Case Report | Open Access
Small Cell Neuroendocrine Carcinoma of the Urinary Bladder: A Rare Entity
Shailaja Shukla1, Neha Suman1, Srijan Srivastav21Department Of Pathology, Lady Hardinge Medical College, New Delhi, India.
2Department Of Pathology, Veer Chandra Singhgarhwali Government Institute Of Medical Sciences & Research, Srinagar, Uttarakhand, India.
Annals of Urologic Oncology 2024, 7(2): 79-82. https://doi.org/10.32948/auo.2024.05.24
Received: 08 May 2024 | Accepted: 31 May 2024 | Published online: 01 Jun 2024
Key words neuroendocrine carcinoma, radical cystoprostatectomy, urothelial carcinoma
The pathogenesis of primary small cell neuroendocrine carcinoma is still subject of debate. Many hypotheses had been given: 1. malignant transformation of bladder neuroendocrine cells gives rise to bladder SCC. This hypothesis was supported by the fact that neuroendocrine cells were found previously in the urinary bladder [5]. 2. urothelial metaplastic changes. 3. the small cell carcinoma of bladder may arise from the totipotent stem cells present in the submucosa of the urinary tract. These cells have the ability to differentiate into various cell types depending on the influence of specific transformation or progression-related gene. This idea of transformation of totipotent cells to tumour cells may explain the coexistence of this tumour with transitional cell carcinoma, and also the heterogeneity of the immune-histochemical expression of cytokeratin and neuroendocrine markers [1, 6, 7]. Here in we report such a case of primary small cell neuroendocrine carcinoma of bladder in an elderly male who presented with symptoms of burning micturition and haematuria.
Grossly, the specimen of urinary bladder, prostate with seminal vesicles, both sided ureters and perivesical adipose tissue altogether measured 11 × 8.5 × 6 cm. A large fragile polypoidal mass was noted on the posterior wall of bladder, measuring 4.5cm in its greatest dimension. Overlying mucosa was ulcerated (Figure 1).
Microscopic examination revealed tumour composed of sheets of small round cells separated by scant stroma. These cells were small with scant cytoplasm, high nuclear-cytoplasmic ratio, moderate nuclear atypia with finely granular chromatin and inconspicuous nucleoli (Figure 2). Large areas of necrosis were seen. Mitosis was ≥ 10/HPF. The tumour was diffusely infiltrating the muscularis propria. No urothelial component was observed even after extensive sampling.
Immunohistochemistry was done. The tumour cells were positive for NSE, CD56, and synaptophysin with 75% Ki-67 index (Figure 3). Pan Cytokeratin, LCA and vimentin were found to be negative.
Based on the histopathological examination and immunohistochemistry, the diagnosis of primary small cell neuroendocrine carcinoma of urinary bladder was rendered.
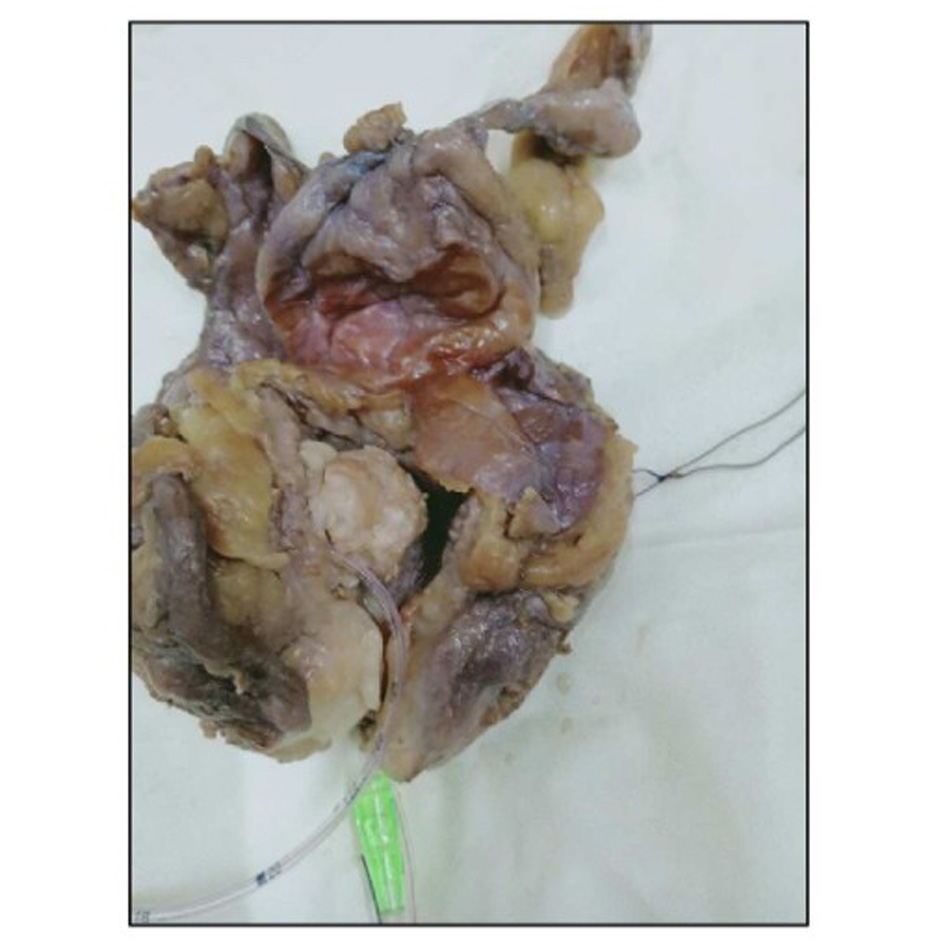 Figure 1. Gross image showing polypoidal solid mass.
Figure 1. Gross image showing polypoidal solid mass.
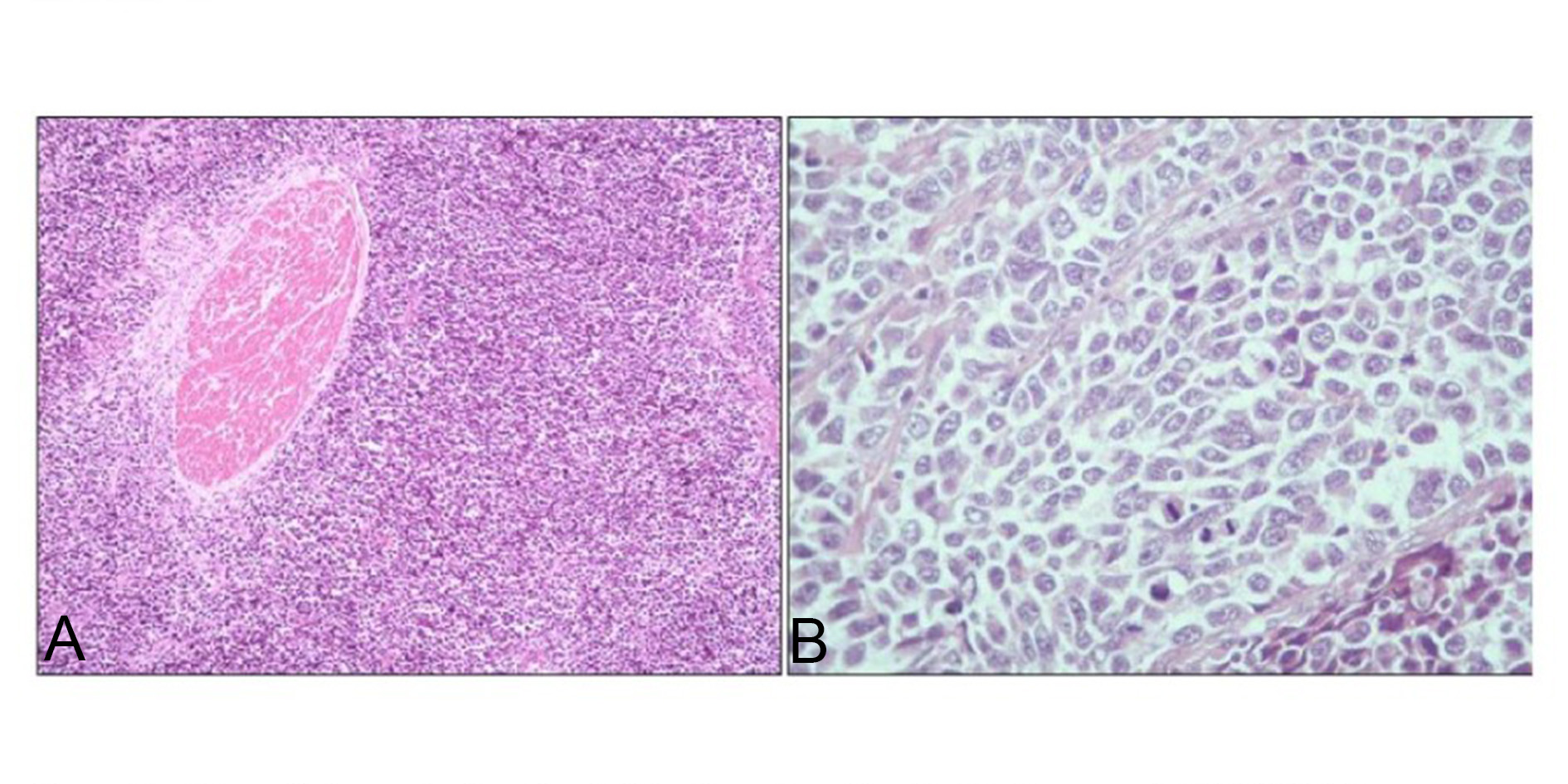 Figure 2. (A) Microphotograph showing infiltration of small cells into the muscle (H&E, 100x); (B) Microphotograph showing sheets of small cells with finely granular chromatin & high mitotic activity (H&E, 400x).
Figure 2. (A) Microphotograph showing infiltration of small cells into the muscle (H&E, 100x); (B) Microphotograph showing sheets of small cells with finely granular chromatin & high mitotic activity (H&E, 400x).
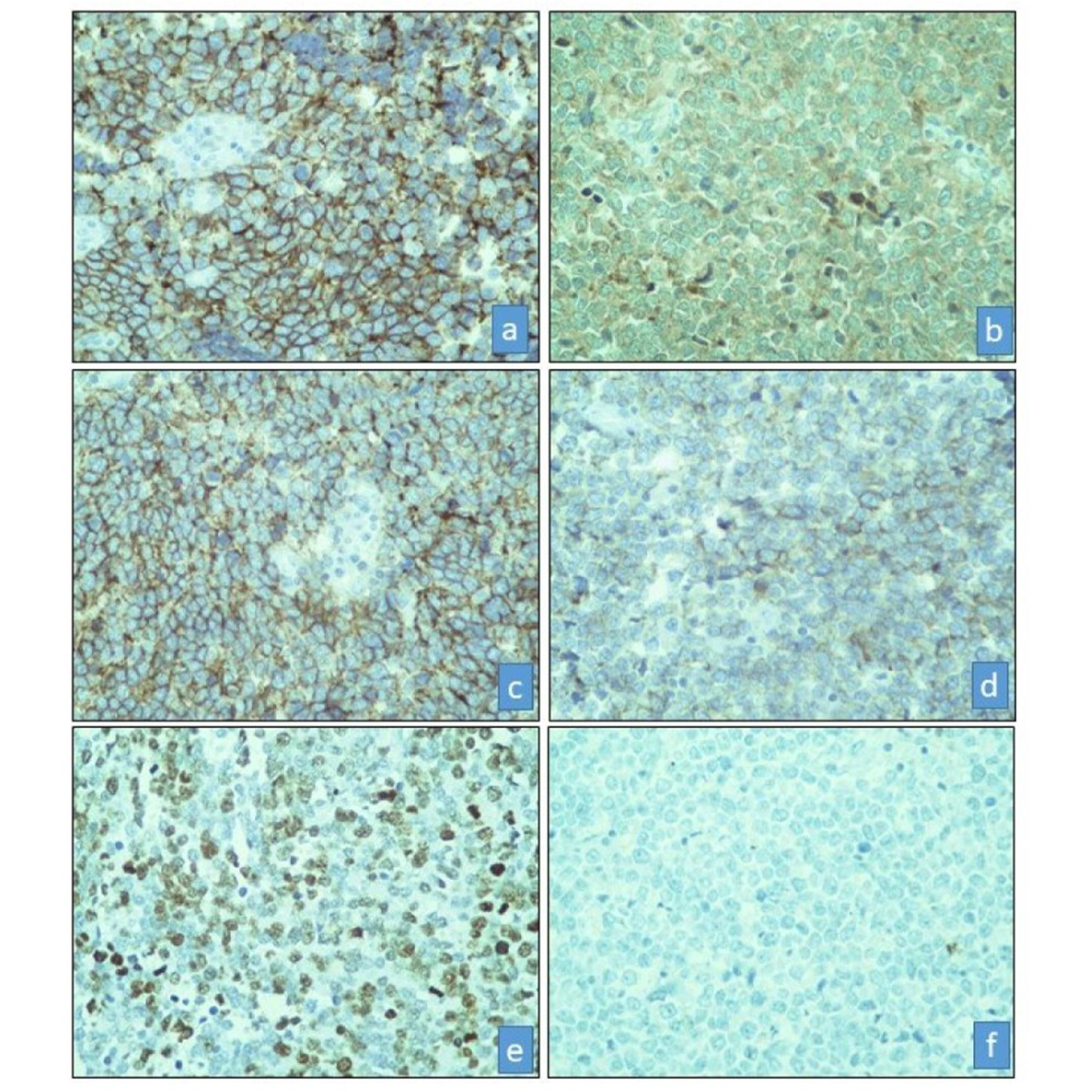 Figure 3. Microphotograph showing positive immunostain for (a) CD56 (CD56, 400x), (b) NSE (NSE, 400x), (c) Synaptophysin (Synaptophysin, 400x), (d) Chromogranin (Chromogranin, 400x), (e) High Ki-67 Index (Ki-67, 400x), (f) Negative immunostain for Cytokeratin (Cytokeratin, 400x).
Figure 3. Microphotograph showing positive immunostain for (a) CD56 (CD56, 400x), (b) NSE (NSE, 400x), (c) Synaptophysin (Synaptophysin, 400x), (d) Chromogranin (Chromogranin, 400x), (e) High Ki-67 Index (Ki-67, 400x), (f) Negative immunostain for Cytokeratin (Cytokeratin, 400x).
The age of presentation varies from 20-91 years. These patients usually present with painless gross hematuria, dysuria and iritative symptoms have also been documented [1]. Our patient presented with hematuria and burning micturition. One of the reasons for hematuria can be the deeply invasive large polypoidal ulcerated lesion.
Diagnosis is mainly by histopathological examination and immunohistochemistry plays a crucial role in differentiating it from its mimickers. The differential diagnosis includes poorly differentiated urothelial carcinoma and non-Hodgkin lymphoma (NHL). Immunohistochemical neuroendocrine markers such as chromogranin, synaptophysin and CD56 are helpful as on microscopy poorly differentiated urothelial carcinoma may also present as sheets of tumour cells, with areas of necrosis and brisk mitoses. NHL shows positive expression for LCA and negative expression for the neuroendocrine markers [5]. Immunohistochemistry was done in the present case also. The tumour cells were positive for neuroendocrine markers - NSE, CD56, and synaptophysin. LCA was found to be negative.
The WHO classification for neuroendocrine neoplasms of urinary bladder includes: 1. Well-differentiated neuroendocrine carcinoma; 2. Small cell neuroendocrine carcinoma; 3. Large cell neuroendocrine carcinoma; 4. Mixed neuroendocrine neoplasms; 5. Paragnaglioma [8]. Recent studies have highlighted the presence of different types of bladder cancers based on tumor heterogeneity. Trancriptional profiling has updated the primary bladder tumors classification as intrinsic basal and luminal molecular subtypes. Kamoun et al described six molecular subtypes of bladder carcinomas as Luminal papillary, Luminal nonspecified, Luminal unstable, Stroma-rich, Basal/Squamous and Neuroendocrine-like (NE-like). They further described few genes for their genomic alterations. Neuroendocrine carcinomas of bladder were associated with mutations in TP53 and RB1. They also found that worst outcome was associated with patients with NE-like carcinomas [9].
Neuroendocrine carcinoma of the urinary bladder is an aggressive tumor with most tumors diagnosed at advanced stages. Radical cystectomy has been proposed in patients with locally advanced disease and adjuvant treatment for patients with stage III and VI (M0) disease [10]. In a multi-institutional review of 64 patients with a muscle invasive disease, a multivariate analysis indicated that neither chemotherapy, nor radiation, nor surgery had any impact on overall survival [11]. Based on the heterogeneity of these tumors, molecular classification will facilitate better treatment options and hence better patient care.
Not applicable.
Ethical policy
Informed patient consent was taken. Any identifying details (such as name) of the patient will not be published.
Availability of data and materials
That data is available from the corresponding author on request.
Author contributions
Shailaja Shukla contribute to study design, manuscript editing and manuscript review. Neha Suman contributed to design, manuscript preparation, literature search and manuscript editing. Srijan Srivastav contributed to manuscript review and literature search. All authors read and approved the manuscript.
Competing interests
The authors have no conflicts of interest to declare.
Funding
None.
- Ismaili N, Ghanem S, Mellas N, Afqir S, Taleb M, Amrani M, Gamra L, Errihani H: Small cell carcinoma of the urinary bladder: A case report and review of the literature. J Can Res Ther 2009, 5(2): 133-136.
- Cramer SF, Aikawa M, Cebelin M: Neurosecretory granules in small cell invasive carcinoma of the urinary bladder. Cancer 1981, 47(4): 724-730.
- Shahab N: Extrapulmonary small cell carcinoma of the bladder. Semin Oncol 2007, 34(1): 15-21.
- Holmang S, Borghede G, Johansson SL: Primary small cell carcinoma of the bladder: A report of 25 cases. J Urol 1995, 153(6): 1820-1822.
- Rao M, Dubey A, Pandey H, Sureka B, Garg PK, Bohra H, Elhence P: Neuroendocrine tumors of the urinary bladder – report of two cases. Autops Case Rep [Internet]. 2021, 11: e2021305.
- Terracciano L, Richter J, Tornillo L, Beffa L, Diener PA, Maurer R, Gasser TC, Moch H, Mihatsch MJ, Sauter G: Chromosomal imbalances in small cell carcinomas of the urinary bladder. J Pathol 1999, 189(2): 230-235.
- Christopher ME, Seftel AD, Sorenson K, Resnick MI: Small cell carcinoma of the genitourinary tract: an immunohistochemical, electron microscopic and clinicopathological study. J Urol 1991, 146(2): 3828.
- Moch H,Humphrey PA,Ulbright TM, Reuter VE: WHO classificationof tumors of the urinary system and male genital organs. 4th ed. Lyon: IARC 2016, P. 117-119.
- Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, Hoadley KA, Groeneveld CS, Al-Ahmadie H, Choi W, et al: Bladder Cancer Molecular Taxonomy Group. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur Urol 2020, 77(4): 420-433.
- Choong NW, Fernando Quevedo J, Kaur JS: Small cell carcinoma of the urinary bladder: The Mayo Clinic experience. Cancer 2005, 103(6): 1172-1178.
- Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Lin H, Kuzel TM, Papavero V, Tretiakova M, Nigro K, et al: Small cell carcinoma of the urinary bladder: A clinicopathologic analysis of 64 patients. Cancer 2004, 101(5): 957-962.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript