Review Article | Open Access
The Application of Exosomes in the Diagnosis and Treatment of Renal Cell Carcinoma
Xian Zhao1, Jian Gong11Research Group of Jian Gong on Pharmacoepidemiology and Clinical Drug Evaluation, School of Life Science and Biopharmaceutics, Shenyang Pharmaceutical University, Shenyang, China.
Correspondence: Jian Gong (School of Life Science and Biopharmaceutics, Shenyang Pharmaceutical University, P.O.Box 88, No.103 Wenhua Road, Shenyang, 110016, P.R. China; Email: gongjian@syphu.edu.cn).
Annals of Urologic Oncology 2023, 6(2): 47-53. https://doi.org/10.32948/auo.2023.05.16
Received: 20 April 2023 | Accepted: 16 May 2023 | Published online: 17 May 2023
Renal cell carcinoma (RCC) is an epithelial tumor originating from the proximal renal tubule of the urinary system. RCC is one of the most common and deadly tumors representing clear cell renal cell carcinoma (ccRCC) (about 75%) as major subtype characterized by high incidence and poor prognosis. There are no obvious clinical symptoms in the early stage ccRCC, but are readily visible in the late stage with >30% patients diagnosed with diffusion and metastasis. The incidence of RCC is higher in males than females and frequent in developed countries than in developing ones. The current treatment for rRCC mainly focuses on surgical excision and chemotherapy, however, it still suffers from high recurrence and poor survival, due to metastatic growth and insensitivity to radiotherapy and chemotherapy. Early diagnosis of RCC is very important and remains a top priority for its treatment. Exosomes are small vesicles with a lipid bilayer membrane structure that are actively secreted by normal and cancer cells in the body, containing bioactive substances such as proteins, nucleic acids, and lipids. The detection of extracellular vesicles in body fluids can play an important role in the early diagnosis of RCC. This article reviews the role of exosomes in the diagnosis and treatment of RCC.
Key words renal cell carcinoma, renal tumors, exosomes, diagnosis
Participate in the construction of tumor microenvironment
Tumor microenvironment is closely related to the biological function of tumor cells, similar to the relationship between seed and soil. Changes in tumor microenvironment can affect proliferation, apoptosis and invasion of tumor cells. With the advancement in studies on cancers, the tumor microenvironment has attracted the attention of researchers and scientists. The effects of tumor microenvironment on malignant cells can be divided into cellular components and non-cellular components [12,13]. In addition to immune cells, some related molecules of the tumor microenvironment also affect the biological behavior of tumor cells [14-16]. Primary tumors can release some biological factors to the areas of preferential metastasis and participate in the formation of metastatic microenvironment. Some studies have shown that exosomes can build a bridge between cells and the microenvironment and can transfer relevant information from the microenvironment to the cell through exosomes. Besides, exosomes can also transmit the genetic signals from the cell to the surrounding microenvironment, maintaining signal communication between cells and the surrounding microenvironment [17]. Fu et al. found that exosomes derived from renal tumor cells can promote cell proliferation and inhibit apoptosis through ERK5 signaling pathway in anoxic environment [17-19]. Exosomes also show certain organ and histiocyte tropism, and this tendency is related to the expression of integrin on the exocrine body surface. The initiation process of ecological niche formation before metastasis is very complex, involving the combination of exosomes secreted by cancer cells and stromal cells, leading to the reprogramming of target cells and the activation of important signal transduction pathways. Finally, the target cells develops the microenvironment before tumor spread, providing a prerequisite for tumor metastasis.
Advantage angiogenesis
Cancer stem cells promote angiogenesis by differentiating endothelial cells. MiRNAs derived from exosomes secreted by tumor cells, such as miR-23a, miR-210, miR-135b, miR-494, miR-1246, and miR-9, can be transferred to endothelial cells to promote the expression of vascular endothelial growth factor, fibroblast growth factor, angiopoietin-1, and accelerate the reconstruction of tumor tissue vascular network. The effect of exosomes on mesenchymal stem cells can promote tumor vascularization and proliferation by increasing the expression levels of MMP1, MMP2, MMP3, CXCR4 and CXCR7 [20]. In addition, exosomes with high expression of carbonic anhydrase IX (CAIX) are associated with vascular remodeling in renal cell carcinoma. Carbonic anhydride 9 (CA9), a hypoxic response induced by hypoxic-inducible factor (HIF1), is overexpressed in RCCs, and CA9 exosomes released by anoxic RCCs may promote angiogenesis in the tumor microenvironment [21]. The formation of a vascular network is not only crucial for the normal growth of tumor tissue, but also provides important means for tumor invasion.
Advantage immune escape
The exosomes secreted by RCC cells can not only induce immune response of T cells, but also induce apoptosis of activated T lymphocytes by activating caspase pathway, weakening cytotoxicity of natural killer cell, and reducing the production of IL-2, IFN- γ, IL-6 and IL-10 contributing to the immune escape. Exosomes derived from tumors contain FasL and PDL-1, which can activate the Fas/FasL and PD-l/PDL-1 pathways. They induce apoptosis of CD8+T cells, inhibit the immune system, and ultimately help cancer cells achieve immune escape [13]. ccRCC derives TGF-β-rich exosomes, which impair the function of TINK cells by activating the TGF-β/SMAD pathway and evade natural immune surveillance, leading to dysfunction of NK cells [22]. The aggregation of myeloid derived suppressor cells (MDSCs) in the tumor microenvironment is one of the most important factors for immune escape. It has been found that Hsp70 is abandunt in RCC exosomes, which can aggregate tumor growth factors and proinflammatory cytokines, inducing the expression of MDSCs through the p-STAT3 pathway promoting tumor growth [14].
Participation in cancer cell invasion and metastasis
The exosomes can change the function of target cells through the transport substance. The tumor-derived exosomes affect urothelium cells and lead to epithelial mesenchymal transformation, which is an important step of tumor metastasis. Renal cancer stem cell exosomes promote EMT migration and invasion, accelerate the proliferation of ccRCC cells in vivo and lung metastasis [23], and the released exosomes promote the formation of lung metastasis through the up-regulation of MMP2, MMP9 and VEGF receptors by lung endothelial cells. Study further show that miR-19a, miR-19b, miR-650 and miR-151 were associated with tumor invasion and metastasis [20]. In addition, miR-25-3p released by exosomes transfers to vascular endothelial cells, leading to increase of the vascular permeability and promoting tumor metastasis.
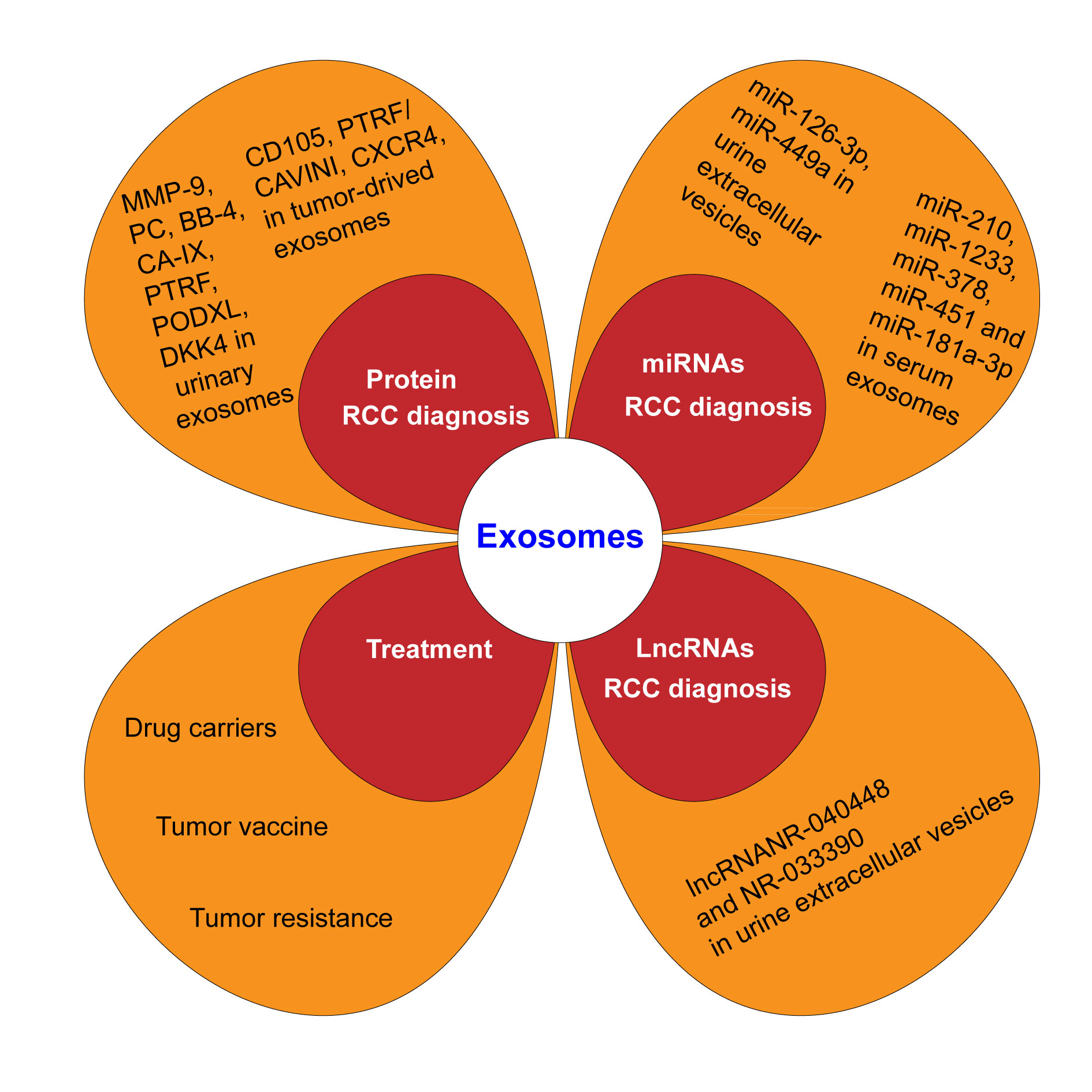 Figure 1. Exosome on diagnosis and treatment of renal cell carcinoma (RCC).
Figure 1. Exosome on diagnosis and treatment of renal cell carcinoma (RCC).
Long chain non coding RNAs (ln cRNAs)
LncRNAs, are RNAs with a length greater than 200 nucleotides thatlack protein encoding ability. Malignant cells can express specific lncRNA markers, indicating that lncRNA can serve as a specific marker for diseases, which is of great significance for the diagnosis of cancer. The presence of lncRNAs in exosomes is particularly abundant, and exosomes can protect the nucleic acids from enzymatic decomposition; Similar to miRNA, lncRNA also plays an important role in the growth, proliferation, invasion, and metastasis of cancer cells. The differential expression of lncRNANR-040448 and NR-033390 in urine extracellular vesicles is most significant, with sensitivity and specificity exceeding 70%, indicating that lncRNA can serve as a potential diagnostic marker for renal cell carcinoma [24].
MicroRNAs (miRNAs)
MiRNAs are small endogenous noncoding RNAs (18-24 nucleotides) in the process of gene post transcriptional protein translation, which are involved in the tumorigenesis and development processes. The miRNA in urine can be produced from various tissues and organs of the urinary system, including tumor tissue, normal urothelium tissue, renal parenchyma, etc. Exosome can be transferred between cells that contains a variety of bioactive components such as miRNA, mRNA, and protein, and is an important intercellular communication molecule. Exosome transport-specific miRNA may play an important role in the pathogenesis of tumors. It has great application foreground in the pathogenesis, diagnosis and treatment of RCC and other malignant tumors of urinary system. The detection through combining urine miR-126-3p with miR-449a can distinguish healthy individuals from RCC patients and can serve as a marker for highly sensitive ccRCC patients. Redova et al. found that the levels of exosomes miR-210, miR-1233, miR-378, and miR-451 in the blood were significantly increased in patients with ccRCC. The increase of miR-181a-3p in serum exosomes of patients with kidney cancer is extremely significant in the early stage, which can be used as a marker for its early diagnosis [25-27]. However,Fan et al.found that the level of exosomes miR-199a-3p,miR-145 were decreased in some patients with ccRCC [28, 29]. Other studies have shown a significant decrease in the expression of miR-210 and miR-1233 in the blood exosomes of renal cancer patients after surgery. Compared with healthy individuals, the expression of miR-183 is increased in kidney cancer patients, and miR-183 levels are positively correlated with poor prognosis in renal cancer patients, playing a role in promoting tumor invasion and metastasis. Combined diagnosis of miR-126-3p and miR-486-5p in exosomes can distinguish benign lesions from ccRCC [30]. miR-19a is overexpressed in renal cancer tissues and up-regulated in metastatic renal cancer and is considered as a possible prognostic marker [20]. Song et al.found significant differences in the expression of miR-30c-5p between ccRCC patients and healthy control individuals, indicating its specificity for ccRCC and potential diagnostic biomarkers for early ccRCC. The intracellular level of miR-224 in primary renal cancer cell lines is significantly up-regulated, and miR-224 in exosomes affects the prognosis of patients, may mediate cell-cell interactions and cancer invasion and metastasis, and is a potential prognostic biomarker in ccRCC patients [31, 32].
Protein
Herreros-Villanueva and Bujanda obtained 261 and 186 types of proteins by isolating the urine exosomes of normal people and renal cancer patients. However, there were not many similarities of these two groups of protein types. Among them, the expression of matrix metalloproteinase 9, podocycalyprotein, black body 4 and CA IX in urinary exosomes of patients with renal cancer is higher than that of normal individuals. Among a group of proteins detected in urinary exosomes of patients with renal cancer, 10 of them may be potential markers of renal cancer. CD105 expression is associated with poor prognosis of renal carcinoma. The high expression of CXCR4 is considered to be a novel marker of CSC, which is associated with the more aggressive and metastatic RCC [20]. The expression of PTRF/CAVINI (transcription releasing factor) is regulated by SHC1 (polymerase), which is significantly overexpressed in high-grade ccRCCS, and abnormally increased PTRF can be detected in urinary exosomes, suggesting that PTRF could be a potential marker for the diagnosis of ccRCC [33]. MMP9, PODXL, DKK4 and CAIX are highly expressed in urinary exosomes of patients with renal cancer and are not lost due to individual differences in patients with renal cancer, which can provide valuable clinical information for the diagnosis and treatment of renal cancer [32, 34].
|
Table 1. Exosome tumor markers in renal cell carcinoma. |
||||
|
Exosome contents |
Tumor biomarkers |
Expression levels |
Exosome source |
Reference |
|
miRNA |
miR-135b |
Increased |
tissue |
[20] |
|
miR-494 |
Increased |
tissue |
[20] |
|
|
miR-1246 |
Increased |
tissue |
[20] |
|
|
miR-9 |
Increased |
tissue |
[20] |
|
|
miR-19a |
Increased |
tissue |
[20] |
|
|
miR-19b |
Increased |
tissue |
[20] |
|
|
miR-650 |
Increased |
tissue |
[20] |
|
|
miR-151 |
Increased |
tissue |
[20] |
|
|
miR-25-3p |
Increased |
tissue |
[20] |
|
|
miR-126-3p |
Increased |
urine |
[25] |
|
|
miR-449a |
Increased |
urine |
[25] |
|
|
miR-1233 |
Increased |
blood |
[25] |
|
|
miR-378 |
Increased |
blood |
[25] |
|
|
miR-451 |
Increased |
blood |
[25] |
|
|
miR-181a-3p |
Increased |
blood |
[25] |
|
|
miR-199a-3p |
Decreased |
urine |
[28, 29] |
|
|
miR-486-5p |
Increased |
urine |
[30] |
|
|
miR-30c-5p |
Increased |
urine |
[31, 32] |
|
|
LncRNA |
lncRNANR-040448 |
Increased |
urine |
[24] |
|
NR-033390 |
Increased |
urine |
[24] |
|
|
Protein |
CAIX |
Increased |
plasma |
[32, 34] |
|
MMP1 |
Increased |
plasma |
[20] |
|
|
MMP2 |
Increased |
plasma |
[20] |
|
|
MMP3 |
Increased |
plasma |
[20] |
|
|
CXCR4 |
Increased |
plasma |
[20] |
|
|
CXCR7 |
Increased |
plasma |
[20] |
|
|
FasL |
Increased |
plasma |
[13] |
|
|
PDL-1 |
Increased |
plasma |
[13] |
|
|
Hsp70 |
Increased |
plasma |
[14] |
|
|
MMP9 |
Increased |
plasma |
[20] |
|
|
CD105 |
Increased |
urine |
[20] |
|
|
black body 4 |
Increased |
urine |
[20] |
|
|
podocycalyprotein |
Increased |
urine |
[20] |
|
|
Matrix metalloproteinase 9 |
Increased |
urine |
[20] |
|
|
PTRF/CAVINI |
Increased |
urine |
[33] |
|
|
PODXL |
Increased |
urine |
[32, 34] |
|
|
DKK4 |
Increased |
urine |
[32, 34] |
|
According to the characteristics of exosomes, they can serve as drug carriers for therapeutic purposes. The lipid bilayer membrane structure of exosomes can protect RNA from being degraded by RNA enzymes, and their size and deformability make it easier to penetrate the biofilm, for accurate delivery of therapeutic genes to the target cells. In addition, the size of exosomes is at the nanoscale, and changing the substances in exosomes can make them a tool for delivering drugs, antigens and genes, with significant advantages such as long half-life, targeting ability, biocompatibility and low toxicity [35]. The anti-tumor substances carried by the exosomes can not only hinder the development of bladder cancer, but also have no obvious damage to the normal urothelium, which indicates that the exosomes are ideal carriers for the treatment of urinary cancer.
Tumor vaccine
Compared with traditional vaccines, exosomes secreted by tumor cells have incomparable effects and higher affinity. Exosomes can express immunosuppressive molecules in the body, thereby reducing their immunogenicity. Zhang Yao et al. found that extracellular vesicles derived from RCC anchored interleukin-12 have more significant anti-tumor effects than that derived from normal cells. In vitro experiments, it was found that this new vaccine has stronger cytotoxic reactions, providing cogent evidence for the treatment of rRCC through extracellular vesicles. These cells can highly express RCC-associated antigen G250 and can effectively induce the body's specific immune response and CTL. This group prepared EXO/IL-12 vaccine. Exosomes produced by antigen-sensitized dendritic cells can activate tumor-specific CTL and produce strong anticyclidal activity, thus exerting anti-renal cancer effect [36]. The tumor suppressor genes loaded and transported by exosomes can inhibit tumor cell growth, providing necessary conditions for exosome-mediated targeted therapy.
Tumor resistance
Tumor resistance is one of the main reasons for clinical treatment failure. Tumor cells that do not develop drug resistance until they receive genetic information related to multidrug-resistant proteins secreted by other tumor cells in exosomes, leading to their acquisition of drug resistance. Qu et al. found that nephrotic drug-resistant cells transmit lncARSR to other cells through exosomes, inducing them to develop drug resistance; LncARSR promotes the expression of AXL/c-MET through competitive combination with miR-34/miR-449, and AXL/c-MET promotes the expression of lncARSR in the form of positive feedback, further promoting drug resistance to RCC cells. In addition, the exosomes miR-210 can mediate drug resistance in gemcitabine sensitive tumor cells. Liu et al. found that overexpression of miR-145 in hUCMSCs can be transferred to RCC cells by exosomes, improving tumor sensitivity to Sunitinib [37-44]. The above research indicates that extracellular vesicles can mediate the development of drug resistance in tumor cells, which not only provides new therapeutic targets for cancer patients, but also predicts the patient's response to anti-tumor drugs through the detection of extracellular vesicle markers, providing important reference for personalized treatment of renal cell carcinoma.
Early diagnosis of RCC is one of the key factors in improving patient survival, and the discovery of exosomes provides a huge possibility. Exosomes can be isolated from the blood, urine, and tumor tissue of RCC patients. There areseveral methods to separate and purify exosomes, and currently the most widely recognized separation method is ultracentrifugation. However, it is time-consuming and requires a number of raw materials, and there is still no reliable and simple separation method that can be applied to the clinical practice. Exosomes derived from tumor carry a large amount of substances, including proteins, nucleic acids, lipids, etc., which can change the biological behavior of target cells and promote cell growth and development. Due to the advantage of simple and fast extraction of exosomes from urine and blood, tumor derived exosomes have incomparable potential in the early diagnosis of RCC. However, the main focus of research is on renal clear cell carcinoma currently, and most of them are retrospective studies. If promoted and applied to clinical practice, it is necessary to combine more extensive research with clinical trials.
We thank Dr. Sanjay Gupta (Case Western Reserve University & UH Cleveland Medical Center) for his proofreading for the review.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
ZX designed the study and was responsible for the writing of the original draft. GX edited and approved the final manuscript.
Competing interests
All authors declare no competing interests.
Funding
None.
- Inamura K: Renal Cell Tumors: Understanding Their Molecular Pathological Epidemiology and the 2016 WHO Classification. Int J Mol Sci 2017, 18(10): 2195.
- Cocucci E, Racchetti G, Meldolesi J: Shedding microvesicles: artefacts no more. Trends Cell Biol 2009, 19(2): 43-51.
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C: Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987, 262(19): 9412-9420.
- Simons M, Raposo G: Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009, 21(4): 575-581.
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M: Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319(5867): 1244-1247.
- Raposo G, Stoorvogel W: Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013, 200(4): 373-383.
- Minciacchi VR, Freeman MR, Di Vizio D: Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 2015, 40: 41-51.
- Matei I, Kim HS, Lyden D: Unshielding Exosomal RNA Unleashes Tumor Growth And Metastasis. Cell 2017, 170(2): 223-225.
- Sahoo S, Mathiyalagan P, Hajjar RJ: Pericardial Fluid Exosomes: A New Material to Treat Cardiovascular Disease. Mol Ther 2017, 25(3): 568-569.
- Sarko DK, McKinney CE: Exosomes: Origins and Therapeutic Potential for Neurodegenerative Disease. Front Neurosci 2017, 11: 82.
- Tomiyama T, Yang GX, Zhao M, Zhang W, Tanaka H, Wang J, Leung PS, Okazaki K, He XS, Lu Q: The modulation of co-stimulatory molecules by circulating exosomes in primary biliary cirrhosis. Cell Mol Immunol 2017, 14(3): 276-284.
- Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N: Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy. Int J Mol Sci 2019, 20(13): 3212.
- Meng W, Xue S, Chen Y: The role of CXCL12 in tumor microenvironment. Gene 2018, 641: 105-110.
- Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, et al: Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun 2018, 9(1): 191.
- Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E, Wang H, Chen Y, Liu K, Shao Z, et al: Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res 2018, 37(1): 242.
- Yang SS, Ma S, Dou H, Liu F, Zhang SY, Jiang C, Xiao M, Huang YX: Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp Cell Res 2020, 391(2): 111983.
- Ludwig N, Whiteside TL, Reichert TE: Challenges in Exosome Isolation and Analysis in Health and Disease. Int J Mol Sci 2019, 20(19): 4684.
- Choudhary GS, Al-Harbi S, Almasan A: Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 2015, 1219: 1-9.
- Yao C, Cao X, Fu Z, Tian J, Dong W, Xu J, An K, Zhai L, Yu J: Boschniakia Rossica Polysaccharide Triggers Laryngeal Carcinoma Cell Apoptosis by Regulating Expression of Bcl-2, Caspase-3, and P53. Med Sci Monit 2017, 23: 2059-2064.
- Grange C, Brossa A, Bussolati B: Extracellular Vesicles and Carried miRNAs in the Progression of Renal Cell Carcinoma. Int J Mol Sci 2019, 20(8): 1832.
- Horie K, Kawakami K, Fujita Y, Sugaya M, Kameyama K, Mizutani K, Deguchi T, Ito M: Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem Biophys Res Commun 2017, 492(3): 356-361.
- Yan X, Chen YG: Smad7: not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J 2011, 434: 1-10.
- Wang L, Yang G, Zhao D, Wang J, Bai Y, Peng Q, Wang H, Fang R, Chen G, Wang Z, et al: CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol Cancer 2019, 18(1): 86.
- Niu Y, Jia X, Wang N, Yuan M, Dong A, Yang Y, Shi X: Identification of exosomes-related lncRNAs in clear cell renal cell carcinoma based on Bayesian spike-and-slab lasso approach. Funct Integr Genomics 2023, 23(1): 62.
- Lei Z, Ma X, Li H, Zhang Y, Gao Y, Fan Y, Li X, Chen L, Xie Y, Chen J, et al: Up-regulation of miR-181a in clear cell renal cell carcinoma is associated with lower KLF6 expression, enhanced cell proliferation, accelerated cell cycle transition, and diminished apoptosis. Urol Oncol 2018, 36(3): 93.e23-93.e37.
- Boguslawska J, Sokol E, Rybicka B, Czubaty A, Rodzik K, Piekielko-Witkowska A: microRNAs target SRSF7 splicing factor to modulate the expression of osteopontin splice variants in renal cancer cells. Gene 2016, 595(2): 142-149.
- Ge YZ, Xu LW, Zhou CC, Lu TZ, Yao WT, Wu R, Zhao YC, Xu X, Hu ZK, Wang M, et al: A BAP1 Mutation-specific MicroRNA Signature Predicts Clinical Outcomes in Clear Cell Renal Cell Carcinoma Patients with Wild-type BAP1. J Cancer 2017, 8(13): 2643-2652.
- Liu J, Liu B, Guo Y, Chen Z, Sun W, Gao W, Wu H, Wang Y: MiR-199a-3p acts as a tumor suppressor in clear cell renal cell carcinoma. Pathol Res Pract 2018, 214(6): 806-813.
- Zhu G, Pei L, Lin F, Yin H, Li X, He W, Liu N, Gou X: Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol 2019, 234(12): 23736-23749.
- Butz H, Nofech-Mozes R, Ding Q, Khella HWZ, Szabó PM, Jewett M, Finelli A, Lee J, Ordon M, Stewart R, et al: Exosomal MicroRNAs Are Diagnostic Biomarkers and Can Mediate Cell-Cell Communication in Renal Cell Carcinoma. Eur Urol Focus 2016, 2(2): 210-218.
- Fujii N, Hirata H, Ueno K, Mori J, Oka S, Shimizu K, Kawai Y, Inoue R, Yamamoto Y, Matsumoto H, et al: Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget 2017, 8(66): 109877-109888.
- van Niel G, D'Angelo G, Raposo G: Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018, 19(4): 213-228.
- Zhao Y, Wang Y, Zhao E, Tan Y, Geng B, Kang C, Li X: PTRF/CAVIN1, regulated by SHC1 through the EGFR pathway, is found in urine exosomes as a potential biomarker of ccRCC. Carcinogenesis 2020, 41(3): 274-283.
- Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, et al: Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 2010, 39(1): 133-144.
- Jella KK, Nasti TH, Li Z, Malla SR, Buchwald ZS, Khan MK: Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines (Basel) 2018, 6(4): 69.
- Li W, Li C, Zhou T, Liu X, Liu X, Li X, Chen D: Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017, 16(1): 145.
- He C, Zheng S, Luo Y, Wang B: Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8(1): 237-255.
- Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M: Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci 2019, 233: 116733.
- Xu W, Hua Y, Deng F, Wang D, Wu Y, Zhang W, Tang J: MiR-145 in cancer therapy resistance and sensitivity: A comprehensive review. Cancer Sci 2020, 111(9): 3122-3131.
- Qiu L, Wang J, Chen M, Chen F, Tu W: Exosomal microRNA‑146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2‑mediated PI3K/Akt axis. Int J Mol Med 2020, 46(2): 609-620.
- Yuan L, Liu Y, Qu Y, Liu L, Li H: Exosomes Derived From MicroRNA-148b-3p-Overexpressing Human Umbilical Cord Mesenchymal Stem Cells Restrain Breast Cancer Progression. Front Oncol 2019, 9: 1076.
- Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, Cui Q, Mei W, Li F: Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett 2019, 442: 351-361.
- Shang M, Wang X, Zhang Y, Gao Z, Wang T, Liu R: LincRNA-ROR promotes metastasis and invasion of esophageal squamous cell carcinoma by regulating miR-145/FSCN1. Onco Targets Ther 2018, 11: 639-649.
- Zheng Z, Chen Z, Zhong Q, Zhu D, Xie Y, Shangguan W, Xie W: CircPVT1 promotes progression in clear cell renal cell carcinoma by sponging miR-145-5p and regulating TBX15 expression. Cancer Sci 2021, 112(4): 1443-1456.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript