Review Article | Open Access
Advances in Immunotherapy and Vaccine for Prostate Cancer
Wei Zhou1, Yikai Zhang2
1No.986 Air Force Hospital, Xi’an City, Shaanxi Province, China.
2Shaanxi Institute of Medical Device Quality, Xi’an City, Shaanxi Province, China.
Correspondence: Yikai Zhang (Shaanxi Institute of Medical Device Quality, No. 6 Jianshe West Road, Xi'an, Shaanxi Province, 710032, China; Email: 462473874@qq.com).
Annals of Urologic Oncology 2023, 6(2): 70-78. https://doi.org/10.32948/auo.2023.05.27
Received: 13 May 2023 | Accepted: 26 May 2023 | Published online: 28 May 2023
Key words prostate cancer, cancer vaccine, sipuleucel-T, immunotherapy
Figure 1 shows that general view of vaccine for prostate cancer.
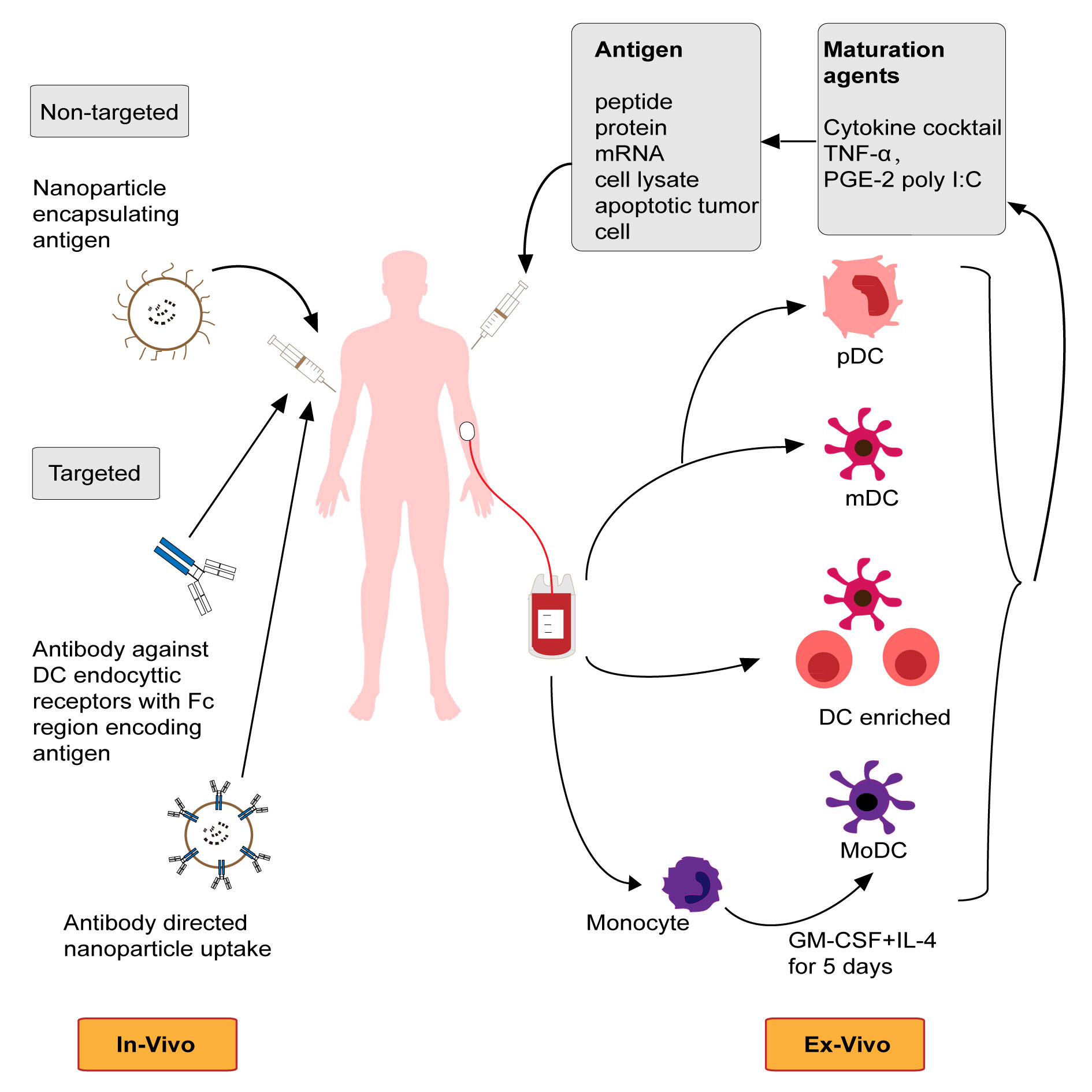 Figure 1. General view of vaccine for prostate cancer (DC, dendritic cell).
Figure 1. General view of vaccine for prostate cancer (DC, dendritic cell).
Sipuleucel-T (Provenge®, Dendreon, USA) is an active immune cell-based immunotherapy that induces an immune response against prostate acid phosphatase (PAP). Prostate acid phosphatase (PAP) is expressed in approximately 95% of prostate cancers and is mainly limited to prostate tissue, which is a target for prostate cancer tumor vaccine development. It is a type of autocellular immunotherapeutic vaccine, composed of peripheral mononuclear cells. Sipuleucel-T consists of autologous peripheral blood mononuclear cells including antigen presenting cells (APCs). Peripheral blood mononuclear cells from patients were obtained by a standard procedure of leukocyte separation approximately 3 days prior to infusion. To increase the immune response to PAP, it is activated by adding PAP and the recombinant fusion protein of immune activator GM-CSF (PAP-GM-CSF) at a specific stage of cell culture. The active components of Sipuleucel-T are autologous APCs and PAP-GM-CSF, in addition to T cells, B cells, natural killer cells, and other cells that specifically bind to PAP expressed in prostate cancer tissue to kill tumor cells. The technology causes patients' own immune system to respond to prostate cancer. In 2010, the US Food and Drug Administration (FDA) approved it into the clinic, and it is the only cell active immune product licensed to treat asymptomatic or mild CRPC [10, 11]. The patient's peripheral blood mononuclear cells (PBMC) rich in antigen-presenting cells (APC) were collected by leukapheresis. After incubating with PA2024 in vitro, the activated cells were injected a new APC, which elicits an antitumor immune response. Among them, PA2024 is the carboxyterminal terminus of PAP and the granulocyte-macrophage colony-stimulating factor (GM-CSF). The amino terminal of GM-CSF was linked to the recombinant protein (PAP-GMCSF). The whole procedure, including leukapheresis, cell activation and reinfusion, repeated every 2 weeks for a total of 3 times (weeks 0, 2 and 4) [12].
IMPACT(NCT00065442) phase III randomized, double-blind, placebo clinical trial [13], where 512 patients were randomly assigned to receive Sipuleucel-T or placebo (2꞉1). After a median follow-up of 34.1 months, the results showed that: Compared with placebo group (121 patients, 70.8%), Sipuleucel-T group (210 patients, 61.6%) had a 22% relative reduction in the risk of death, and the adjusted hazard ratio for death was 0.78 (95%CI: 0.61-0.98, P=0.03). The median overall survival (OS) was improved by 4.1 months (25.8 months vs 21.7 months). The estimated 36-month survival rate was 31.7% (vs. 23.0% with placebo); In another exploratory study, baseline PSA was found to be a predictor of Sipuleucel-T efficacy [14]. In this study, all patients were divided into four groups on average, with 128 patients in each group. Baseline PSA values (ng/ml) were ≤ 22.1, > 22.1-50.1, > 50.1-134.1, and > 134.1, respectively. The group with the best Sipuleucel-T efficacy was the group with the lowest PSA interval. The median OS in the treatment and control groups was 41.3 and 28.3 months, respectively, while the median OS in the treatment and control groups for the highest PSA interval was 18.4 and 15.6 months.. The study concluded that while Sipuleucel-T is effective in all patients, it is most effective in patients predicted to have a lower risk of progression, so the earlier Sipuleucel-T is used, the better is the treatment response. Sipuleucel-T can only be used by the patient. Before infusion, the patient's identity must be confirmed to match that marked on the infusion bag. When given 3 times, about 2 weeks apart from each treatment, OS of patients with advanced prostate cancer can be significantly improved and the average survival time can be extended by more than 4 months. The most common adverse events (incidence ≥15%) included chills, fatigue, fever, back pain, nausea, joint pain, and headache. Sipuleucel-T was well tolerated, and the most common adverse events were fever and influenza-like symptoms. However, it is worth noting that there was no significant difference in progression-free survival (PFS) or prostate specific antigen (PSA) decline between the two groups. Less than 3% of Sipuleucel-T patients had a PSA decline of 50% or more. Based on the IMPACT [13], D9901 (NCT00005947) [15], D9902A(NCT01133704) [16], double-blind, controlled, multicenter phase III randomized trials, in April 2010, the FDA officially approved Sipuleucel-T as the first prostate cancer vaccine for the treatment of asymptomatic or mildly symptomatic mCRPC [8]. The clinical use of this drug has greatly promoted the development of cancer vaccines, but unfortunately, prostate cancer vaccines other than Sipuleucel-T have not shown promising clinical benefit in large, randomized phase II and III trials.
Immunologic mechanism of action of Sipuleucel-T
The immune response induced by Sipuleucel-T is multifaceted, and APC-mediated immune response is the basis of its action, that is, it induces the body to produce PAP specific T cells and B cells, thereby activating the body's immune response to prostate cancer and generating an anticancer effect. The specific mechanisms of sipuleucel-T are as follows: 1) APC activation and immune enhancement. Increased cumulative APC activation, a measure of product potency and immune activation, was statistically significantly associated with improved OS. Sipuleucel-T infusions at weeks 2 and 4 resulted in increased APC activation. This indicates that the first infusion (week 0) primes the immune system and subsequent treatments enhance the immune response. Meanwhile, APC activation is more effective in patients with early prostate cancer [17]. 2) Generate specific immune responses against PAP and PA2024. 3) Antigen diffusion. After an initial immune response to a specific target antigen, Sipuleucel-T expands its response to other antigens expressed by the tumor, which is known as antigen diffusion. In this process, tumor cells lysed by antigen-specific T cells release additional tumor-associated antigens (TAA), which are eventually expressed by the tumor-associated antigens. APC processing and presentation to induce B cells and T cells to produce immune responses against secondary antigens (such as E-RAS, KLK2, K-RAS, LGALS3 and LGALS8, etc.), which are closely related to the improved survival benefits of Sipuleucel-T [18, 19]. 4) T cell reactive transfer and cytotoxic T lymphocyte (CTL) activity. Fong et al.[20] pointed out that neoadjuvant Sipuleucel-T could induce systemic antigen-specific T cell response and activation effect before radical prostatectomy.
T cells should be recruited to the prostate tumor microenvironment to enhance the immune effect. Antonarakis et al. [21] measured the expression of CD107a on CD8+ T cells specific for PAP or PA2024 and demonstrated that Sipuleucel-T could generate antigen-specific CTL responses in prostate cancer patients. The study found that this response was associated with improved OS after Sipuleucel-T treatment. Kibel et al. [22] documented the cytolytic activity of CTLS against target cells expressing PAP by video, demonstrating tumor induction by antigen-specific CD8+ cells, where tumor lysis is an important part of the mechanism of action of Sipuleucel-T. At the same time, Sipuleucel-T treatment alters the B cell receptor repertoire and induces the development of long-term immune memory in the organism, making it more useful for killing tumors [23].
Clinical application of Sipuleucel-T
Efficacy evaluation. The IMPACT study [13] showed that Sipuleucel-T could prolong the median OS of mCRPC patients. Schellhammer et al. [14] pointed out that PSA was a strong predictor of Sipuleucel-T treatment response (P<0.0001), and with the decrease of baseline PSA, Sipuleucel-T treatment response would be greater. The hazard ratio for OS was 0.51(95% CI: 0.31-0.85) in patients with baseline PSA ≤ 22.1 ng/mL and 0.84 (95%CI: 0.31-0.85) in patients with PSA >134 ng/mL. (0.55 to 1.29), indicating a benefit of Sipuleucel-T at an earlier stage of cancer. Early use of Sipuleucel-T has less tumor immunosuppression and more APC activation, which is more conducive to the long-term stimulation of immune response and long-term clinical benefit. Another notable clinical feature of Sipuleucel-T is the delayed effect, in which proximal end points such as PSA levels, objective disease progression, and the occurrence of disease-related pain are not altered. In contrast, there was an improvement in the distal end points [24]. Small et al. [25] found that Sipuleucel-T treatment was not associated with time to disease-related pain (TDRP) (HR=0.819, 95% CI: 0.616-1.089, P=0.170), which was associated with delayed time to first use of opioid analgesics (TFOA) (HR=0.755, 95%CI: 0.579-0.985, P=0.038). These changes in late outcomes may be related to the time required to exert the immune antitumor effect after Sipuleucel-T treatment. Sipuleucel-T results were also variable in different ethnic groups.
The PROCEED study [26] showed that compared with whites, African Americans had a higher median OS after Sipuleucel-T treatment (HR= 0.81, 95%CI: 0.68-0.97, P=0.03). This may be related to the differences in the immune system between different ethical backgrounds [27].
The value of drug combinations. Prostate cancer has a low mutation burden and a small number of tumor infiltrating CD8+ T cells, which makes prostate cancer a "cold tumor" of immune response, resulting in a far worse effect of immunotherapy than other solid tumors [28]. Therefore, combination therapy is commonly used in prostate cancer immunotherapy. Theoretically, combination of Sipuleucel-T with the activation and spread of the immune response, combined with the durable efficacy and safety profile, offers the promise of prolonged anticancer activity and is of great value. A randomized phase II open-label trial evaluated Sipuleucel-T versus androgen deprivation therapy (ADT) in the treatment of patients with biochemical recurrence. The results showed that the safety of different administration sequences was good. The PA2024-specific T cell response of Sipuleucel-T first was higher than that of ADT first (P= 0.001), which could induce greater anti-tumor immune response [29]. The results of a mathematical model for Sipuleucel-T and ADT response in prostate cancer developed by Jain et al. [30] showed that 2 doses of vaccine administered before ADT was optimal, reducing cancer mortality by approximately 45% and maximizing median OS. Small et al. [31] randomized phase II trial evaluating Sipuleucel-T combined with abiraterone in the treatment of mCRPC showed that simultaneous administration did not attenuate or change the immune effect of Sipuleucel-T, and the combination was well tolerated. Radiotherapy (hereafter referred to as radiotherapy) and chemotherapy (hereafter referred to as chemotherapy) may synergize with immunotherapy to enhance and expand antitumor immune responses. However, in a randomized phase II trial of Sipuleucel-T combined with radiotherapy, irradiation of up to 30 Gy to a single metastatic site in asymptomatic or minimally symptomatic mCRPC patients did not enhance the immune response associated with Sipuleucel-T treatment [32]. With respect to combination chemotherapy, few studies have been conducted, and two clinical trials evaluating the sequencing of Sipuleucel-T plus docetaxel (NCT02793219 and NCT02793765) were withdrawn with reduction in demand. In addition, a randomized phase II trial of Sipuleucel-T combined with radium 223 in the treatment of bone metastatic mCRPC showed that the combination therapy could improve clinical efficacy, but the immune response was low [33]. Due to the lack of effector T cell infiltration in the tumor microenvironment (TME), the efficacy of immune checkpoint inhibitors (ICI) monotherapy in the treatment of prostate cancer is not good. More recently, Sinha et al. [34] combined Sipuleucel-T and ipilimumab, showing that combination therapy produced modest clinical responses without changing antigen-specific responses. The phase Ib study by Dorff et al. [35] on mCRPC patients who received different sequential regimens of atezolizumab, and Sipuleucel-T showed that the combination therapy was safe and may be more beneficial regardless of the order of administration. At the same time, other emerging therapies, such as the combination of cytokine IL-7, have also achieved encouraging results in phase II trials [36]. Currently, compared with other immunotherapies, Sipuleucel-T is not widely used, in part because its clinical success rate is limited, and the cost associated with its treatment is not acceptable to most patients. However, the immunologic benefit of Sipuleucel-T in combination therapy still sets the stage for additional large trials.
Clinical limitations "A survey of factors associated with the use of Sipuleucel-T in mCRPC patients in the United States [37] showed that only 730 of 7272 patients treated received Sipuleucel-T, and ethnicity, region of residence, income, and professional recommendation were associated with its use." From a cost perspective, Sipuleucel-T requires three infusions over a period of 1 month. However, the cost-effectiveness analysis for the treatment of mCRPC patients found that the treatment strategy without sipuleucel-T could show the most favorable incremental cost-effectiveness ratios (ICER) [38]. At the same time, the delayed effect of Sipuleucel-T, resulting in the lack of PSA or objective response in clinical trials, affects the assessment of disease progression. Currently, Sipuleucel-T is not widely adopted outside the United States. This drug has been discontinued in Europe because of its complex management, low survival benefit, and high price [39]. Its owner, Dendreon, went bankrupt due to production capacity and financial problems, and was completely acquired by China's Sanpower Group.
PSA-TRICOM
Psa-tricom (Prostvac) is a viral vector-based vaccine produced by inserting a recombinant plasmid with a PSA transgene into a poxvirus together with a plasmid encoding three viral T-cell costimulatory molecules (TRICOM). Patients first receive primary immunization with recombinant vaccinia (Prostvac-V), followed by multiple booster immunization with recombinant avian pox (Prostvac-F) [48]. TRICOM, which is composed of B7.1, ICAM-1 and LFA-3, is mainly used to improve T cell affinity and increase tumor cell lysis [49]. The results of phase II trial showed that patients receiving Prostvac-V/F had a prolonged median OS of 8.5 months (25.1 months vs 16.6 months), with an estimated hazard ratio of 0.56 (95%CI: 0.37-0.85, P=0.0061) and a 44% reduction in mortality [50]. This result strongly encourages subsequent phase III trials. The development of cancer vaccines has also set off a new climax. However, the subsequent phase III trial showed that there was no difference in OS between Prostvac group, proSTVAC-GM-CSF group and placebo group, and the trial was terminated prematurely [51].
Adenovirus /PSA
Adenoviruses were originally used as vectors for gene therapy. In recent years, with the higher safety and high immunogenicity of genetically modified products and the development of next-generation vectors, their utility as vaccine vectors has been increasing [52]. Animal experiments have shown that adenovirus /PSA (Ad5-PSA) can induce immune response against PSA and target killing tumor cells [53]. A phase I clinical trial showed that the treatment of mCRPC patients with adenovirus /PSA was safe, and most patients could produce anti-PSA T cell responses. However, the current research on this vaccine is still in phase I/II trials with small samples, and there are few related phase III trials [54].
Individualized peptide vaccines
Personalized peptide vaccines (PPV) are tumor vaccines that are produced by selecting individual peptides for vaccination based on individual genetic structure and functional differences. The mechanism of PPV is that the specific tumor antigen peptide is delivered to the major histocompatibility complex (MHC) on the surface of APC, which is degraded into short peptides in APC and forms peptide-MHC-TCR complex, which is recognized by T cells. The corresponding CTL response is stimulated, thereby carrying out anti-tumor immunity [55]. A randomized, double-blind, placebo-controlled phase III trial of PPV in patients with CRPC who progressed after docetaxel chemotherapy [56] showed that PPV did not prolong OS (P=0.77). Subgroup analysis showed that patients with lower percentage of neutrophils or higher percentage of lymphocytes at baseline could benefit from PPV treatment.
GX301
GX301 is a vaccine consisting of four telomerase peptides and two immune adjuvants (Montanide ISA-51 and imiquimod). It was found to be safe and immunogenic in phase I trials [57]. The results of a multicenter, randomized, parallel-group, open-label phase II trial showed that GX301 vaccine was safe and immunogenic in patients with mCRPC, and the immune response rate was related to the number of immunizations, which provided a reference for treatment options in future studies [58].
DNA vaccines are closed circular DNA plasmids designed using target antigens, encoding antigens under the action of a strong mammalian promoter, and improving immunogenicity through targeted antigen presentation to kill tumor cells. Currently, DNA-based prostate cancer vaccines mainly include vaccines encoding PAP, PSMA, PSA, etc.[59]. A phase I/IIa trial of encoded PAP [60] showed that the vaccine had a good safety profile, with 14% (3/22) of patients producing PAP-specific IFN-γ-secreting CD8+ T cells immediately after treatment. PAP specific CD4+ and/or CD8+ T cell proliferation was observed in 41% (9/22) patients, and the difference in PSADT was statistically significant (P<0.05). Similarly, the safety and efficacy of DNA vaccine encoding PSMA were also confirmed in phase I/II trials, and its PSADT was significantly increased (P=0.0417) [61]. A recent study [62] showed that PSMA or T-cell receptor γ alternate reading frame protein, peptides of TARP, incorporated into spherical nucleic acid (SNA) vaccines with optimized structures, can significantly affect adaptive immune responses to clinically used prostate cancer targets.
This may become a new research direction in the future. The mRNA vaccine is a synthetic protein antigen encoded by the mRNA sequence delivered to the body to cause the body to express the corresponding protein and induce the body to produce an immune response against the protein to achieve the purpose of disease prevention and treatment [63-65]. Among them, safe and efficient delivery system is the core key technology of mRNA vaccine development [66]. Currently, mRNA vaccines for prostate cancer are under development. To address the bottleneck of tumor antigen screening and vaccine candidates, Zheng et al. [67] showed that KLHL17, CPT1B, IQGAP3, LIME1, YJEFN3, KIAA1529, MSH5, and CELSR3 are important markers of prostate adenocarcinoma. Patients with PRAD immune subtype (PIS) 2 and 3 are more suitable for vaccination. The mechanisms of action and study results of these different types of vaccines are shown in Table 1.|
Table 1. Overview of prostate cancer vaccines. |
||||||
|
Vaccine |
Type |
Mechanism of action |
Clinical stage |
Results |
State |
Reference |
|
Sipuleucel-T |
Monocte vaccine |
Immunotherapy based on active immune cells can induce targeting prostatic acid phosphatase prostate acid phosphatase (PAP) anti-tumor immune response. |
Stage Ⅲ |
Improve median survival Phase 4.1 months |
On the market |
[7-9], [11-39] |
|
DCVAC/PCa |
Dendritic Cellular vaccine |
Peripheral blood mononuclear cells (mononuclear cells) obtained by apheresis cell, PBMC) is pulsed with killed prostate cancer cells (LNCaP) and re-skinned mature dendritic cells are injected below to obtain an antitumor immune response. |
Stage Ⅲ |
Unprolonged patient Median survival |
Finished |
[40-43] |
|
GVAX/PCa |
Cancer cell vaccine |
After gene transfection, LNCaP and PC-3 cell lines were used to secrete granulocyte-macrophage factor(granulocyte-macrophage colony-stimulating factor, GM-CSF) to produce an antitumor immune response. |
Stage Ⅲ |
Unprolonged patient Median survival |
Early termination |
[44-47] |
|
PROSTVAC (PSA-TRICOM) |
Viral vector vaccine |
A plasmid with PSA-TRICOM is inserted into a poxvirus. Patients first receive an initial vaccine for recombinant vaccinia (Prostvac-V), followed by multiple booster immunizations with recombinant avian pox (Prostvac-F). |
Stage Ⅲ |
Unprolonged patient Median survival |
Early termination |
[48-51] |
|
Adenovirus /PSA |
Viral vector vaccine |
Adenovirus /PSA can be used to induce an immune response against PSA and target the killing of tumor cells. |
StageⅠ/Ⅱ |
The vaccine is immunogenic |
Be in progress |
[52-54] |
|
PPV |
Polypeptide vaccine |
Will feed specific tumor antigen peptide antigen presenting cells (antigen presenting cell, APC) on the surface of the major histocompatibility complex, forming peptide - MHC - which is complex, so as to stimulate cytotoxic T lymphocyte (cytotoxic T lymphocyte, CTL) response, producing antitumor immunity. |
Stage Ⅲ |
Unprolonged patient Median survival |
Finished |
[55, 56] |
|
GX301 |
Polypeptide vaccine |
A vaccine consisting of four telomerase peptides and two immune adjuvants (Montanide ISA-51 and imiquimod). |
Stage Ⅱ |
The vaccine is immunogenic |
Be in progress |
[57, 58] |
|
DNA/mRNA vaccine |
DNA/mRNA vaccine |
DNA vaccine is a closed circular DNA plasmid designed by the target antigen, which encodes the antigen under the action of the powerful promoter of mammals, and improves the immunogenicity through the targeted antigen presentation. mRNA vaccines deliver mRNA sequences that encode protein antigens into the body, causing the body to express the corresponding protein and inducing an immune response against the protein. |
StageⅠ/Ⅱ |
The vaccine is immunogenic |
Be in progress |
[59-67] |
We thank Dr. Sanjay Gupta (Case Western Reserve University & UH Cleveland Medical Center) for his proofreading for the review.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
ZW designed the study and was responsible for the writing of the original draft. ZY edited and approved the final manuscript.
Competing interests
All authors declare no competing interests.
Funding
None.
- Carlsson SV, Vickers AJ: Screening for Prostate Cancer. Med Clin North Am 2020, 104(6): 1051-1062.
- Dörr M, Hölzel D, Schubert-Fritschle G, Engel J, Schlesinger-Raab A: Changes in prognostic and therapeutic parameters in prostate cancer from an epidemiological view over 20 years. Oncol Res Treat 2015, 38(1-2): 8-14.
- Siegel RL, Miller KD, Fuchs HE, Jemal A: Cancer statistics, 2022. CA Cancer J Clin 2022, 72(1): 7-33.
- Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N et al: Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022, 135(5): 584-590.
- Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, Zeng HM, Wei WW, He J: [Cancer statistics in China, 2016]. Zhonghua Zhong Liu Za Zhi 2023, 45(3): 212-220.
- Merseburger AS, Alcaraz A, von Klot CA: Androgen deprivation therapy as backbone therapy in the management of prostate cancer. Onco Targets Ther 2016, 9: 7263-7274.
- DeFrancesco L: Landmark approval for Dendreon's cancer vaccine. Nat Biotechnol 2010, 28(6): 531-532.
- Marcus L, Lemery SJ, Keegan P, Pazdur R: FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin Cancer Res 2019, 25(13): 3753-3758.
- Movassaghi M, Chung R, Anderson CB, Stein M, Saenger Y, Faiena I: Overcoming Immune Resistance in Prostate Cancer: Challenges and Advances. Cancers (Basel) 2021, 13(19): 4757.
- Cheever MA, Higano CS: PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 2011, 17(11): 3520-3526.
- Quinn DI, Shore ND, Egawa S, Gerritsen WR, Fizazi K: Immunotherapy for castration-resistant prostate cancer: Progress and new paradigms. Urol Oncol 2015, 33(5): 245-260.
- Claps M, Mennitto A, Guadalupi V, Sepe P, Stellato M, Zattarin E, Gillessen SS, Sternberg CN, Berruti A, De Braud FGM et al: Immune-checkpoint inhibitors and metastatic prostate cancer therapy: Learning by making mistakes. Cancer Treat Rev 2020, 88: 102057.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB et al: Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010, 363(5): 411-422.
- Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW: Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology 2013, 81(6): 1297-1302.
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM: Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006, 24(19): 3089-3094.
- Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW: Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009, 115(16): 3670-3679.
- Mulders PF, De Santis M, Powles T, Fizazi K: Targeted treatment of metastatic castration-resistant prostate cancer with sipuleucel-T immunotherapy. Cancer Immunol Immunother 2015, 64(6): 655-663.
- Gulley JL, Madan RA, Pachynski R, Mulders P, Sheikh NA, Trager J, Drake CG: Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. J Natl Cancer Inst 2017, 109(4): djw261.
- GuhaThakurta D, Sheikh NA, Fan LQ, Kandadi H, Meagher TC, Hall SJ, Kantoff PW, Higano CS, Small EJ, Gardner TA et al: Humoral Immune Response against Nontargeted Tumor Antigens after Treatment with Sipuleucel-T and Its Association with Improved Clinical Outcome. Clin Cancer Res 2015, 21(16): 3619-3630.
- Fong L, Carroll P, Weinberg V, Chan S, Lewis J, Corman J, Amling CL, Stephenson RA, Simko J, Sheikh NA et al: Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014, 106(11): dju268.
- Antonarakis ES, Small EJ, Petrylak DP, Quinn DI, Kibel AS, Chang NN, Dearstyne E, Harmon M, Campogan D, Haynes H et al: Antigen-Specific CD8 Lytic Phenotype Induced by Sipuleucel-T in Hormone-Sensitive or Castration-Resistant Prostate Cancer and Association with Overall Survival. Clin Cancer Res 2018, 24(19): 4662-4671.
- Kibel AS, Inman BA, Pachynski RK, Vu T, Sheikh NA, Petrylak DP: Videos of Sipuleucel-T Programmed T Cells Lysing Cells That Express Prostate Cancer Target Antigens. J Natl Cancer Inst 2022, 114(2): 310-313.
- Zhang L, Kandadi H, Yang H, Cham J, He T, Oh DY, Sheikh NA, Fong L: Long-term Sculpting of the B-cell Repertoire following Cancer Immunotherapy in Patients Treated with Sipuleucel-T. Cancer Immunol Res 2020, 8(12): 1496-1507.
- Madan RA, Antonarakis ES, Drake CG, Fong L, Yu EY, McNeel DG, Lin DW, Chang NN, Sheikh NA, Gulley JL: Putting the Pieces Together: Completing the Mechanism of Action Jigsaw for Sipuleucel-T. J Natl Cancer Inst 2020, 112(6): 562-573.
- Small EJ, Higano CS, Kantoff PW, Whitmore JB, Frohlich MW, Petrylak DP: Time to disease-related pain and first opioid use in patients with metastatic castration-resistant prostate cancer treated with sipuleucel-T. Prostate Cancer Prostatic Dis 2014, 17(3): 259-264.
- Sartor O, Armstrong AJ, Ahaghotu C, McLeod DG, Cooperberg MR, Penson DF, Kantoff PW, Vogelzang NJ, Hussain A, Pieczonka CM et al: Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis 2020, 23(3): 517-526.
- Vidal AC, Howard LE, de Hoedt A, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Taioli E, Fowke JH et al: Neutrophil, lymphocyte and platelet counts, and risk of prostate cancer outcomes in white and black men: results from the SEARCH database. Cancer Causes Control 2018, 29(6): 581-588.
- Steele KE, Tan TH, Korn R, Dacosta K, Brown C, Kuziora M, Zimmermann J, Laffin B, Widmaier M, Rognoni L et al: Measuring multiple parameters of CD8+ tumor-infiltrating lymphocytes in human cancers by image analysis. J Immunother Cancer 2018, 6(1): 20.
- Antonarakis ES, Kibel AS, Yu EY, Karsh LI, Elfiky A, Shore ND, Vogelzang NJ, Corman JM, Millard FE, Maher JC et al: Sequencing of Sipuleucel-T and Androgen Deprivation Therapy in Men with Hormone-Sensitive Biochemically Recurrent Prostate Cancer: A Phase II Randomized Trial. Clin Cancer Res 2017, 23(10): 2451-2459.
- Jain HV, Sorribes IC, Handelman SK, Barnaby J, Jackson TL: Standing Variations Modeling Captures Inter-Individual Heterogeneity in a Deterministic Model of Prostate Cancer Response to Combination Therapy. Cancers (Basel) 2021, 13(8): 1872.
- Small EJ, Lance RS, Gardner TA, Karsh LI, Fong L, McCoy C, DeVries T, Sheikh NA, GuhaThakurta D, Chang N et al: A Randomized Phase II Trial of Sipuleucel-T with Concurrent versus Sequential Abiraterone Acetate plus Prednisone in Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2015, 21(17): 3862-3869.
- Twardowski P, Wong JYC, Pal SK, Maughan BL, Frankel PH, Franklin K, Junqueira M, Prajapati MR, Nachaegari G, Harwood D et al: Randomized phase II trial of sipuleucel-T immunotherapy preceded by sensitizing radiation therapy and sipuleucel-T alone in patients with metastatic castrate resistant prostate cancer. Cancer Treat Res Commun 2019, 19: 100116.
- Marshall CH, Fu W, Wang H, Park JC, DeWeese TL, Tran PT, Song DY, King S, Afful M, Hurrelbrink J et al: Randomized Phase II Trial of Sipuleucel-T with or without Radium-223 in Men with Bone-metastatic Castration-resistant Prostate Cancer. Clin Cancer Res 2021, 27(6): 1623-1630.
- Sinha M, Zhang L, Subudhi S, Chen B, Marquez J, Liu EV, Allaire K, Cheung A, Ng S, Nguyen C et al: Pre-existing immune status associated with response to combination of sipuleucel-T and ipilimumab in patients with metastatic castration-resistant prostate cancer. J Immunother Cancer 2021, 9(5): e002254.
- Dorff T, Hirasawa Y, Acoba J, Pagano I, Tamura D, Pal S, Zhang M, Waitz R, Dhal A, Haynes W et al: Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J Immunother Cancer 2021, 9(8): e002931.
- Pachynski RK, Morishima C, Szmulewitz R, Harshman L, Appleman L, Monk P, Bitting RL, Kucuk O, Millard F, Seigne JD et al: IL-7 expands lymphocyte populations and enhances immune responses to sipuleucel-T in patients with metastatic castration-resistant prostate cancer (mCRPC). J Immunother Cancer 2021, 9(8): e002903.
- Caram MEV, Ross R, Lin P, Mukherjee B: Factors Associated With Use of Sipuleucel-T to Treat Patients With Advanced Prostate Cancer. JAMA Netw Open 2019, 2(4): e192589.
- Pollard ME, Moskowitz AJ, Diefenbach MA, Hall SJ: Cost-effectiveness analysis of treatments for metastatic castration resistant prostate cancer. Asian J Urol 2017, 4(1): 37-43.
- Tsaur I, Brandt MP, Juengel E, Manceau C, Ploussard G: Immunotherapy in prostate cancer: new horizon of hurdles and hopes. World J Urol 2021, 39(5): 1387-1403.
- Saad F, Shore N, Zhang T, Sharma S, Cho HK, Jacobs IA: Emerging therapeutic targets for patients with advanced prostate cancer. Cancer Treat Rev 2019, 76: 1-9.
- Podrazil M, Horvath R, Becht E, Rozkova D, Bilkova P, Sochorova K, Hromadkova H, Kayserova J, Vavrova K, Lastovicka J et al: Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015, 6(20): 18192-18205.
- Fucikova J, Podrazil M, Jarolim L, Bilkova P, Hensler M, Becht E, Gasova Z, Klouckova J, Kayserova J, Horvath R et al: Phase I/II trial of dendritic cell-based active cellular immunotherapy with DCVAC/PCa in patients with rising PSA after primary prostatectomy or salvage radiotherapy for the treatment of prostate cancer. Cancer Immunol Immunother 2018, 67(1): 89-100.
- Vogelzang NJ, Beer TM, Gerritsen W, Oudard S, Wiechno P, Kukielka-Budny B, Samal V, Hajek J, Feyerabend S, Khoo V et al: Efficacy and Safety of Autologous Dendritic Cell-Based Immunotherapy, Docetaxel, and Prednisone vs Placebo in Patients With Metastatic Castration-Resistant Prostate Cancer: The VIABLE Phase 3 Randomized Clinical Trial. JAMA Oncol 2022, 8(4): 546-552.
- Comiskey MC, Dallos MC, Drake CG: Immunotherapy in Prostate Cancer: Teaching an Old Dog New Tricks. Curr Oncol Rep 2018, 20(9): 75.
- Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift SM, Hege KM, Ando DG, Piantadosi S et al: Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin Cancer Res 2006, 12(11 Pt 1): 3394-3401.
- Higano CS, Corman JM, Smith DC, Centeno AS, Steidle CP, Gittleman M, Simons JW, Sacks N, Aimi J, Small EJ: Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 2008, 113(5): 975-984.
- Sonpavde G, Slawin KM, Spencer DM, Levitt JM: Emerging vaccine therapy approaches for prostate cancer. Rev Urol 2010, 12(1): 25-34.
- Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL: Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs 2009, 18(7): 1001-1011.
- Sharp DW, Lattime EC: Recombinant Poxvirus and the Tumor Microenvironment: Oncolysis, Immune Regulation and Immunization. Biomedicines 2016, 4(3): 19.
- Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J et al: Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010, 28(7): 1099-1105.
- Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J et al: Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2019, 37(13): 1051-1061.
- Chang J: Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune Netw 2021, 21(1): e6.
- Elzey BD, Siemens DR, Ratliff TL, Lubaroff DM: Immunization with type 5 adenovirus recombinant for a tumor antigen in combination with recombinant canarypox virus (ALVAC) cytokine gene delivery induces destruction of established prostate tumors. Int J Cancer 2001, 94(6): 842-849.
- Bilusic M, McMahon S, Madan RA, Karzai F, Tsai YT, Donahue RN, Palena C, Jochems C, Marté JL, Floudas C et al: Phase I study of a multitargeted recombinant Ad5 PSA/MUC-1/brachyury-based immunotherapy vaccine in patients with metastatic castration-resistant prostate cancer (mCRPC). J Immunother Cancer 2021, 9(3): e002374.
- Kimura T, Egawa S, Uemura H: Personalized peptide vaccines and their relation to other therapies in urological cancer. Nat Rev Urol 2017, 14(8): 501-510.
- Noguchi M, Mine T, Komatsu N, Suekane S, Moriya F, Matsuoka K, Yutani S, Shichijo S, Yamada A, Toh U et al: Assessment of immunological biomarkers in patients with advanced cancer treated by personalized peptide vaccination. Cancer Biol Ther 2010, 10(12): 1266-1279.
- Noguchi M, Fujimoto K, Arai G, Uemura H, Hashine K, Matsumoto H, Fukasawa S, Kohjimoto Y, Nakatsu H, Takenaka A et al: A randomized phase III trial of personalized peptide vaccination for castration‑resistant prostate cancer progressing after docetaxel. Oncol Rep 2021, 45(1): 159-168.
- Fenoglio D, Traverso P, Parodi A, Tomasello L, Negrini S, Kalli F, Battaglia F, Ferrera F, Sciallero S, Murdaca G et al: A multi-peptide, dual-adjuvant telomerase vaccine (GX301) is highly immunogenic in patients with prostate and renal cancer. Cancer Immunol Immunother 2013, 62(6): 1041-1052.
- Filaci G, Fenoglio D, Nolè F, Zanardi E, Tomasello L, Aglietta M, Del Conte G, Carles J, Morales-Barrera R, Guglielmini P et al: Telomerase-based GX301 cancer vaccine in patients with metastatic castration-resistant prostate cancer: a randomized phase II trial. Cancer Immunol Immunother 2021, 70(12): 3679-3692.
- Zahm CD, Colluru VT, McNeel DG: DNA vaccines for prostate cancer. Pharmacol Ther 2017, 174: 27-42.
- McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R et al: Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol 2009, 27(25): 4047-4054.
- Chudley L, McCann K, Mander A, Tjelle T, Campos-Perez J, Godeseth R, Creak A, Dobbyn J, Johnson B, Bass P et al: DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol Immunother 2012, 61(11): 2161-2170.
- Teplensky MH, Dittmar JW, Qin L, Wang S, Evangelopoulos M, Zhang B, Mirkin CA: Spherical Nucleic Acid Vaccine Structure Markedly Influences Adaptive Immune Responses of Clinically Utilized Prostate Cancer Targets. Adv Healthc Mater 2021, 10(22): e2101262.
- Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG: The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol 2022, 40(6): 840-854.
- Li M, Wang Z, Xie C, Xia X: Advances in mRNA vaccines. Int Rev Cell Mol Biol 2022, 372: 295-316.
- Karam M, Daoud G: mRNA vaccines: Past, present, future. Asian J Pharm Sci 2022, 17(4): 491-522.
- Zheng X, Xu H, Yi X, Zhang T, Wei Q, Li H, Ai J: Tumor-antigens and immune landscapes identification for prostate adenocarcinoma mRNA vaccine. Mol Cancer 2021, 20(1): 160.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript