Research Article | Open Access
Value of Immunological Biomarkers in Early Prediction of Bacillus Calmette-Guerin Failure in High-Risk Non-muscle-invasive Bladder Cancer
Mohamed G.A. El-Gazzar1, Hassan Abol-Enein2, Amira Awadalla3, Ahmed El-Assmy2, Ahmed M. Harraz2, 4, Mohamed S. El-Ghreb4, Lamiaa A.A. Barakat4 and A.F. Abdel-Aziz1
1Medical analysis specialist, Port Said medical center, Suez Canal authority, Port Said, Egypt.
2Urology and Nephrology Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
3Center of Excellence for Genome and Cancer Research, Urology and Nephrology Center, Mansoura University, Mansoura, Egypt.
4Department of chemistry, Faculty of Science, University of Port Said, Egypt.
Correspondence: Ahmed El-Assmy (Urology and nephrology center, Mansoura University, 35516, Egypt; Email: a_assmy@yahoo.com).
Annals of Urologic Oncology 2023, 6(3): 111-120. https://doi.org/10.32948/auo.2023.10.03
Received: 02 Sep 2023 | Accepted: 30 Sep 2023 | Published online: 04 Oct 2023
Patients and Methods Patients who underwent transurethral resection of bladder tumors for NMIBC were assessed for study eligibility. Urine and blood samples were taken from patients at baseline (immediately before the first dose of induction). Urine samples were evaluated for interleukin (IL)-6, IL-8, IL-10, IL-11, and interferon- γ by solid-phase enzyme-linked immunosorbent assay (ELISA). Blood samples were evaluated for epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor-2 (HER2) using quantitative reverse transcriptase-polymerase chain reaction analysis. Each marker was assessed in relation to tumor recurrence.
Results Between June 2016 and December 2019, 160 patients were included. Tumor recurrence occurred in 47 (29.38%) patients over a median (IQR) follow-up of 24 (12: 49) months. Using univariate analysis, the following urinary cytokines were associated with higher recurrence: urinary IL-6, 8, 10, 11, and interferon-γ. Also, serum EGFR and HER2 were associated with higher recurrence. On multivariate Cox regression analysis, significant variables include HER2 [HR (95%CI): 2.675 (1.367-5.233), p= 0.004], and IL-11 [HR (95%CI): 0.889 (0.825-0.957), p= 0.002].
Conclusions Serum HER2 and urinary IL-11 could be applied in clinical practice to predict BCG failure in patients with high-risk NMIBC, so those patients could be offered other modalities (radical cystectomy) early with better survival. Further studies are recommended to establish their exact role.
Key words BCG failure, biomarkers, high risk, non muscle invasive
For patients with intermediate-risk and high-risk tumors, intravesical Bacillus Calmette-Guerin (BCG) instillation is recommended as the first choice of treatment [3]. Nevertheless, BCG response is inconstant with recurrence and progression rates seemingly ranging from 32% to 43% and 9.5% to 14%, respectively [4]. In addition, the need for frequent cystoscopies, biopsies, and long-term surveillance of NMIBC imposes high costs on the patient and society and is associated with significant patient anxiety. Furthermore, early radical cystectomy in patients with BCG failure gives better survival [5]. Therefore, the identification of prognostic factors that could predict BCG failure is of the highest importance to offer patients another line of treatment with better outcomes.
Prior attempts to predict BCG response focused on clinicopathological characteristics, such as stage, grade, and concomitant CIS. However, tumors with similar histopathological appearance can show large differences in disease aggressiveness and outcome. It is now believed that the most important determinant of BCG response is the patient’s capability to generate an adequate immune response [6].
Intravesical BCG instillation induces the activation of urothelial cells and antigen-presenting cells (APCs), which produce cytokines and chemokines that attract granulocytes and mononuclear cells to the bladder. These events are characterized by the typical epithelioid and giant cell granulomas found in the bladder wall after BCG instillation, which contain macrophages, dendritic cells, lymphocytes, neutrophils, and fibroblasts. Several cytokines have been implicated in this cell-mediated response, including interleukin (IL)-1, IL-2, IL-6, IL-8, IL-10, IL-12, tumor necrosis factor-α, and interferon (IFN) [7].
Over the last decade, several studies evaluated the prognostic values of different immunological markers after BCG instillation and their impact on treatment outcomes [8-10]. However, these studies were limited by either a small sample size, short follow-up, or retrospective nature. These limitations triggered us to assess the predictive value of different immunological markers on treatment outcomes after BCG induction in high-risk NMIBC in a larger number of patients with adequate follow-up in a prospective fashion.
This is a prospective observational study that included patients with high-risk NMIBC who were treated by transurethral resection (TURBT). The study protocol was approved by the institutional review board. The experimental procedures were carried out in accordance with the ethical standards of the Helsinki Declaration and informed consent was obtained from all participants.
Exclusion Criteria
Patients with previous BCG instillation, benign pathology, variant histology, non-urothelial carcinoma, upper tract urothelial tumors, detrusor muscle invasion, and low- or intermediate-risk NMIBC were all excluded.
Primary TURBT procedures, postoperative care, and repeat biopsy
TURBT procedures were done using a resectoscope by an expert urologist Histopathological assessment of resected tumor specimens was evaluated by expert uro-pathologists. Repeat biopsy was taken in all patients at 4-6 weeks after the initial primary procedures. Then, 2 weeks later, all patients included in the study received 80 mg intravesical BCG for 6 consecutive weeks as an induction regimen of intravesical BCG. At 4 weeks after the induction phase, a ‘check’ cystoscopy was done, and patients with no tumor were scheduled for a maintenance regimen of intravesical BCG for 3 consecutive weeks at 3 and 6 months, and 6 monthly thereafter for a period of 3 years.
Specimen handling, processing, and assay details
Urine and blood samples were collected at baseline (immediately before the first instillation of the induction regimen). These samples were collected according to standard operating procedures at our center for similar studies.
Cytokines measurements
Urinary IL-6, IL-8, IL-10, IL-11, and IFN-γ levels were measured in the supernatants. Cytokines concentrations were determined in the urine of all patients by solid phase ELISA Immunoassay (R&D Systems, Minneapolis, MN, USA). All samples were measured in duplicate in accordance with the manufacturer’s recommendations.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) analysis
RNA was extracted from blood samples using QIAamp RNA Blood Mini kit (Qiagen, Hilden, Germany). In all, 1 microgram of total RNA was reverse transcribed with random primers, using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). The RT-qPCR analysis was carried out with SYBER Green PCR Master Mix (Applied Biosystems). Primers for EGFR and HER2 with glycerldehyde-3-phosphate dehydrogenase were used as the PCR control.
Follow-up and outcomes
During the maintenance regimen, all patients underwent cystoscopy/cytology at 3-month intervals for 2 years and at 6-month intervals thereafter. Upper tract imaging was done whenever indicated. An initial complete response (ICR) was defined as tumor-free cystoscopy at 3 months after biopsy. Recurrence was defined as the development of new tumor in patients with an ICR. Progression was defined as upstaging to muscle invasion.
Statistical analysis
Data were described by the median (IQR) and category frequencies for scale and nominal variables, respectively. P-values were calculated using the Mann-Whitney U and the Chi-square tests, respectively. Spearman rho was used to estimate the strength and direction of the correlation between the markers. Survival curves were drawn using the Kaplan Meier method with the log-rank test used to provide statistical significance. Cut-off values of scale variables were selected based on the maximally selected rank statistic method in survminer and maxstat R packages. Predictors of recurrence-free survival were estimated using the univariate and multivariate Cox regression models. Because of the significant correlation between the independent variables, the variation inflation factor (VIF) and tolerance were used to exclude multiple collinearities in the multivariate analysis. Statistical analyses were used using the R programming language (4.1.2) with a p-value < 0.05 is considered statistically significant.
Between June 2014 and December 2019, A total of 160 patients with a mean (SD) age of 67.01 (7.92) years were included in this study. The main study population was males [139 (86.88%)]. Tumor recurrence occurred in 47 (29.38%) patients over a median (IQR) follow-up of 24 (12: 49) months. The median (IQR) follow-up was 30 (14: 54) months. Table 1a-b shows the characteristics of the study population.
Factors associated with recurrence
Significant variables include EGFR median (IQR) [2.1 (1.9: 2.3) versus 3.8 (3.5: 7.1), p< 0.001 MW], HER2 median (IQR) [1.967 (1.87: 2.01) versus 3.8 (3.455: 5.45), p< 0.001 MW], IFN-γ median (IQR) [0.66 (0.54: 0.74) versus 0.1 (0.07: 0.2), p< 0.001 MW], IL-10 median (IQR) [21 (20: 22) versus 38 (35.5: 61), p< 0.001 MW], IL-11 median (IQR) [55 (51: 56) versus 31 (30: 32.5), p< 0.001 MW], IL-6 median (IQR) [37 (33: 44) versus 69 (66:91), p< 0.001 MW], IL-8 median (IQR) [203 (163: 277) versus 501 (452.5: 699), p< 0.001 MW], prior recurrence [primary: 105 (92.92%) versus 12 (25.53%), recurrent: 8 (7.08%) versus 35 (74.47%), p< 0.001 X2] for Recurrence no versus yes, respectively. Table 2 shows the characteristics of patients with recurrence. Figure 1 shows the levels of the tumor markers stratified according to the recurrence status.
Correlation between tumor markers
EGFR has positive correlations with HER2 [strong], IL-10 [moderate], IL-6 [strong], IL-8 [moderate] and has negative correlation with IFN-γ [strong], IL-11 [moderate]. HER2 has positive correlations with IL-10 [moderate], IL-6 [strong], IL-8 [moderate] and has negative correlation with IFN-γ [strong], IL-11 [moderate]. IFN-γ has positive correlations with IL-11 [moderate] and has negative correlation with IL-10 [moderate], IL-6 [strong], IL-8 [moderate]. IL-10 has positive correlations with IL-6 [moderate], IL-8 [moderate] and has negative correlation with IL-11 [moderate]. IL-11 and has negative correlation with IL-6 [moderate], IL-8 [moderate]. IL-6 has positive correlations with IL-8 [moderate]. Results are shown in Figure 2.
Survival curves for recurrence-free survival
Kaplan Meier survival curves are shown in Figure 3. Significant variables comparing low versus high levels (survival at 12 months and 60 months, p-value) were EGFR (100% vs 73%, 100% vs 22%, p< 0.001), HER2 (100% vs 71%, 98% vs 18%, p< 0.001), IFN-γ (73% vs 100%, 22% vs 100%, p< 0.001), IL-10 (99% vs 75%, 97% vs 23%, p< 0.001), IL-11 (72% vs 100%, 22% vs 96%, p<0.001), IL-6 (100% vs 73%, 98% vs 22%, p< 0.001), IL-8 (98% vs 73%, 98% vs 15%, p< 0.001), and type: primary vs recurrent (96% vs 74%, 85% vs 20%, p< 0.001), respectively. Cut-off values used for stratifying scale variables were, 2.54 for EGFR, 3.1 for HER2, 0.35 for IFN-γ, 31 for IL-10, 34 for IL-11, 55 for IL-6, and 398 for IL-8.
Univariate and multivariate COX regression analysis
Significant variables include EGFR [HR (95%CI): 1.69 (1.47-1.944), p< 0.001], HER2 [HR (95%CI): 2.238 (1.857-2.698), p< 0.001], IFN-γ [HR (95%CI): 0.003 (0-0.016), p< 0.001], IL-10 [HR (95%CI): 1.063 (1.046-1.081), p< 0.001], IL-11 [HR (95%CI): 0.848 (0.804-0.895), p< 0.001], IL-6 [HR (95%CI): 1.054 (1.038-1.069), p< 0.001], IL-8 [HR (95%CI): 1.001 (1-1.001), p= 0.002], prior recurrence: recurrent [HR (95%CI): 8.836 (4.583-17.038), p< 0.001]. Multicollinearity exists in 4 variables: EGFR (VIF: 19.2, Tolerance: 0.052), HER2 (VIF: 16.2, Tolerance: 0.062), IL-10 (VIF: 10.7, Tolerance: 0.093), IL-6 (VIF: 10.8, Tolerance: 0.093), after the exclusion of EGFR from the multivariate analysis, multicollinearity between the predictors disappeared. On multivariate Cox regression analysis, significant variables include HER2 [HR (95%CI): 2.675 (1.367-5.233), p= 0.004], and IL-11 [HR (95%CI): 0.889 (0.825-0.957), p= 0.002]. Univariate and multivariate analyses are shown in Table 3.
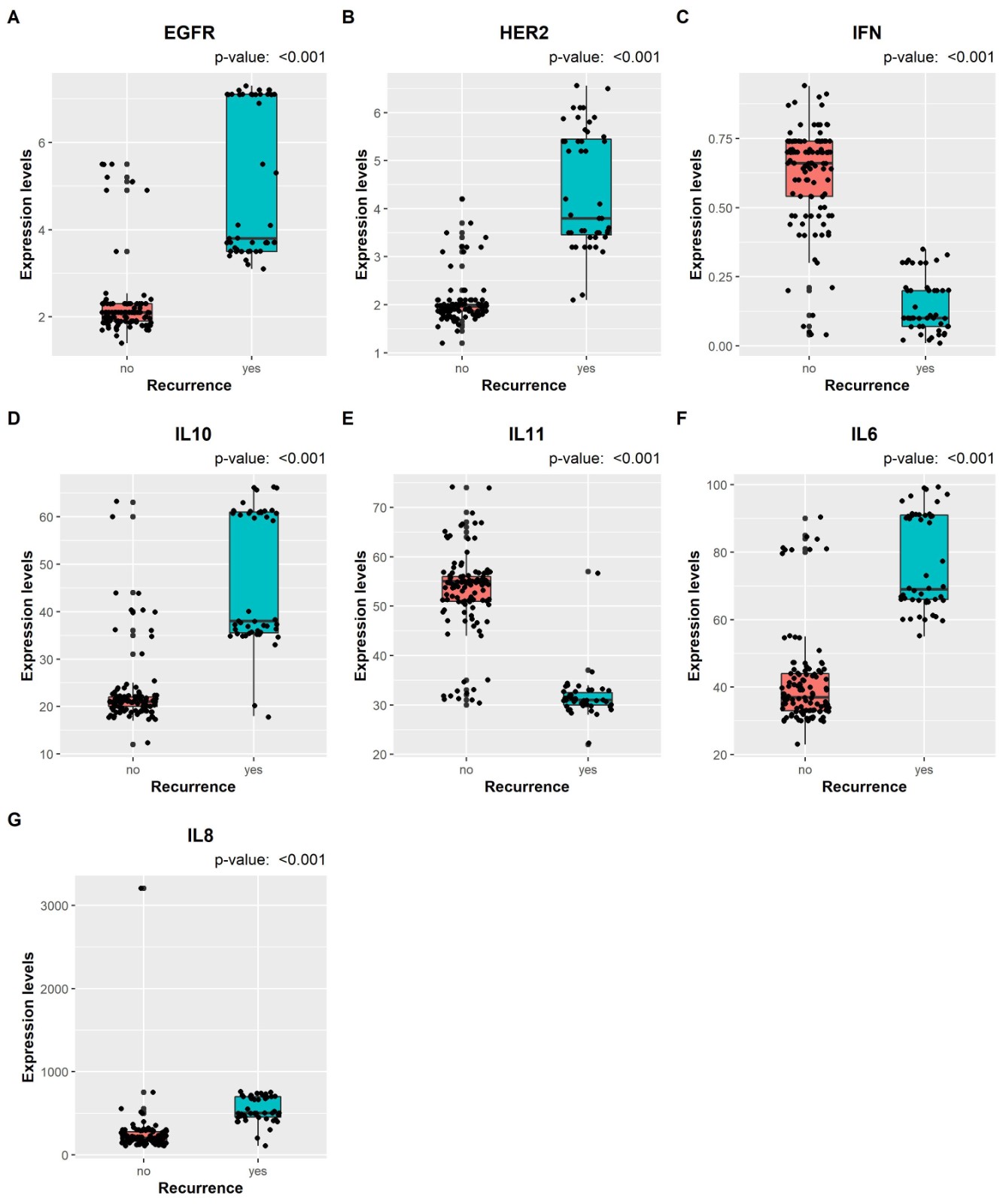 Figure 1. The levels of each tumor markers (EGFR, HER2, IFN-γ, IL-10, IL-11, IL-6, IL-8) stratified according to the recurrence status.
Figure 1. The levels of each tumor markers (EGFR, HER2, IFN-γ, IL-10, IL-11, IL-6, IL-8) stratified according to the recurrence status.
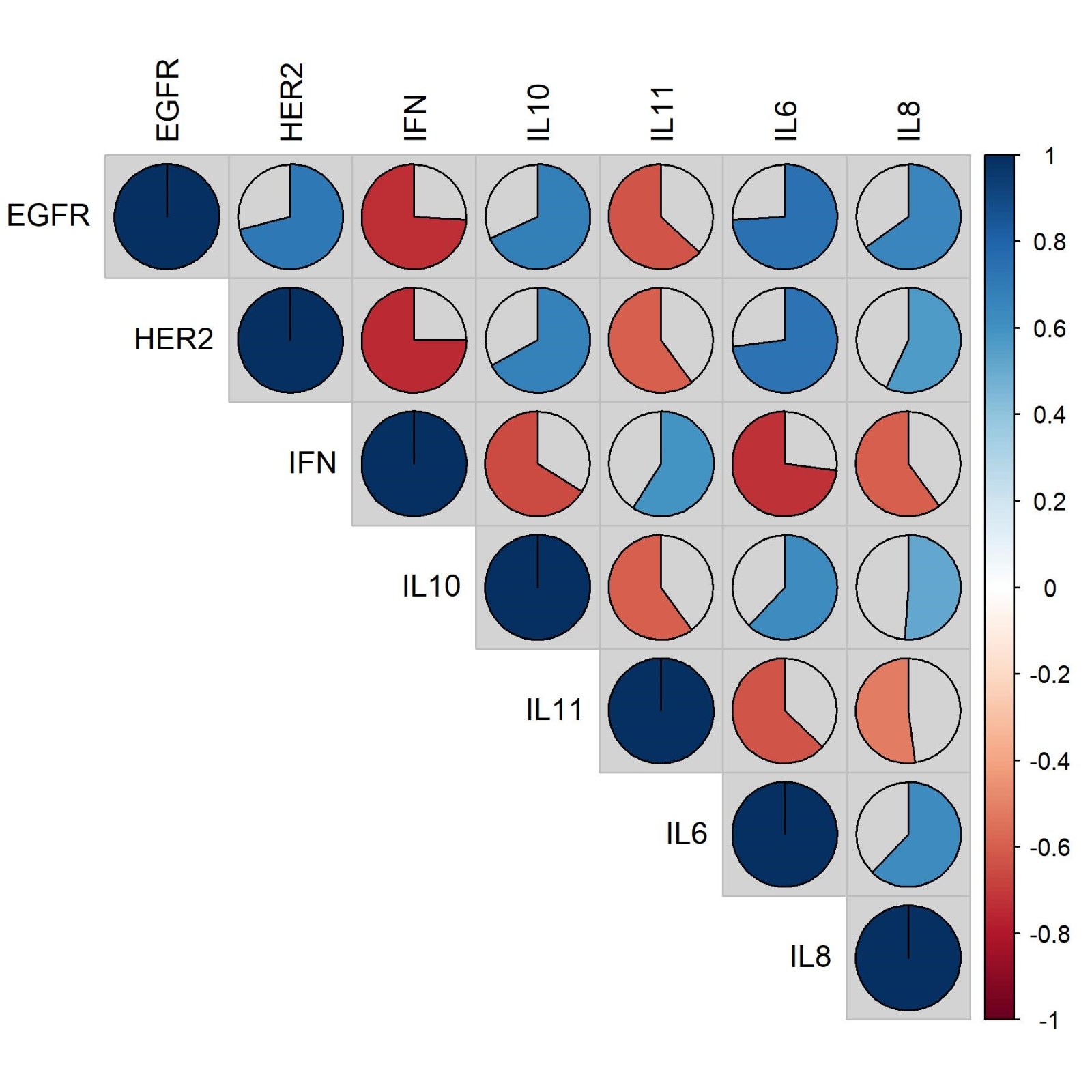 Figure 2. Correlation between each tumor markers (EGFR, HER2, IFN-γ, IL-10, IL-11, IL-6, IL-8).
Figure 2. Correlation between each tumor markers (EGFR, HER2, IFN-γ, IL-10, IL-11, IL-6, IL-8).
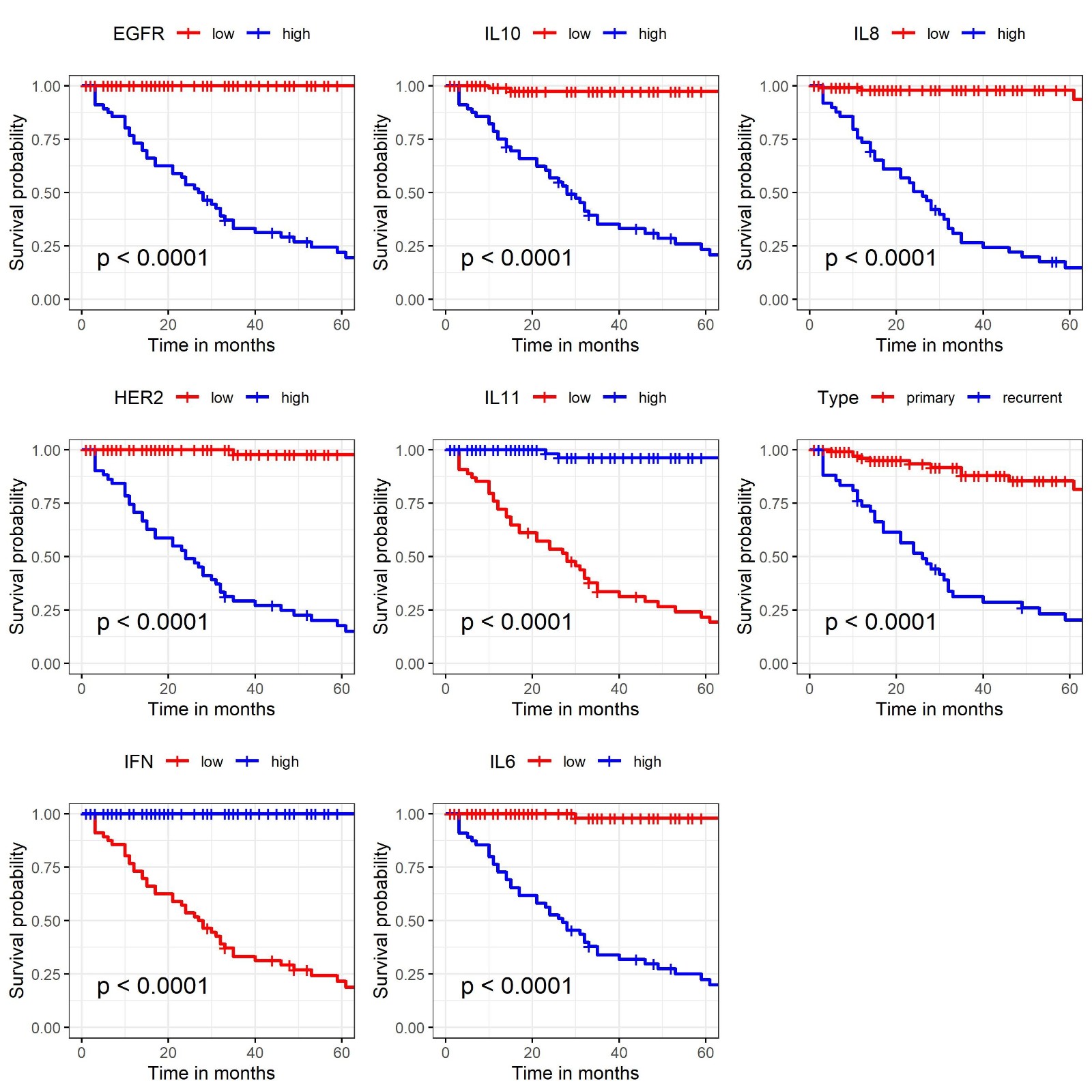 Figure 3. Kaplan Meier survival curves for recurrence-free survival in each tumor markers (EGFR, HER2, IFN-γ, IL-10, IL-11, IL-6, IL-8).
Figure 3. Kaplan Meier survival curves for recurrence-free survival in each tumor markers (EGFR, HER2, IFN-γ, IL-10, IL-11, IL-6, IL-8).
|
Table 1a. Patients characteristics. |
||
|
Items |
Variable |
Cases (160 in total, 100%) |
|
Recurrence |
no |
113 (70.62%) |
|
yes |
47 (29.38%) |
|
|
Time to recurrence, months |
24 (12:49) |
|
|
age |
67.01 (7.92) |
|
|
CIS |
no |
156 (97.5%) |
|
yes |
4 (2.5%) |
|
|
Number |
single |
85 (53.12%) |
|
2-7 |
68 (42.5%) |
|
|
8 or more |
7 (4.38%) |
|
|
Prior recurrence |
primary |
117 (73.12%) |
|
recurrent |
43 (26.88%) |
|
|
Site: lateral |
No |
74 (46.25%) |
|
Yes |
86 (53.75%) |
|
|
Site: posterior and trigone |
No |
59 (36.88%) |
|
Yes |
101 (63.12%) |
|
|
Size |
<3 cm |
61 (38.12%) |
|
3 or more cm |
99 (61.88%) |
|
|
Sex |
Male |
139 (86.88%) |
|
Female |
21 (13.12%) |
|
|
Cystectomy |
no |
149 (93.12%) |
|
yes |
11 (6.88%) |
|
|
Table 1b. Patients characteristics in each tumor markes. |
|||
|
Patients |
Samples |
Tumor markers |
Median (IQR) |
|
Recurrence |
Blood |
EGFR |
2.15 (1.98:3.61) |
|
Blood |
HER2 |
2.01 (1.87:3.4) |
|
|
Urinary |
IFN-γ |
0.54 (0.2:0.7) |
|
|
Urinary |
IL-10 |
22 (20:36) |
|
|
Urinary |
IL-11 |
51 (32:55) |
|
|
Urinary |
IL-6 |
43 (35:66) |
|
|
Urinary |
IL-8 |
232 (187.75:451.75) |
|
|
Table 2. The difference in characteristics between patients who developed recurrence versus no recurrence. |
||||
|
Recurrence |
no |
yes |
p-value |
|
|
Age mean (SD) |
67.381 (8.226) |
66.106 (7.118) |
0.3ST |
|
|
EGFR median (IQR) |
2.1 (1.9:2.3) |
3.8 (3.5:7.1) |
<0.001MW |
|
|
HER2 median (IQR) |
1.967 (1.87:2.01) |
3.8 (3.455:5.45) |
<0.001 MW |
|
|
IFN-γmedian (IQR) |
0.66 (0.54:0.74) |
0.1 (0.07:0.2) |
<0.001MW |
|
|
IL-10 median (IQR) |
21 (20:22) |
38 (35.5:61) |
<0.001MW |
|
|
IL-11 median (IQR) |
55 (51:56) |
31 (30:32.5) |
<0.001 MW |
|
|
IL-6 median (IQR) |
37 (33:44) |
69 (66:91) |
<0.001MW |
|
|
IL-8 median (IQR) |
203 (163:277) |
501 (452.5:699) |
<0.001MW |
|
|
CIS |
no |
109 (96.46%) |
47 (100%) |
0.3F |
|
yes |
4 (3.54%) |
0 (0%) |
||
|
Number |
single |
61 (53.98%) |
24 (51.06%) |
0.3F |
|
2-7 |
49 (43.36%) |
19 (40.43%) |
||
|
8 or more |
3 (2.65%) |
4 (8.51%) |
||
|
Prior recurrence |
primary |
105 (92.92%) |
12 (25.53%) |
<0.001X2 |
|
recurrent |
8 (7.08%) |
35 (74.47%) |
||
|
Site: lateral |
No |
52 (46.02%) |
22 (46.81%) |
1 X2 |
|
Yes |
61 (53.98%) |
25 (53.19%) |
||
|
Site: posterior and trigone |
No |
39 (34.51%) |
20 (42.55%) |
0.4X2 |
|
Yes |
74 (65.49%) |
27 (57.45%) |
||
|
Size |
<3 cm |
43 (38.05%) |
18 (38.3%) |
1X2 |
|
3 or more cm |
70 (61.95%) |
29 (61.7%) |
||
|
MW: Mann Whitney U test; X2: Chi-square test; F: Fischer’s exact test; ST: Student t test. |
||||
|
Table 3. Univariate and multivariate Cox regression analysis for the predictors of recurrence-free survival. |
||||
|
Cox regression analysis |
Variables |
Hazards ratio |
95%CI |
p-value |
|
Univariate |
Age |
0.982 |
(0.947-1.018) |
0.3 |
|
EGFR |
1.69 |
(1.47-1.944) |
< 0.001 |
|
|
HER2 |
2.238 |
(1.857-2.698) |
< 0.001 |
|
|
IFN-γ |
0.003 |
(0-0.016) |
< 0.001 |
|
|
IL-10 |
1.063 |
(1.046-1.081) |
< 0.001 |
|
|
IL-11 |
0.848 |
(0.804-0.895) |
< 0.001 |
|
|
IL-6 |
1.054 |
(1.038-1.069) |
< 0.001 |
|
|
IL-8 |
1.001 |
(1-1.001) |
0.002 |
|
|
Number 2-7 |
1.169 |
(0.636-2.15) |
0.6 |
|
|
Number 8 or more |
2.038 |
(0.705-5.893) |
0.2 |
|
|
Prior recurrence (recurrent vs primary) |
8.836 |
(4.583-17.038) |
< 0.001 |
|
|
Site: lateral (Yes vs no) |
0.989 |
(0.557-1.755) |
1 |
|
|
Site: posterior and trigone (Yes vs no) |
0.863 |
(0.484-1.541) |
0.6 |
|
|
Size 3 or more cm |
0.869 |
(0.482-1.568) |
0.6 |
|
|
Multivariate |
HER2 |
2.675 |
(1.367-5.233) |
0.004 |
|
IFN-γ |
0.441 |
(0.015-12.687) |
0.6 |
|
|
IL-10 |
0.943 |
(0.889-1.001) |
0.05 |
|
|
IL-11 |
0.889 |
(0.825-0.957) |
0.002 |
|
|
IL-6 |
0.992 |
(0.942-1.045) |
0.8 |
|
|
IL-8 |
1.001 |
(0.999-1.002) |
0.2 |
|
|
Prior recurrence (recurrent vs primary) |
1.83 |
(0.834-4.017) |
0.1 |
|
Our study found that lower levels of IL-11 and higher levels of HER2 were significant predictors of RFS on multivariate analysis. IL-11 is a secreted cytokine with an anti-inflammatory nature probably achieved by reducing the number of macrophages at the inflammation site [16]. The potential link between inflammation and the pathogenesis of cancer might explain the IL-11 role in cancer development [17]. In one report, IL-11 was downregulated in human bladder cancer cell lines and was associated with tumor grade and stage [18]. The authors proposed that lower levels might be a mechanism of bladder tumor carcinogenesis and progression and more active treatment should be administered because of the more aggressive biology of these tumors [18].
HER2, a type I trans-membrane 185 kDa tyrosine kinase receptor, is normally responsible for regulating cell proliferation, increasing angiogenesis, inhibiting apoptosis, and decreasing cellular adhesion by the activation of various signaling pathways [19]. In addition to bladder cancer, HER2 overexpression has been identified in several tumor types, including breast and ovarian cancers. The 5-year disease-free survival was significantly reduced (9.7% vs 48.5%) in patients with positive HER2 and the expression of HER2 was linked to decreased disease-specific survival [20] which is in concordance with the current findings.
In our study, all other studied markers were significantly associated with RFS on survival and univariate analyses. These findings, together with others, imply their significance in the pathogenesis of bladder cancer. IL-8 is an angiogenic factor that is expressed in inflammation and carcinogenesis with elevated urinary protein levels have been shown in patients with urothelial carcinoma [21-23]. Elevated urinary levels were linked to higher disease stages [21], increased probability of recurrence [22], and reduced efficacy of intravesical therapies such as mitomycin C and BCG [24]. Likewise, IL-6, a 26 kDa glycoprotein, plays an important role in the pathogenesis of several malignancies by inducing angiogenesis and inhibiting apoptosis of cancer cells [25]. In bladder cancer patients, it was reported elevated serum levels of IL-6 when compared to non-cancer patients suggesting a possible role in urothelial carcinoma [26].
IL-10 is a cytokine synthesis inhibitory factor (CSIF) that has been shown to inhibit cellular immune responses via several mechanisms. IL-10 can block the macrophage accumulation at the tumor site, and down-regulate the expression of MHC-II on these cells, thus suppressing the induction of immune responses [27]. Consequently, blocking IL-10 activity could enhance BCG induction of Th1 immunity and improve the outcome. Previous evidence suggested that IL-10 was upregulated in high-grade and advanced tumor stages [28] which confirms the findings of the current report. On the contrary, it has been shown that higher levels of IFN-γ might predict an enhanced T-cell mediated antitumor immune response and a better prognosis. In one study evaluating a group of patients with metastatic, muscle-invasive, and non-muscle-invasive bladder cancer, IFN-γ was associated with a decreased risk of mortality with improved survival rates though the significance was only delineated in the muscle-invasive group only [29].
The last biomarker evaluated in the current study is the EGFR which is a transmembrane protein that upregulation produced uncontrolled cell division and has been linked to several cancers including lung, anal, and head and neck cancers [30]. In our study, the effect of EGFR was not evaluated in the multivariate analysis because of the multicollinearity between its level and other evaluated biomarkers. This significant correlation would ruin the statistical analysis and hence it was omitted from the final analysis.
While the current study shed the light on some potential prognostic factors for BCG response and elected the most significant factors, it has some limitations that deserve mention. First, it is a single-center study reflecting certain patients’ demographics and cancer characteristics with might not be identical to the results from patients of different geographical distribution. In addition, the lack of exclusion of concurrent urinary tract infections during or after BCG induction may alter urinary cytokines levels by its local inflammatory response. For the survival analysis, we have identified the cut-off value based on a single statistical method among many while there is no known optimal method to determine the cut-off values. However, the Cox analysis is included as it does not depend on stratifying factors based on cut-offs. Several potential prognostic factors were not evaluated with might enhance the findings of the current study. The results of the current study need to be validated on a larger scale and in a multi-institutional setting.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
MGE, HA and AA: Conception, design of study and manuscript preparation; MSE, LAB, AF and AMH: Data collection and analysis; AE: Approval for the final version of the manuscript and funding supports.
Competing interests
The authors have no competing interest.
Funding
None.
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, et al: European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol 2022, 81(1): 75-94.
- Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, Guo CC, Lotan Y, Kassouf W: Bladder cancer. Lancet 2016, 388(10061): 2796-2810.
- Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FC, Oosterlinck W, Prescott S, et al: EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive Stage Ta–T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guerin. Eur Urol 2016, 69(1): 60-69.
- van den Bosch S, Witjes JA: Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol 2011, 60(3): 493-500.
- Singer S, Ziegler C, Schwalenberg T, Hinz A, Götze H, Schulte T: Quality of life in patients with muscle invasive and non-muscle invasive bladder cancer. Support Care Cancer 2013, 21(5): 1383-1393.
- Videira PA, Calais FM, Correia M, Ligeiro D, Crespo HJ, Calais F, Trindade H: Efficacy of bacille Calmette-Guerin immunotherapy predicted by expression of antigen-presenting molecules and chemokines. Urology 2009, 74(4): 944-950.
- Jackson AM, Alexandroff AB, Kelly RW, Skibinska A, Esuvaranathan K, Prescott S, Chisholm GD, James K: Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guerin (BCG) immunotherapy. Clin Exp Immunol 1995, 99(3): 369-375.
- Watanabe E, Matsuyama H, Matsuda K, Ohmi C, Tei Y, Yoshihiro S, Ohmoto Y, Naito K: Urinary interleukin-2 may predict clinical outcome of intravesical bacillus Calmette-Guerin immunotherapy for carcinoma in situ of the bladder. Cancer Immunol Immunother 2003, 52(8): 481-486.
- Saint F, Kurth N, Maille P, Vordos D, Hoznek A, Soyeux P, Patard JJ, Abbou CC, Chopin DK: Urinary IL-2 assay for monitoring intravesical bacillus Calmette-Guerin response of superficial bladder cancer during induction course and maintenance therapy. Int J Cancer 2003, 107(3): 434-440.
- Kamat AM, Briggman J, Urbauer DL, Svatek R, Nogueras González GM, Anderson R, Grossman HB, Prat F, Dinney CP: Cytokine panel for response to intravesical therapy (CyPRIT): Nomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette-Guerin. Eur Urol 2016, 69(2): 197-200.
- Fernandez-Gomez J, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, Hernandez R, Madero R, Ojea A, Pertusa C, Rodriguez-Molina J, et al: Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guerin: multivariate analysis of data from four randomized CUETO trials. Eur Urol 2008, 53(5): 992-1001.
- Kamat AM, Dickstein RJ, Messetti F, Anderson R, Pretzsch SM, Gonzalez GN, Katz RL, Khanna A, Zaidi T, Wu X, et al: Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guerin therapy for bladder cancer: results of a prospective trial. J Urol 2012, 187(3): 862-867.
- Pages F, Flam TA, Vieillefond A, Molinie V, Abeille X, Lazar V, Bressac-de Paillerets B, Mosseri V, Zerbib M, et al: p53 status does not predict initial clinical response to bacillus Calmette-Guerin intravesical therapy in T1 bladder tumors. J Urol 1998, 159(3): 1079-1084.
- Elsawy AA, Harraz AM, Ghobrial FK, Abdullatef M, Ali-El-Dein B: Diagnostic performance and predictive capacity of early urine cytology after transurethral resection of nonmuscle invasive bladder cancer: A prospective study. Urol Oncol 2020, 38: 935.e1-8.
- Jackson AM, Ivshina AV, Senko O, Kuznetsova A, Sundan A, O'Donnell MA, Clinton S, Alexandroff AB, Selby PJ, James K, et al: Prognosis of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer by immunological urinary measurements: statistically weighted syndromes analysis. J Urol 1998, 159(3): 1054-1063.
- Putoczki T and Ernst M: More than a sidekick: the IL-6 family cytokine IL-11 links inflammation to cancer. J Leukoc Biol 2010, 88(6): 1109-1117.
- Coussens LM and Werb Z: Inflammation and cancer. Nature 2002, 420(6917): 860-867.
- Wu D, Tao J, Ding J, Qu P, Lu Q, Zhang W: Interleukin-11, an interleukin-6-like cytokine, is a promising predictor for bladder cancer prognosis. Mol Med Rep 2013, 7(2): 684-688.
- Tschui J, Vassella E, Bandi N, Baumgartner U, Genitsch V, Rotzer D, Seiler R, Thalmann GN and Fleischmann A: Morphological and molecular characteristics of HER2 amplified urothelial bladder cancer. Virchows Arch 2015, 466(6): 703-710.
- Sanguedolce F, Russo D, Mancini V, Selvaggio O, Calo B, Carrieri G, Cormio L: Prognostic and therapeutic role of HER2 expression in micropapillary carcinoma of the bladder (Review). Mol Clin Oncol 2019, 10(2): 205-213.
- Sheryka E, Wheeler MA, Hausladen DA, Weiss RM: Urinary interleukin-8 levels are elevated in subjects with transitional cell carcinoma. Urology 2003, 62(1): 162-166.
- Sagnak L, Ersoy H, Ozok U, Senturk B, Ercil H, Bahar G, Ozturk E: Predictive value of urinary interleukin-8 cutoff point for recurrences after transurethral resection plus induction bacillus Calmette-Guerin treatment in non-muscle-invasive bladder tumors. Clin Genitourin Cancer 2009, 7(2): E16-23.
- Koçak H, Oner-Iyidoğan Y, Koçak T, Oner P: Determination of diagnostic and prognostic values of urinary interleukin-8, tumor necrosis factor-alpha, and leukocyte arylsulfatase-A activity in patients with bladder cancer. Clin Biochem 2004, 37(8): 673-678.
- Kumar A, Dubey D, Bansal P, Mandhani A, Naik S: Urinary interleukin-8 predicts the response of standard and low dose intravesical bacillus Calmette-Guerin (modified Danish 1331 strain) for superficial bladder cancer. J Urol 2002, 168(5): 2232-2235.
- Mastorakos, G and Ilias, I: Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann N Y Acad Sci 2007, 1088: 373-381.
- Abdulmohymen N and Ashoor Z: Serum interleukin-6 level using ELISA in patients with bladder cancer and having urinary tract infection. Iraqi J Comm Med 2010, 4: 251-256.
- Chan C, John S and Abraham S: Mast Cell Interleukin-10 Drives Localized Tolerance in Chronic Bladder Infection. Immunity 2013, 10(16): 10-19.
- Luo Y, Eric J, Skeland A, Mark R and O’Donnell M: Role of IL-10 in Urinary Bladder Carcinoma and Bacillus Calmette-Guerin Immunotherapy. Am J Immunol 2012, 8(1): 1-9.
- Gillezeau C, Movva N, van Gerwen M, Rabon-Stith K, Shire N, Brohawn PZ, Taioli E, Fryzek J: Interferon gamma expression and mortality in unselected cohorts of urothelial bladder cancer patients. PLoS One 2022, 17(8): e027133.
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al: Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004, 350(21): 2129-2139.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript