need for further research into combination therapies, such as pairing atezolizumab with other treatments like chemotherapy and BCG, to improve efficacy, reduce toxicity, and enhance disease-free rates.
Similar to atezolizumab, a fully human monoclonal antibody, targets PD-L1 to block its interaction with the PD-1 receptor [56]. By preventing this binding, durvalumab helps to reactivate T-cells, thereby enhancing the immune response and potentially reducing tumor progression. A phase II study assessed the effectiveness of 1500 mg of durvalumab administered intravenously every 4 weeks for 12 months in 17 patients who were unresponsive to BCG [57]. The study found a 12% complete response rate at 6 months, and 41% of patients experienced immune-related adverse events. The observed resistance to durvalumab may be attributed to challenges with the immune system's ability to fully restore its activity and effectively target cancer cells, a phenomenon known as incomplete activation. Although both atezolizumab and durvalumab target the same ligand, there is less clinical research supporting the efficacy of durvalumab. Further investigation into durvalumab, particularly when administered intravesically, could help address systemic side-effects and improve its therapeutic potential. Additionally, studying durvalumab in combination with other checkpoint inhibitors or treatments might provide clues to potential resistance issues and enhance treatment outcomes.
In summary, immune checkpoint inhibitors—such as pembrolizumab, nivolumab, atezolizumab, and durvalumab—show promise for treating NMIBC. Future research should focus on comparative studies to evaluate the relative efficacy of different checkpoint inhibitors and explore combination therapies involving immunotherapy, chemotherapy, BCG, or radiation. Additionally, investigating intravesical delivery methods for immune checkpoint inhibitors could help mitigate adverse side-effects. Identifying and analyzing biomarkers associated with immunotherapy responses may further enhance patient outcomes and optimize treatment strategies.
Antibody-drug conjugates
Antibody-drug conjugates (ADCs) are complex molecules composed of an antibody, a linker, and a cytotoxic drug. These ADCs bind to specific antigens on the surface of cancer cells. Once internalized in the cell, the cytotoxic drug is released and exerts its toxic effects, leading to the destruction of the cancer cells [58]. Vicinium is a fusion protein designed to target cancer cells by binding to the overexpressed epithelial cell adhesion molecule (EpCAM) on their surface [59]. Once internalized in the cancer cell, vicinium releases the cytotoxic agent Pseudomonas exotoxin A, which inhibits protein synthesis and leads to cell death. Kowalski et al [60]. evaluated the efficacy and tolerability of vicinium in 46 bladder cancer patients. The study found that the complete response rate at 3 months was 44%, and the disease-free rate was 16%, indicating promising results. A phase III trial evaluated vicinium administered intravesically twice a week for 6 weeks, followed by weekly doses for an additional 6 weeks. The trial demonstrated a 40% complete response rate at 3 months and a 52% disease-free rate at 12 months, further supporting the protein's efficacy. However, despite these promising results, vicinium was not approved by the FDA in 2021 due to insufficient clinical data, leading to a decrease in research interest regarding its potential for treatment of NMIBC [61].
The recently FDA-approved protein N-803 binds to the IL-15 receptor on the surface of CD8+ T cells and NK cells [62]. This binding activates and enhances the production of these immune cells, which are crucial for immune responses. The increased and activated immune cells are then better equipped to detect and destroy cancer cells. The approval of N-803 was supported by data from the phase II/III Quilt-3.032 study, which investigated its use in combination with BCG for patients with high-grade NMIBC, specifically carcinoma in situ (CIS), who were unresponsive to BCG alone [63]. The study reported a complete response rate of 71% after a median follow-up of 26.6 months, highlighting the protein's significant efficacy. Another study explored the effects of combining BCG with N-803 on 9 patients. In this study, patients received weekly doses of 50 mg of BCG for 6 weeks, along with escalating doses of N-803 (100, 200, or 400 micrograms per installation) [64]. Remarkably, after 6 years of treatment, 100% of the patients remained disease-free. Given the risks and benefits of combining BCG with N-803, the favorable outcomes make it a compelling option. The clinical trials and data demonstrate that the combination is both significantly effective and durable. Compared to vicinium, which was not granted FDA approval, N-803 has proven to be a more effective and promising treatment for NMIBC. ADCs like vicinium and N-803 target cancer cells using distinct mechanisms, offering varying levels of efficacy while minimizing damage to normal tissues [65]. Future research should focus on elucidating the specific mechanisms of ADCs, particularly vicinium, to enhance their effectiveness and optimize treatment strategies. Understanding these pathways in greater detail could lead to improved designs and more effective therapies.
Device-assisted therapy
Device-assisted therapies offer innovative methods to enhance the effectiveness of cancer treatments by using physical devices to improve drug delivery or activation [66]. Chemohyperthermia, electromotive drug administration, and photodynamic therapy are examples of device-assisted therapies for NMIBC.
Chemohyperthermia combines chemotherapy with localized heat to enhance the treatment of bladder cancer. This approach aims to improve drug efficacy and reduce recurrence rates by leveraging the synergistic effects of heat and chemotherapy. Mitomycin C has demonstrated lower efficacy and a higher recurrence rate compared to other chemotherapy drugs such as gemcitabine, as evidenced by a Phase III trial [67]. To address this limitation, mitomycin C is often administered through device-assisted therapies such as chemohyperthermia [68]. This technique involves delivering the chemotherapy drug and then applying localized heat to the tumor, typically raising the temperature to 40-44°C. Methods for applying this heat include intravesical microwave-induced heating, conductive heating, or loco-regional heating [69]. The elevated temperature increases the permeability of cancer cell membranes, allowing the drug to penetrate more effectively. Additionally, the heat enhances the cytotoxic effects of the chemotherapy drug, thereby improving its ability to kill cancer cells. Chemohyperthermia with mitomycin C was evaluated against BCG in an open-label, multicenter trial [70]. The results showed that the device-assisted therapy achieved a recurrence-free survival rate of 78.1%, compared to 64.8% for BCG, highlighting the potential advantages of chemohyperthermia in improving treatment outcomes. A Phase II trial [71] compared the efficacy and safety of chemohyperthermia combined with mitomycin C versus mitomycin C alone, yielding contradictory results. At 24 months, the disease-free rates for chemohyperthermia with mitomycin C and the drug alone were 61% and 60%, respectively. While chemohyperthermia demonstrated similar efficacy to mitomycin C alone, it was associated with more short-term adverse effects and a lower treatment completion rate. While chemohyperthermia has demonstrated a higher success rate as an alternative treatment after BCG failure, it cannot yet be recommended as a standard treatment over intravesical chemotherapy. Its potential benefits are tempered by a higher incidence of short-term adverse effects and a lower treatment completion rate. Further studies exploring chemohyperthermia with mitomycin C and other drugs are needed to provide more definitive conclusions and assess its overall efficacy and safety in treating NMIBC.
Electromotive drug administration (EMDA) is a technique designed to enhance the delivery and efficacy of chemotherapy drugs like mitomycin C [72, 73]. The process involves inserting a catheter into the bladder and applying electric currents to facilitate the movement of the drug through the bladder lining. This electrical stimulation increases the drug's concentration at the site of the cancer cells, potentially improving its effectiveness in treating NMIBC. Busseto et al. [74] conducted a study on 80 NMIBC patients, comparing outcomes between those who received a second round of BCG alone and those who received a second round of BCG combined with EMDA of mitomycin C. After a median follow-up of 38 months, the results showed that the group treated with EMDA and mitomycin C had a higher recurrence-free survival rate compared to the group that received only BCG. This suggests that EMDA can enhance the efficacy of mitomycin C and potentially improve outcomes for patients with NMIBC who have failed prior BCG treatments. Another study compared the impact of EMDA of mitomycin C combined with BCG versus BCG alone [75]. The results indicated that while the NMIBC progression rate was slightly higher for the BCG-only group compared to the group receiving EMDA with mitomycin C, the difference was not statistically significant. This suggests that EMDA might offer some advantage in terms of progression rates, but further research is needed to confirm its overall efficacy and benefits in combination with BCG therapy.
In summary, device-assisted therapies such as chemohyperthermia and EMDA represent promising approaches to enhance the efficacy of treatments for NMIBC. While the outcomes are not impressively high, it shows better results compared to BCG alone. More research on these techniques compared to other types of treatments such as immunotherapy or intravesical chemotherapy could provide additional data on the efficacy and safety of EMDA. For future purposes, identifying specific patient subgroups that would benefit the most from these therapies could help in tailoring treatment plans to maximize patient outcomes.
Gene therapy
Gene therapy for NMIBC involves delivering nucleic acids to cancer cells to modify their genes and produce therapeutic effects [76]. The manipulation of genes through nadofaragene firadenovec or CG0070 is conducted to produce therapeutic benefits for cancer patients.
Nadofaragene firadenovec is engineered to treat NMIBC by leveraging the immune system's capabilities. The therapy labeled as nadofaragene firadenovec uses a viral vector to deliver the gene encoding interferon alpha-2b (IFN-a2b) protein directly into tumor cells within the bladder [77]. This protein activates various immune cells to target and attack cancer cells, stimulates the production of cytokines, block angiogenesis, and induces apoptosis (programmed cell death) in cancer cells, leading to their destruction. In a phase III trial [78], 151 patients received 75 ml of nadofaragene firadenovec at the start, and then at 3, 6, and 9 months to evaluate its effect on patient outcomes. At the 3-month mark, 53.4% of patients exhibited a complete response. Of those who responded completely at 3 months, 45.5% maintained their complete response at 12 months. The most frequently reported drug-related side effect was micturition urgency, with no treatment-related deaths occurring. This study's findings led to the FDA approval of nadofaragene firadenovec in 2022 [79]. This gene therapy offers a promising alternative for patients who have failed BCG treatment, with several advantages over traditional options. Its recent approval underscores its effectiveness, but further research is needed to validate its long-term benefits.
The CG0070 adenovirus, which carries a granulocyte-macrophage colony-stimulating factor (GM-CSF) gene, targets cancer cells with dysfunctional retinoblastoma (RB) protein [80]. This therapy stimulates the immune system to generate anti-tumor responses. In a phase II trial [81], involving 45 patients with carcinoma in situ, CG0070 demonstrated a notable 50% complete response rate at 6 months with manageable toxicity. Current research is ongoing to assess the safety of this promising treatment, under clinical trial number NCT06253845. The encouraging results from previous studies, combined with ongoing investigations, highlight CG0070's potential as a viable option for treating NMIBC. Expanding research could offer a less toxic alternative for patients who prefer not to undergo or are ineligible for radical cystectomy, paralleling the recent acceptance of nadofaragene firadenovec. In conclusion, gene therapy represents a promising advancement in the treatment of NMIBC, demonstrating encouraging results. Ongoing research into nadofaragene firadenovec and CG0070 could further solidify their roles, potentially leading to widespread endorsement by clinicians.
Radiotherapy
While radiotherapy is a well-established cancer treatment, its application in managing NMIBC has not been extensively studied. However, both external beam radiotherapy and brachytherapy have shown promising results in specific circumstances.
External beam radiotherapy is used in patients who have failed BCG or intravesical chemotherapy [82]. This method employs advanced imaging technologies to direct x-rays or protons precisely at the bladder, minimizing damage to surrounding normal tissues. Current research is evaluating the effects of intravesical durvalumab alone, durvalumab combined with BCG, and durvalumab combined with external beam radiotherapy [83]. The 3-month complete response rates for these treatments are 33%, 85%, and 50%, respectively. These results highlight the potential of external beam radiotherapy, though other treatment combinations currently show higher efficacy. Despite its relatively lower response rate compared to other treatments, external beam radiotherapy holds promise for improved outcomes when used in conjunction with other drugs and therapies.
Brachytherapy involves placing a radioactive source directly in the bladder to target cancerous cells [84]. This method allows for higher doses of radiation to be delivered to the tumor while minimizing exposure to surrounding tissues. The radiation damages the DNA of cancer cells, leading to apoptosis. A study comparing brachytherapy with and without external beam radiation to radical cystectomy followed 301 patients over 28 years [85]. With a median follow-up of 10 years, disease-specific survival rates were 67% for brachytherapy and 65% for radical cystectomy. While the outcomes are similar, brachytherapy shows a slightly better result than surgery. Although radiotherapy is not commonly used for NMIBC, combining it with other treatments, such as intravesical chemotherapy, could yield significant benefits.
|
Table 3. Treatment options post-BCG failure for NMIBC. |
|||||
|
Type of Treatment/ Therapy |
Variable |
Route of Administration |
FDA Approval |
Mechanism of Action |
Ref. |
|
Intravesical chemotherapy |
Valrubicin |
Intravesical |
Yes |
Arrests the cell cycle in the G2 phase by inhibiting topoisomerase II and the fusion of nucleotides in DNA |
10 |
|
Intravesical chemotherapy |
Gemcitabine |
Intravesical |
No |
Causes apoptosis in tumour cells by blocking ribonucleotide reductase and inhibiting DNA synthesis |
11 |
|
Intravesical chemotherapy |
Docetaxel |
Intravesical |
No |
Inhibits certain proteins and microtubule breakdown which prevents cell division, leading to apoptosis |
12 |
|
Intravesical chemotherapy |
Gemcitabine + Mitomycin C |
Intravesical |
No |
Mitomycin c is administered after gemcitabine |
13,14 |
|
Intravesical chemotherapy |
Gemcitabine + Docetaxel |
Intravesical |
No |
Docetaxel is administered after gemcitabine |
15 |
|
Intravesical chemotherapy |
Paclitaxel |
Intravesical |
No |
Stabilizes microtubules to prevent cell division, leading to apoptosis |
16 |
|
Immunotherapy |
Pembrolizumab |
Intravenous |
Yes |
Binds to programmed cell death 1 receptor to prevent the deactivation of T-cells |
17 |
|
Immunotherapy |
Nivolumab |
Intravenous |
No |
Targets programmed cell death 1 receptor to maintain T-cell activation |
18 |
|
Immunotherapy |
Atezolizumab |
Intravenous |
No |
Attaches to protein programmed death-ligand 1 to trigger an immune response |
19 |
|
Immunotherapy |
Durvalumab |
Intravenous + intravesical |
No |
Blocks the bing of PD-L1 with its receptor, to produce an immune response against cancer cells |
20 |
|
Device-Assisted Therapy |
chemohyperthermia |
Intravesical |
No |
The tumor is heated to make it easier for the inserted drug to kill cancer cells |
21 |
|
Device-Assisted Therapy |
Electromotive drug administration |
Catheter insertion |
No |
Applies electric currents to allow chemotherapy drugs to reach cancer cells in high concentrations |
22 |
|
Antibody-Drug Conjugates |
Vicinium |
Intravesical |
No |
Attaches to epithelial cell adhesion molecules to release cytotoxic drugs, killing cancer cells |
23 |
|
Antibody-Drug Conjugates |
N-803 |
Intravesical |
Yes |
Connects to the IL-15 receptor to activate immune cells, helping them recognize and destroy cancer cells |
24 |
|
Gene therapy |
Nadofaragene Firadenovec |
Intravesical |
Yes |
Delivers a protein-encoding gene that enhances the immune system and destroys cancer cells |
25 |
|
Gene therapy |
CG0070 |
Intravesical |
No |
Replicates in cancer cells with dysfunctional retinoblastoma protein to boost immune system and induce apoptosis |
26 |
|
Radiotherapy |
External Beam Radiotherapy |
External |
No |
Sends x-rays or protons through advanced imaging technologies to target the cancer cells in the bladder |
27 |
|
Radiotherapy |
Brachytherapy |
Internal |
No |
Radioactive source placed in bladder to damage cancer cell DNA and induce apoptosis |
28 |
 Figure 2. Intravesical chemotherapy drugs and their combinations approved or are currently being studied in bladder cancer.
Figure 2. Intravesical chemotherapy drugs and their combinations approved or are currently being studied in bladder cancer.
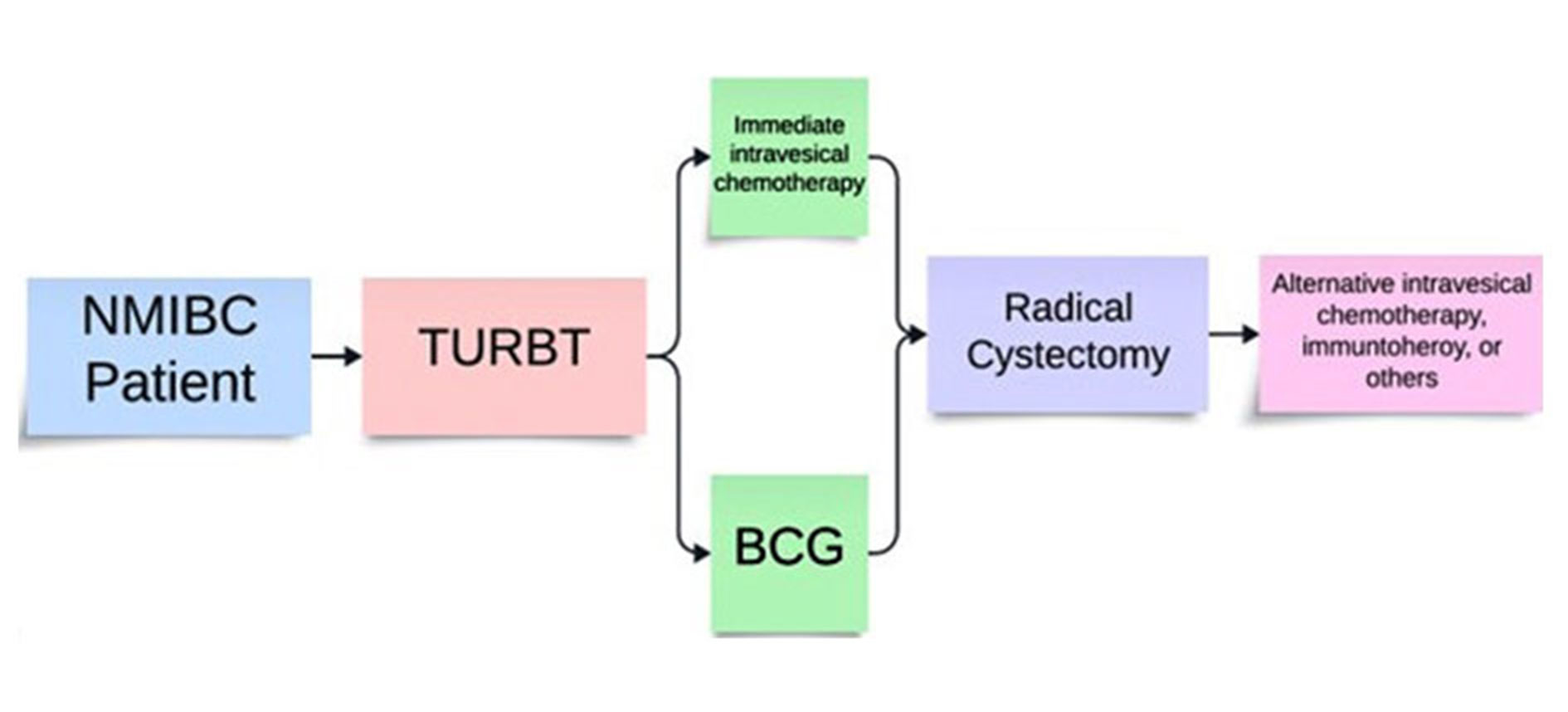 Figure 3. Recommended order of treatment for NMIBC according to NCCN guidelines.
Figure 3. Recommended order of treatment for NMIBC according to NCCN guidelines.
Clinical trials performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consents were obtained from individual participants included in the study.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
Conceptualization: AG and SG; Methodology: AG, SV, and SG; Data Curation: AG and SG; Writing to Original Draft Preparation: AG; Writing to Review & Editing: AG, SV, and SG; Visualization: AG and SG; Supervision: SG; Project Administration: SG.
Competing interests
All authors declare no competing interests.
Funding
None.
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, et al: European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol 2022, 81(1): 75-94.
- Mukherjee N, Wheeler KM, Svatek RS: Bacillus Calmette-Guérin treatment of bladder cancer: a systematic review and commentary on recent publications. Curr Opin Urol 2019, 29(3): 181-188.
- Han J, Gu X, Li Y, Wu Q: Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed Pharmacother 2020, 129: 110393.
- Kodera A, Mohammed M, Lim P, Abdalla O, Elhadi M: The Management of Bacillus Calmette-Guérin (BCG) Failure in High-Risk Non-muscle Invasive Bladder Cancer: A Review Article. Cureus 2023, 15(6): e40962.
- Kates M, Matoso A, Choi W, Baras AS, Daniels MJ, Lombardo K, Brant A, Mikkilineni N, McConkey DJ, Kamat AM, et al: Adaptive Immune Resistance to Intravesical BCG in Non-Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin Cancer Res 2020, 26(4): 882-891.
- Orihuela E, Herr HW, Pinsky CM, Whitmore WF Jr: Toxicity of intravesical BCG and its management in patients with superficial bladder tumors. Cancer 1987, 60(3): 326-333.
- Psutka SP, Barocas DA, Catto JWF, Gore JL, Lee CT, Morgan TM, Master VA, Necchi A, Rouprêt M, Boorjian SA: Staging the Host: Personalizing Risk Assessment for Radical Cystectomy Patients. Eur Urol Oncol 2018, 1(4): 292-304.
- Shibutani K, Ishikawa K, Mori N: Uncommon but Clinically Significant: Bacillus Calmette-Guerin (BCG) Infection of the Urinary Tract and its Impact on Quality of Life. Am J Case Rep 2023, 24: e940375.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Bladder Cancer. Version 4.2024. available at: www.nccn.org/patients. Accessed May 21, 2024.
- Dinney CP, Greenberg RE, Steinberg GD: Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol 2013, 31(8): 1635-1642.
- Mini E, Nobili S, Caciagli B, Landini I, Mazzei T: Cellular pharmacology of gemcitabine. Ann Oncol 2006, 17 Suppl 5: v7-12.
- Shantharam G, Amin A, Pereira J, Kott O, Mueller-Leonhard C, Mega A, Golijanin D, Gershman B: Intravesical docetaxel for high-risk non-muscle invasive bladder cancer after Bacillus Calmette-Guérin failure. Curr Urol 2021, 15(1): 33-38.
- Skinner EC, Goldman B, Sakr WA, Petrylak DP, Lenz HJ, Lee CT, Wilson SS, Benson M, Lerner SP, Tangen CM, et al: SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guérin. J Urol 2013, 190(4): 1200-1204.
- Li R, Li Y, Song J, Gao K, Chen K, Yang X, Ding Y, Ma X, Wang Y, Li W, et al: Intravesical gemcitabine versus mitomycin for non-muscle invasive bladder cancer: a systematic review and meta-analysis of randomized controlled trial. BMC Urol 2020, 20(1): 97.
- Steinberg RL, Thomas LJ, O'Donnell MA, Nepple KG: Sequential Intravesical Gemcitabine and Docetaxel for the Salvage Treatment of Non-Muscle Invasive Bladder Cancer. Bladder Cancer 2015, 1(1): 65-72.
- Mugabe C, Hadaschik BA, Kainthan RK, Brooks DE, So AI, Gleave ME, Burt HM: Paclitaxel incorporated in hydrophobically derivatized hyperbranched polyglycerols for intravesical bladder cancer therapy. BJU Int 2009, 103(7): 978-986.
- Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, Krieger LEM, Singer EA, Bajorin DF, Grivas P, et al: Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol 2021, 22(7): 919-930.
- Tomita Y, Kobayashi K, Kimura G, Oya M, Uemura H, Nishiyama H, Galsky MD, Nasroulah F, Collette S, Broughton E, et al: Adjuvant nivolumab versus placebo following radical surgery for high-risk muscle-invasive urothelial carcinoma: a subgroup analysis of Japanese patients enrolled in the phase 3 CheckMate 274 trial. Jpn J Clin Oncol 2023, 53(1): 16-25.
- Roumiguié M, Kamat AM, Bivalacqua TJ, Lerner SP, Kassouf W, Böhle A, Brausi M, Buckley R, Persad R, Colombel M, et al: International Bladder Cancer Group Consensus Statement on Clinical Trial Design for Patients with Bacillus Calmette-Guérin-exposed High-risk Non-muscle-invasive Bladder Cancer. Eur Urol 2022, 82(1): 34-46.
- Moe A, Liow E, Redfern A, Swarbrick N, Ferguson T, Davis ID, Hayne D: A phase I open label dose-escalation study to evaluate the tolerability, safety, and immunological efficacy of sub-urothelial durvalumab injection in adults with muscle-invasive or high-risk non-muscle-invasive bladder cancer (SUBDUE-1, SUB-urothelial DUrvalumab injection-1 study): clinical trial protocol. BJU Int 2021, 128 Suppl 1: 9-17.
- Liem EI, Crezee H, de la Rosette JJ, de Reijke TM: Chemohyperthermia in non-muscle-invasive bladder cancer: An overview of the literature and recommendations. Int J Hyperthermia 2016, 32(4): 363-373.
- Min JWS, Saeed N, Coene A, Adriaens M, Ceelen W: Electromotive Enhanced Drug Administration in Oncology: Principles, Evidence, Current and Emerging Applications. Cancers (Basel) 2022, 14(20): 4980.
- Fragkoulis C, Glykas I, Bamias A, Stathouros G, Papadopoulos G, Ntoumas K: Novel treatments in BCG failure. Where do we stand today? Arch Esp Urol 2021, 74(7): 681-691.
- Cody JW, Ellis-Connell AL, O'Connor SL, Pienaar E: Mathematical modeling of N-803 treatment in SIV-infected non-human primates. PLoS Comput Biol 2021, 17(7): e1009204.
- Narayan VM, Meeks JJ, Jakobsen JS, Shore ND, Sant GR, Konety BR: Mechanism of action of nadofaragene firadenovec-vncg. Front Oncol 2024, 14: 1359725.
- Grandi P, Darilek A, Moscu A, Pradhan A, Li R: Intravesical Infusion of Oncolytic Virus CG0070 in the Treatment of Bladder Cancer. Methods Mol Biol 2023, 2684: 303-317.
- Koka K, Verma A, Dwarakanath BS, Papineni RVL: Technological Advancements in External Beam Radiation Therapy (EBRT): An Indispensable Tool for Cancer Treatment. Cancer Manag Res 2022, 14: 1421-1429.
- Skowronek J: Current status of brachytherapy in cancer treatment - short overview. J Contemp Brachytherapy 2017, 9(6): 581-589.
- Lenfant L, Pinar U, Rouprêt M, Seisen T: First-line Intravesical Chemotherapy for Non-muscle-invasive Bladder Cancer: Chimera or "Ne Plus Ultra"? Eur Urol 2023, 84(1): 1-3.
- Tan WS, Steinberg G, Witjes JA, Li R, Shariat SF, Roupret M, Babjuk M, Bivalacqua TJ, Psutka SP, Williams SB, et al: Intermediate-risk non-muscle-invasive Bladder Cancer: Updated Consensus Definition and Management Recommendations from the International Bladder Cancer Group. Eur Urol Oncol 2022, 5(5): 505-516.
- Sharma P, Zargar-Shoshtari K, Sexton WJ: Valrubicin in refractory non-muscle invasive bladder cancer. Expert Rev Anticancer Ther 2015, 15(12): 1379-1387.
- Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M: Efficacy, and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol 2000, 163(3): 761-767.
- Cookson MS, Chang SS, Lihou C, Li T, Harper SQ, Lang Z, Tutrone RF: Use of intravesical valrubicin in clinical practice for treatment of nonmuscle-invasive bladder cancer, including carcinoma in situ of the bladder. Ther Adv Urol 2014, 6(5): 181-91.
- Piska K, Koczurkiewicz P, Bucki A, Wójcik-Pszczoła K, Kołaczkowski M, Pękala E: Metabolic carbonyl reduction of anthracyclines - role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents. Invest New Drugs 2017, 35(3): 375-385.
- Ansari Djafari A, Javanmard B, Razzaghi M, Hojjati SA, Razzaghi Z, Faraji S, Rahavian A, Garoosi M: Intravesical Gemcitabine versus Intravesical Bacillus Calmette-Guerin for the Treatment of Intermediate-Risk Non-Muscle Invasive Bladder Cancer: A Randomized Controlled Trial. Urol J 2023, 20(2): 123-128.
- Albany C, Sonpavde G: Docetaxel for the treatment of bladder cancer. Expert Opin Investig Drugs 2015, 24(12): 1657-1664.
- Barlow LJ, McKiernan JM, Benson MC: Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guérin therapy. J Urol 2013, 189(3): 834-839.
- Bass PD, Gubler DA, Judd TC, Williams RM: Mitomycinoid alkaloids: mechanism of action, biosynthesis, total syntheses, and synthetic approaches. Chem Rev 2013, 113(8): 6816-6863.
- Breyer BN, Whitson JM, Carroll PR, Konety BR: Sequential intravesical gemcitabine and mitomycin C chemotherapy regimen in patients with non-muscle invasive bladder cancer. Urol Oncol 2010, 28(5): 510-514.
- Lightfoot AJ, Breyer BN, Rosevear HM, Erickson BA, Konety BR, O'Donnell MA: Multi-institutional analysis of sequential intravesical gemcitabine and mitomycin C chemotherapy for non-muscle invasive bladder cancer. Urol Oncol 2014, 32(1): 35e15-9.
- Chevuru PT, McElree IM, Mott SL, Steinberg RL, O'Donnell MA, Packiam VT: Long-term follow-up of sequential intravesical gemcitabine and docetaxel salvage therapy for non-muscle invasive bladder cancer. Urol Oncol 2023, 41(3): 148.e1-148.e7.
- Alqahtani FY, Aleanizy FS, El Tahir E, Alkahtani HM, AlQuadeib BT: Paclitaxel. Profiles Drug Subst Excip Relat Methodol 2019, 44: 205-238.
- Robins DJ, Sui W, Matulay JT, Ghandour R, Anderson CB, DeCastro GJ, McKiernan JM: Long-term Survival Outcomes with Intravesical Nanoparticle Albumin-bound Paclitaxel for Recurrent Non-muscle-invasive Bladder Cancer After Previous Bacillus Calmette-Guérin Therapy, Urology 2017, 103: 149-153.
- McKiernan JM, Holder DD, Ghandour RA, Barlow LJ, Ahn JJ, Kates M, Badalato GM, Roychoudhury A, Decastro GJ, Benson MC: Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guérin treatment failure. J Urol 2014, 192(6): 1633-1638.
- Pettenati C, Ingersoll MA: Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol 2018, 5(10): 615-625.
- Lobo N, Martini A, Kamat AM: Evolution of immunotherapy in the treatment of non-muscle-invasive bladder cancer. Expert Rev Anticancer Ther 2022, 22(4): 361-370.
- Pfail JL, Katims AB, Alerasool P, Sfakianos JP: Immunotherapy in non-muscle-invasive bladder cancer: current status and future directions. World J Urol 2021, 39(5): 1319-1329.
- Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL: Pembrolizumab (Keytruda). Hum Vaccin Immunother 2016, 12(11): 2777-2789.
- Necchi A, Roumiguié M, Kamat AM, Shore ND, Boormans JL, Esen AA, Lebret T, Kandori S, Bajorin DF, Krieger LEM, et al: Pembrolizumab monotherapy for high-risk non-muscle-invasive bladder cancer without carcinoma in situ and unresponsive to BCG (KEYNOTE-057): a single-arm, multicentre, phase 2 trial. Lancet Oncol 2024, 25(6): 720-730.
- Meghani K, Cooley LF, Choy B, Kocherginsky M, Swaminathan S, Munir SS, Svatek RS, Kuzel T, Meeks JJ: First-in-human Intravesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Eur Urol 2022, 82(6): 602-610.
- Rendon A, Rayi A: Nivolumab. 2024 Feb 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2024.
- Bristol-Myers Squibb: A Phase 2, Randomized, Open-label Study of Nivolumab or Nivolumab/BMS-986205 Alone or Combined with Intravesical BCG in Participants With BCG-Unresponsive, High-Risk, Non-Muscle Invasive Bladder Cancer [Internet]. clinicaltrials.gov. 2023. available from: https://classic.clinicaltrials.gov/ct2/show/results/NCT03519256
- Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, et al: Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020, 383(14): 1328-1339.
- Black PC, Tangen CM, Singh P, McConkey DJ, Lucia MS, Lowrance WT, Koshkin VS, Stratton KL, Bivalacqua TJ, Kassouf W, et al: Phase 2 Trial of Atezolizumab in Bacillus Calmette-Guérin-unresponsive High-risk Non-muscle-invasive Bladder Cancer: SWOG S1605. Eur Urol 2023, 84(6): 536-544.
- Inman BA, Hahn NM, Stratton K, Kopp R, Sankin A, Skinner E, Pohar K, Gartrell BA, Pham S, Rishipathak D, et al: A Phase 1b/2 Study of Atezolizumab with or Without Bacille Calmette-Guérin in Patients with High-risk Non-muscle-invasive Bladder Cancer. Eur Urol Oncol 2023, 6(3): 313-320.
- Fung S, Syed YY: Durvalumab: A Review in Advanced Biliary Tract Cancer. Target Oncol 2023, 18(6): 965-972.
- Li R, Sexton WJ, Dhillon J, Berglund A, Naidu S, Borjas G, Rose K, Kim Y, Wang X, Conejo-Garcia JR, et al: A Phase II Study of Durvalumab for Bacillus Calmette-Guerin (BCG) Unresponsive Urothelial Carcinoma in Situ of the Bladder. Clin Cancer Res 2023, 29(19): 3875-3881.
- Hafeez U, Parakh S, Gan HK, Scott AM: Antibody-Drug Conjugates for Cancer Therapy. Molecules 2020, 25(20): 4764.
- Jeong SH, Ku JH: Treatment strategies for the Bacillus Calmette-Guérin-unresponsive non-muscle invasive bladder cancer. Investig Clin Urol 2023, 64(2): 103-106.
- Kowalski M, Guindon J, Brazas L, Moore C, Entwistle J, Cizeau J, Jewett MA, MacDonald GC: A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin. J Urol 2012, 188(5): 1712-1718.
- FDA Does Not Approve Vicinium for BCG-unresponsive NMIBC [Internet]. Targeted Oncology. 2021. available from: https://www.targetedonc.com/view/fda-does-not-approve-vicinium-for-bcg-unresponsive-nmibc
- Knudson KM, Hicks KC, Ozawa Y, Schlom J, Gameiro SR: Functional and mechanistic advantage of the use of a bifunctional anti-PD-L1/IL-15 superagonist. J Immunother Cancer 2020, 8(1): e000493.
- Chamie K, Chang SS, Kramolowsky EV, Gonzalgo ML, Huang M, Bhar P, Spilman P, Sender L, Reddy SK, Soon-Shiong P: Quality of Life in the Phase 2/3 Trial of N-803 Plus Bacillus Calmette-Guérin in Bacillus Calmette-Guérin‒Unresponsive Nonmuscle-Invasive Bladder Cancer. Urol Pract 2024, 11(2): 367-375.
- Rosser CJ, Tikhonenkov S, Nix JW, Chan OTM, Ianculescu I, Reddy S, Soon-Shiong P: Safety, Tolerability, and Long-Term Clinical Outcomes of an IL-15 analogue (N-803) Admixed with Bacillus Calmette-Guérin (BCG) for the Treatment of Bladder Cancer. Oncoimmunology 2021, 10(1): 1912885.
- Ponziani S, Di Vittorio G, Pitari G, Cimini AM, Ardini M, Gentile R, Iacobelli S, Sala G, Capone E, Flavell DJ, et al: Antibody-Drug Conjugates: The New Frontier of Chemotherapy. Int J Mol Sci 2020, 21(15): 5510.
- Hendricksen K: Device-assisted intravesical therapy for non-muscle invasive bladder cancer. Transl Androl Urol 2019, 8(1): 94-100.
- Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, Miragliuolo A, Guarrasi R, Lanna M, Cennamo G, et al: Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol 2010, 28(4): 543-548.
- Volpe A, Racioppi M, D'Agostino D, Cappa E, Filianoti A, Bassi PF: Mitomycin C for the treatment of bladder cancer. Minerva Urol Nefrol 2010, 62(2): 133-44.
- Elias DM, Ouellet JF: Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am 2001, 10(4): 915-933.
- Arends TJ, Nativ O, Maffezzini M, de Cobelli O, Canepa G, Verweij F, Moskovitz B, van der Heijden AG, Witjes JA: Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guérin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. Eur Urol 2016, 69(6): 1046-1052.
- Tan WS, Prendergast A, Ackerman C, Yogeswaran Y, Cresswell J, Mariappan P, Phull J, Hunter-Campbell P, Lazarowicz H, Mishra V, et al: Adjuvant Intravesical Chemohyperthermia Versus Passive Chemotherapy in Patients with Intermediate-risk non-muscle-invasive Bladder Cancer (HIVEC-II): A Phase 2, Open-label, Randomised Controlled Trial. Eur Urol 2023, 83(6): 497-504.
- Hashemi S, Sahai A, Malde S: Applications of electromotive drug administration in urology. Urol Ann 2020, 12(4): 301-308.
- Di Stasi SM, Liberati E, Dutto L, Verri C: Intravesical electromotive drug administration of mitomycin-C for non-muscle invasive bladder cancer. Arch Ital Urol Androl 2008, 80(4): 157-161.
- Busetto GM, Finati M, Chirico M, Cinelli F, D'Altilia N, Falagario UG, Sanguedolce F, Del Giudice F, De Berardinis E, Ferro M, et al: Conservative treatment for high-risk NMIBC failing BCG treatment: who benefits from adding electromotive drug administration (EMDA) of mitomycin C (MMC) to a second BCG induction cycle? World J Urol 2023, 41(5): 1329-1335.
- Zazzara M, Nazaraj A, Scarcia M, Cardo G, Carando R, Ludovico GM: Electromotive Drug Administration of Mitomycin C (EMDA/MMC) versus Intravesical Immunotherapy with Bacillus Calmette-Guérin (BCG) in Intermediate and High-Risk Non-Muscle Invasive Bladder Cancer. Urol Int 2023, 107(1): 64-71.
- Narayan VM, Dinney CPN: Intravesical Gene Therapy. Urol Clin North Am 2020, 47(1): 93-101.
- Martini A, Tholomier C, Mokkapati S, Dinney CPN: Interferon gene therapy with nadofaragene firadenovec for bladder cancer: from bench to approval. Front Immunol 2023, 14: 1260498.
- Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, Bivalacqua TJ, Montgomery JS, Lerner SP, Busby JE, et al: Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol 2021, 22(1): 107-117.
- Lee A: Nadofaragene Firadenovec: First Approval. Drugs 2023, 83(4): 353-357.
- Fukuhara H, Ino Y, Todo T: Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci 2016, 107(10): 1373-1379.
- Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL 3rd, Clark W, Kroeger M, Dumbadze I, Chamie K, et al: An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol 2018, 36(10): 440-447.
- Bostrom PJ, Soloway MS, Manoharan M, Ayyathurai R, Samavedi S: Bladder cancer after radiotherapy for prostate cancer: detailed analysis of pathological features and outcome after radical cystectomy. J Urol 2008, 179(1): 91-95.
- Hahn NM, O'Donnell MA, Efstathiou JA, Zahurak M, Rosner GL, Smith J, Kates MR, Bivalacqua TJ, Tran PT, Song DY, et al: A Phase 1 Trial of Durvalumab in Combination with Bacillus Calmette-Guerin (BCG) or External Beam Radiation Therapy in Patients with BCG-unresponsive Non-Muscle-Invasive Bladder Cancer: The Hoosier Cancer Research Network GU16-243 ADAPT-BLADDER Study. Eur Urol 2023, 83(6): 486-494.
- Voskuilen CS, Bosschieter J, van Werkhoven E, Hendricksen K, Vis AN, Witteveen T, Pieters BR, Burger M, Bex A, van der Poel HG, et al: Long-term survival and complications following bladder-preserving brachytherapy in patients with cT1-T2 bladder cancer. Radiother Oncol 2019, 141: 130-136.
- Bos MK, Marmolejo RO, Rasch CR, Pieters BR: Bladder preservation with brachytherapy compared to cystectomy for T1-T3 muscle-invasive bladder cancer: a systematic review. J Contemp Brachytherapy 2014, 6(2): 191-199.
- Jarow JP, Lerner SP, Kluetz PG, Liu K, Sridhara R, Bajorin D, Chang S, Dinney CP, Groshen S, Morton RA, et al: Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: report of a Food and Drug Administration and American Urological Association public workshop. Urology 2014, 83(2): 262-264.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript