Research Article | Open Access
Is Robotic-assisted Partial Nephrectomy an Efficacious and Safe Procedure for Removal of Stage T1 Renal Tumors?
Deshraj Choudhary1, Shams Tabrej Asgarali Ansari2, Ershad Hussain Galeti3, Saqib Shahab4
1Dept of urology, Jaslok Hospital, Mumbai, India.
2Dept of urology, DR BSAMCH Hospital, Delhi, India.
3Dept of urology, Narayana Medical College, Nellore, India.
4Department of Surgery, RDJM Medical College, Muzaffarpur, Bihar, India.
Correspondence: Ershad Hussain Galeti (Dept of urology, Narayana medical college, Nellore, Andhra Pradesh, India; Email: dr.ershadhussain@gmail.com).
Annals of Urologic Oncology 2022, 5(1): 8-19. https://doi.org/10.32948/auo.2022.03.23
Received: 08 Mar 2022 | Accepted: 21 Mar 2022 | Published online: 13 Apr 2022
Materials & Methods It is a prospective observational study over 18 months in patients aged more > 18 years with a renal tumour of clinical stage T1 coming for robotic partial nephrectomy. A total of 40 patients were included in the study who underwent robotic-assisted partial nephrectomy for renal tumours less than 7 cm in size. The duration of the study was from July- 2018 to December-2019 and their follow up period was 3 months post-surgery.
Results Of the 40 patients, the mean age was 52.3 ± 11.91 years. 28 (70%) patients were males and remaining 12 (30%) were females. 11 (27.5%) tumours were situated in the anterior upper pole, 9 (22.5%) in the anterior lower pole, 8 (20%) in the posterior upper pole, 7 (17.5%) in the posterior lower pole and interpolar tumours were 5 in number (12.5%). 24 (60%) tumors were ≥ 50% exophytic, 11 (27.5%) were < 50% exophytic and 5 (12.5%) were purely endophytic in nature. Maximum nephrometry score was 5a amounting to 10 (25%) patients and 5p tumours were the second most common amounting to 7 (17.5%) patients. The mean console time was noted to be 84.40± 12.05 mins. The mean total operative time was noted to be 167.00 ± 21.116 minutes. Mean Warm Ischemia Time (WIT) was recorded to be 27.28 ± 5.923 minutes. The mean blood loss was 145.75±61.075 ml. The mean length of hospital stay was 4.27 ± 0.78 days. None of the cases was converted to open partial/radical nephrectomy and none had positive surgical margins in the histopathology reports.
Conclusion Our study shows that Robotic Assisted Partial Nephrectomy (RAPN) is an efficacious and safe surgery in stage T1 renal tumours (tumour size <7 cm) to achieve complete oncological clearance by minimal access technique.
Key words Partial nephrectomy, renal cell carcinoma, robotic surgery
“The European Organization for Research and Treatment of Cancer” provided the first Level I evidence that long-term oncologic outcomes between partial nephrectomy and radical nephrectomy were equivalent, allowing partial nephrectomy to become a standard of care for small renal masses [2]. Although open partial nephrectomy is an efficacious procedure, a minimally invasive approach might be more attractive to patients [3].
Robotic surgery has now evolved as an emerging tool for better and easy operative techniques. Though robotic has high costing value, their outcome has superseded cost analysis and is being widely used for its outcome, thus the limitation of laparoscopy has been overcome by a robot. As the technique of RAPN (robotic-assisted partial nephrectomy) continues to develop and mature, intraoperative & perioperative outcomes continue to be reported [4].
The introduction of robotics added a new dimension to minimally invasive surgery with three-dimensional (3D) visual capabilities and advanced instrumentation having freedom of movement similar to that of the human wrist [5]. Recently there have been several reports indicating that robotic surgery facilitates the resection of the tumour and intracorporeal suturing thus decreasing the warm ischemia time [6-8]. With the availability of robots, even large size tumours have been operated on to preserve the nephrons and with good postoperative outcomes.
The quest for trifecta comprising warm ischemia time less than 25 minutes, negative surgical margins and no perioperative complications seems to be better accomplished by robotic partial nephrectomy, which is likely to become the new standard for minimally invasive partial nephrectomy [9]. Compared to open partial nephrectomy (OPN), RAPN had lower odds than LPN for most studied outcomes except hospital charges. RAPN has now supplanted LPN as the most common minimally invasive approach for partial nephrectomy[10].
In our centre, where we have a high volume of cases, we have started doing renal tumours of size up to 7 cm with robotic partial nephrectomy. This study aims to see the outcome of robotic partial nephrectomy for renal tumours of size up to 7 cm.
Objectives
To assess efficacy and safety of Robotic partial nephrectomy for renal tumour clinical stage T1 in terms of warm ischemia time (WIT), estimated blood loss (EBL), total operative time (TOT), console time (CT), positive surgical margins (PSM), perioperative complications and length of hospital stay (LOH).
Patients and methods
It is a prospective observational study over 18 months in patients aged
more > 18 years with a renal tumour of clinical stage T1 coming for
robotic partial nephrectomy.
The sample size was calculated at a
95% confidence level assuming a standard deviation of 8.9 minutes in
warm ischemia time as a result of a reference study of Ener et al [11].
Inclusion criteria were all patients of age more than 18 years with
renal tumours equal to or less than 7 cm undergoing robotic partial
nephrectomy and willing to participate in the study and giving consent.
Exclusion criteria were Patients with Renal tumours > 7 cm and
Patients refusing to give consent. This study was carried out only after
the clearance from the Ethics Committee. All eligible patients
fulfilling inclusion criteria were approached by the investigator
himself and explained the nature and purpose of the study. After
obtaining their informed consent, detailed history regarding
socio-demographic characteristics, symptoms and clinical profile was
taken and a thorough general and systemic examination were done. Routine
and specific investigations required for preoperative evaluation was
done in the identified lab and by designated experts. If amenable to
partial nephrectomy, patients were offered RAPN with the Da Vinci Si HD
surgical system (Figure 1 and 2). Port placement and docking of robotic
is done as shown in figure 3 and 4. Intra-operative details such as
total operative time, warm ischemia time, blood loss, and complications
and post-operative data such as blood transfusions and length of
hospital stay recorded by the investigator himself and surgical specimen
taken and sent in the designated laboratory to designated experts. The
surgical pathology reports and the margin clearance were collected
prospectively. Data thus collected recorded on pre-designed
semi-structured study proforma.
Patients who had grade < 2 Clavien Dindo complications, negative surgical margins, and warm ischemia time [WIT] ≤25 minutes were accepted to fit the strict Trifecta outcomes.
Statistical analysis
Linear variables were described as mean and standard deviation and analysed by using unpaired t-test and one-way Anova test. Nominal/categorical variables expressed as proportions (%) and analysed by using Chi-square test / Fisher exact test. Ordinal variables are summarized as median and range. P-value < 0.05 taken as significant. Medcalc 16.4 version software is used for all statistical calculations.
 Figure 1. Our da Vinci Si HD Robot.
Figure 1. Our da Vinci Si HD Robot.
 Figure 2. Operating surgeon console of the da Vinci Robot.
Figure 2. Operating surgeon console of the da Vinci Robot.
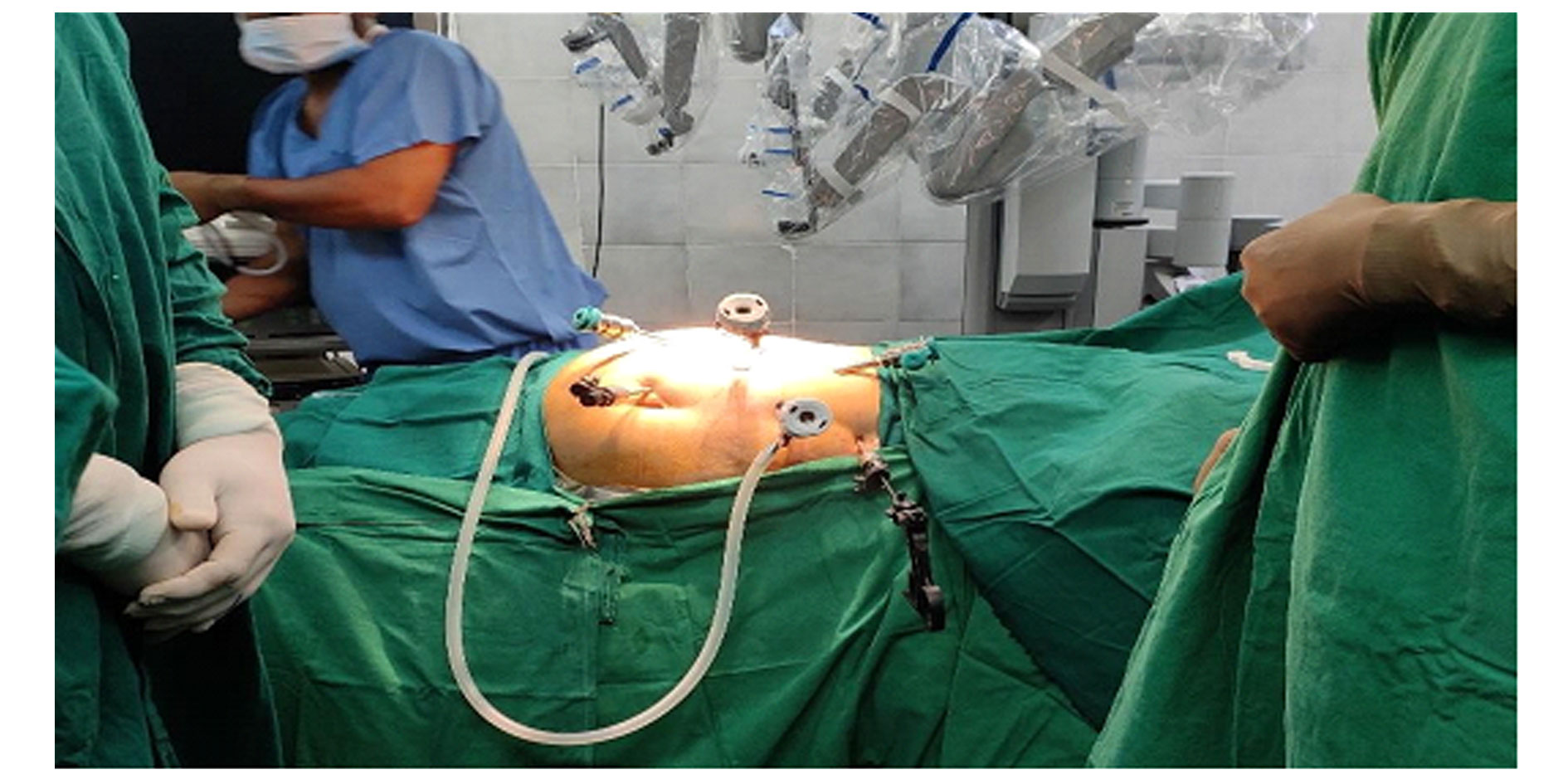 Figure 3. Normal placement of the laparoscopic assistant and Robotic ports for right RAPN.
Figure 3. Normal placement of the laparoscopic assistant and Robotic ports for right RAPN.
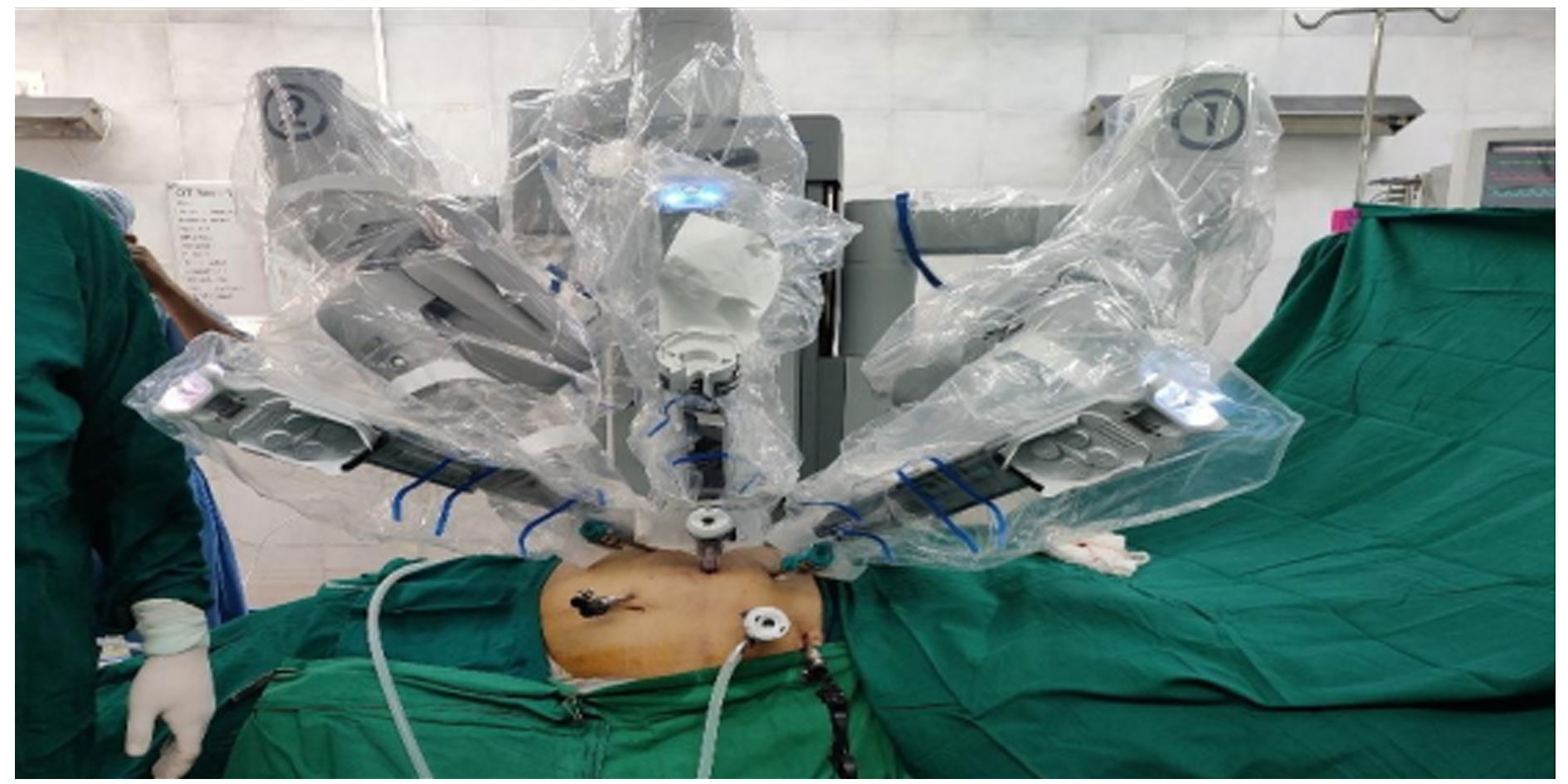 Figure 4. Post docking of Robot with the instruments in position.
Figure 4. Post docking of Robot with the instruments in position.
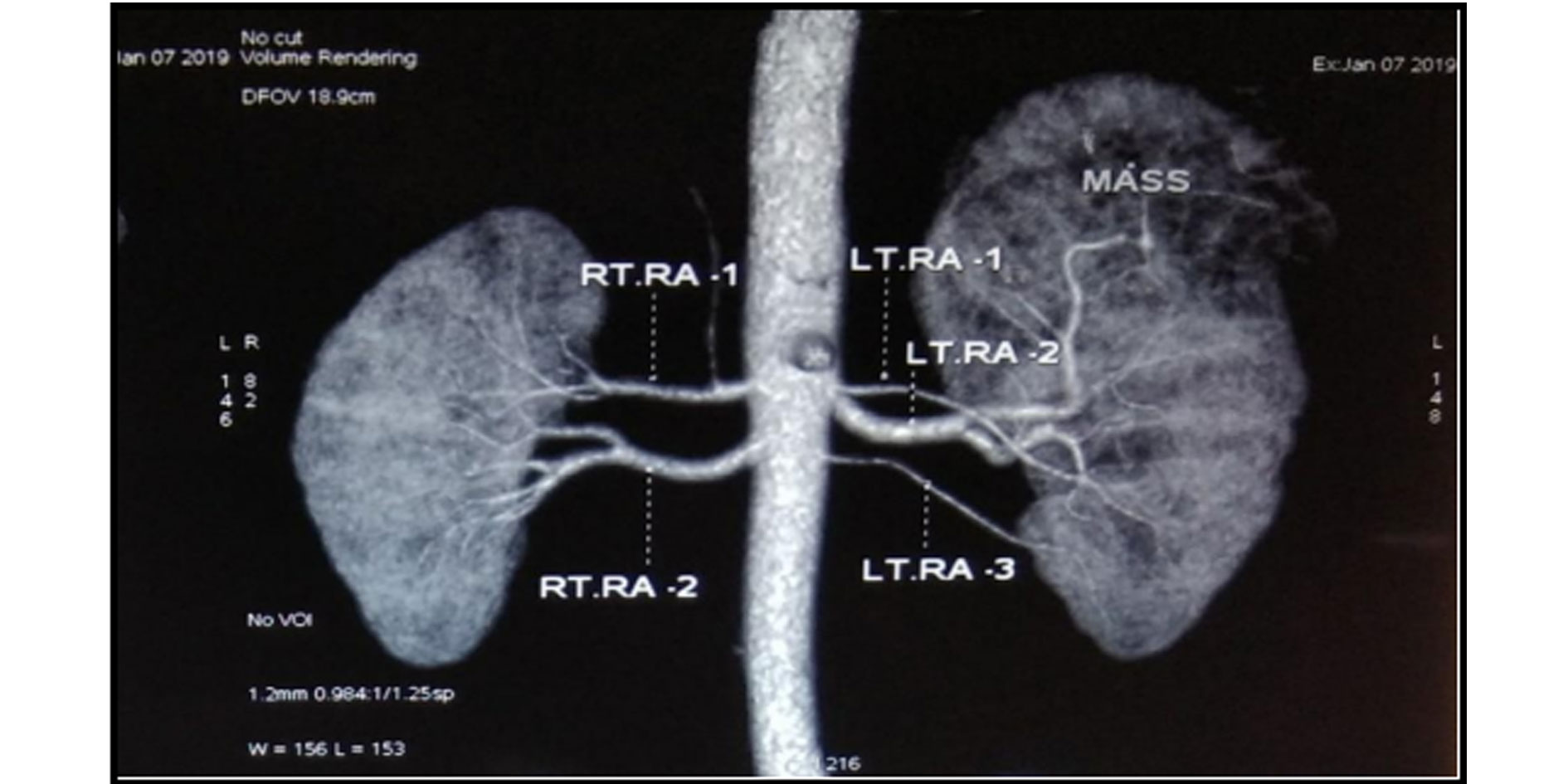 Figure 5. CT Renal angiogram showing dual arteries supply to right kidney and three artery supply to left kidney. The mass lesion is supplied from superior segmental branch of left renal artery.
Figure 5. CT Renal angiogram showing dual arteries supply to right kidney and three artery supply to left kidney. The mass lesion is supplied from superior segmental branch of left renal artery.
Genderwise distribution: Out of the 40 patients, 28 (70%) patients were males. The remaining 12 (30%) patients were females.
Clinical presentation: Out of a total number of 40 patients, 6 patients (15%) were presented with pain, 3 patients (7.5%) were presented with hematuria, 7 patients (17.5%) presented with lower urinary tract symptoms, 21 patients incidentally presented and 3 patients presented with other symptoms (fatigue, urinary tract infection).
Co-morbid conditions: Out of the 40 patients in the studied period, 13 (32.5%) were diabetic. 22 (45%) were hypertensive. 5 (12.5%) had Coronary Artery Disease. 8 (20%) patients had other comorbid conditions like hypothyroidism, Bronchial Asthma & COPD.
Size of tumour: Mean tumour size was 4.70 ± 0.847 cm with max tumour size being 6.7cm & min of 2.8cm.
Side of tumour: The distribution of the side of tumours was fairly well distributed with 22 (55%) tumours being right-sided & the remaining 18 (45%) being left-sided.
Tumour location: 11 (27.5%) tumours were situated in the anterior upper pole. 9 (22.5%) tumours were located in the anterior lower pole, 8 (20%) were in the posterior upper pole, 7 (17.5%) were in the posterior lower pole. Interpolar tumours were 5 in number (12.5%).
Phyticity: 24 (60%) tumors were ≥ 50% exophytic, 11 (27.5%) tumors were < 50% exophytic. 5 (12.5%) tumors were purely endophytic in nature.
Renal nephrometry score: Maximum number of tumours being 5a amounting to 10 (25%). 5p tumours were the second most common amounting to 7 (17.5%).
24 patients (60%) had low renal scores, 14 patients (35%) had a moderate renal score and the remaining 2 patients (5%) had high renal scores.
Renal vasculature on affected side: All the cases that underwent the surgery had a preoperative contrast-enhanced CT Scan with 3D vascular reconstruction done. It was noted 33 (82.5%) out of the 40 tumours had a single renal artery, whereas the other 7 (17.5%) had two or more arteries supplying the affected kidney (Figure 5).
Console time (ct): The mean console time was noted to be 84.40± 12.05 mins. The minimum console time was 65 minutes & the maximum was 110 minutes.
Total operative time: The mean total operative time was noted to be 167.00 ± 21.116 minutes. The minimum time was 130 minutes & the maximum was 240 minutes.
Warm ischemia time: Mean Warm Ischemia Time (WIT) was recorded to be 29.28 ± 5.923 minutes. Minimum WIT was 25 minutes & maximum time was 40 minutes.
Hilar clamping: Hilar clamping was done for all the cases. Only the artery was clamped in 32 cases (80%). Both vein & artery were clamped in the remaining 8 (20%) cases.
Estimated blood loss: The mean blood loss was 145.75±61.075 ml. Minimum blood loss was 75 ml and maximum blood loss was 460 ml. An overwhelming 32 (80%) cases had blood loss in the range of 100-200 ml. 5 cases (12.5%) had blood loss greater than 200 ml. 3 (7.5%) cases had blood loss <100 ml.
Opening of the pelvicalyceal system: Pelvicalyceal system opening was considered an intra-operative event. 12 (30%) of the cases had an opening of the Pelvicalyceal system, all of which were repaired with vicryl 3-0. In the remaining 28 (70%) cases Pelvicalyceal system was intact.
Placement of DJ stent: DJ Stent was placed in 17 (42.5%) cases and 23 (57.5%) cases were without DJ stents. Most DJ stents were placed antegrade over a hydrophilic guidewire.
Blood transfusion: Only 1 (2.5%) patients had required blood transfusion. The remaining 39 (97.5%) patients did not require a blood transfusion.
Haemoglobin: Preoperative HB and immediate postoperative HB, Preoperative HB and postoperative 3 months HB are positively correlated and there was a significant difference between Preoperative HB and postoperative HB (t=9.296), Preoperative HB and postoperative 3 months HB (t=5.290). So, in 95% CI, preoperative HB is better than postoperative HB and 3 months postoperative HB. But 3 months postoperative HB is better than postoperative HB (t= -2.275)
Serum creatinine: 36 (90%) patients had normal serum creatinine values at the time of diagnosis. 4 (10%) patients were found to be azotemic. All the patients had azotemia secondary to medical renal disease with no evidence of obstructive uropathy. All of the azotemic patients had stable chronic kidney disease, with none of the patients being on dialysis. The creatinine values of the patients who had elevated pre-operative creatinine ranged from 1.3 mg/dl to 3.79 mg/dl.
Preoperative serum creatinine level is lower than postoperative immediate level (t: -6.231) & 3 months level (t: -6.302), but the immediate postoperative and 3 months serum creatinine levels were almost the same (t: 0.00).
Length of hospital stay: The mean length of hospital stay was 4.27 ± 0.78 days. The minimum stay recorded was 3 days & the maximum stay was 7 days.
Postoperative infection: 1 patient (2.5%) developed fever due to wound infection which was managed by intravenous antibiotics and conservative management. 39 (97.5%) patients had no infection.
Histology of resected mass: 27 (67.5%) tumours had histopathology report as clear cell carcinoma. Next common was papillary cell carcinoma 6 (15%), 3 (7.5%) tumours as chromophobe cell carcinoma 2 (5.0%) as oncocytoma and 2 (5.0%) as other histology as angiomyolipoma and adenoma.
Warm ischemia time with a renal score: Out of the total of 40 patients, 24 patients had a low renal score. Mean warm ischemia time for low renal score patient was 23.75 ± 4.4 minutes, whereas moderate renal score patients (14 in number) had to mean warm ischemia time of 31.64 ± 2.3 minutes and high renal score patients (2 in number) had a mean warm ischemia time of 39.00 ± 1.4 minutes with highly significant p-value (0.000) suggesting strong positive correlation in between RENAL score and warm ischemia time.
None of the cases was converted to open partial/radical nephrectomy.
None of the cases had positive surgical margins in the histopathology reports.
There were no reported cases of major vessel injury during any of the surgeries performed.
No secondary haemorrhage was noted in any of the cases that were performed in the study period.
No urinary leak was noted in postoperative cases in all patients.
There were no reported incidents of the technical fault with the robot in any of the cases.
Clavien Dindo grade of complications: 1 (2.5%) patients had Grade 1 complications in the form of mild fever in the post-operative period due to wound infection, whereas the remaining 39 (97.5%) patients had no complications in the postoperative period according to clavien Dindo grade.
32 (80%) patients had strict Trifecta outcomes. Because all patients had negative surgical margins, Trifecta outcomes were based on WIT and complications. One patient (2.5%) had grade 1 Clavien-Dindo complications, 8 (20%) had WIT longer than 25 minutes (Table 6).
|
Table 1a. Demographic and peri-operative data in percentage. |
||
|
|
Frequency (n=40) |
Percententage |
|
Age Distribution (years) |
||
|
Upto 40 |
5 |
12.5% |
|
41-50 |
15 |
37.5% |
|
51-60 |
10 |
25.0% |
|
61 years and above |
10 |
25.0% |
|
Gender-wise Distribution |
||
|
Male |
28 |
70.0% |
|
Female |
12 |
30.0% |
|
Clinical Presentation |
||
|
Pain |
6 |
15.0% |
|
Haematuria |
3 |
7.5% |
|
LUTS |
7 |
17.5% |
|
Other |
3 |
7.5% |
|
Incidental |
21 |
52.5% |
|
Co-morbid Conditions |
||
|
Diabetes |
13 |
32.5% |
|
Hypertensive |
22 |
45.0% |
|
Coronary artery disease |
5 |
12.5% |
|
Others |
8 |
20.0% |
|
Side of Tumor |
||
|
Right |
22 |
55.0% |
|
Left |
18 |
45.0% |
|
Tumor Location |
||
|
Anterior upper pole |
11 |
27.5% |
|
Anterior lower pole |
9 |
22.5% |
|
Posterior upper pole |
8 |
20.0% |
|
Posterior lower pole |
7 |
17.5% |
|
Interpolar |
5 |
12.5% |
|
Phyticity |
||
|
≥50% Exophytic |
24 |
60.0% |
|
<50% Exophytic |
11 |
27.5% |
|
100% endophytic |
5 |
12.5% |
|
Table 1b. Demographic and peri-operative data in percentage. |
||
|
Renal Nephrometry score-range |
||
|
Low Renal score (4-6) |
24 |
60% |
|
Moderate Renal score (7-9) |
14 |
35% |
|
High Renal Score (10-12) |
2 |
5% |
|
Renal Vasculature on Affected Side |
||
|
Single artery |
33 |
82.5 |
|
Two or more arteries |
7 |
17.5 |
|
Hilar Clamping |
||
|
Only artery |
32 |
80.0 |
|
Both vein & artery |
8 |
20.0 |
|
Opening of Pelvicalyceal System |
||
|
No |
28 |
70.0 |
|
Yes |
12 |
30.0 |
|
Placement of DJ Stent |
||
|
No |
23 |
57.5 |
|
Yes |
17 |
42.5 |
|
Blood Transfusion |
||
|
No |
39 |
97.5 |
|
Yes |
1 |
2.5 |
|
Pre-operative Serum Creatinine |
||
|
NORMAL |
36 |
90% |
|
AZOTEMIC |
4 |
10% |
|
Post-operative Infection |
||
|
No |
39 |
97.5 |
|
Yes |
1 |
2.5 |
|
Histopathology of Resected Mass |
||
|
Clear cell carcinoma |
27 |
67.5 |
|
Papillary carcinoma |
6 |
15.0 |
|
Chromophobe carcinoma |
3 |
7.5 |
|
Oncocytoma |
2 |
5.0 |
|
Others |
2 |
5.0 |
|
Table 2. Demographic and peri-operative data in SD. |
|||||
|
|
Number (n) |
Minimum |
Maximum |
Mean |
Standard. Deviation |
|
Age (years) |
40 |
26 |
77 |
52.30 |
±11.91 |
|
Size (cm) |
40 |
2.8 |
6.7 |
4.70 |
±0.847 |
|
Console Time (min) |
40 |
65 |
110 |
84.40 |
±12.049 |
|
Total operative time (min) |
40 |
130 |
240 |
167.00 |
± 21.116 |
|
Warm ischaemia time (min) |
40 |
25 |
40 |
29.28 |
±5.923 |
|
Estimated blood loss (ml) |
40 |
75 |
460 |
145.75 |
±61.075 |
|
Length of hospital stay (days) |
40 |
3 |
7 |
4.27 |
±0.784 |
|
Table 3. Renal Nephrometry Score. |
||
|
Renal nephrometry score |
Frequency(n=40) |
Percentage (%) |
|
4a |
2 |
5.0 |
|
4p |
2 |
5.0 |
|
5a |
10 |
25.0 |
|
5p |
7 |
17.5 |
|
6a |
2 |
5.0 |
|
6p |
1 |
2.5 |
|
7a |
2 |
5.0 |
|
7p |
1 |
2.5 |
|
7x |
2 |
5.0 |
|
8a |
2 |
5.0 |
|
8p |
1 |
2.5 |
|
9a |
1 |
2.5 |
|
9p |
2 |
5.0 |
|
9x |
3 |
7.5 |
|
10a |
1 |
2.5 |
|
10p |
1 |
2.5 |
|
Total |
40 |
100.0 |
|
Table 4. Preoperative and Postoperative Values of Haemoglobin. |
|||||
|
|
Mean |
N |
Std. Deviation |
t-value |
p value |
|
PRE OP HB gm POST OP HB gm |
13.257 |
40 |
0.8837 |
9.296 |
.000 |
|
12.5550 |
40 |
1.05319 |
|||
|
PRE OP HB gm POST OP 3 months HB gm |
13.257 |
40 |
0.8837 |
5.290 |
.000 |
|
12.7750 |
40 |
1.08054 |
|||
|
POST OP HB gm POST OP 3 months HB gm |
12.5550 |
40 |
1.05319 |
2.275 |
.028 |
|
12.7750 |
40 |
1.08054 |
|||
|
Table 5. Preoperative and Postoperative Values of Creatinine. |
|||||
|
|
Mean |
N |
Std. Deviation |
t-value |
p value |
|
PRE OP Sr.CR (mg/dl) POST OP Sr. CR (mg/dl) |
1.0093 |
40 |
.49583 |
6.231 |
.000
|
|
1.1975 |
40 |
.48277 |
|||
|
PRE OP Sr.CR (mg/dl) Post Op 3 Months Sr.Cr (mg/dl) |
1.0093 |
40 |
.49583 |
6.302 |
.000 |
|
1.1975 |
40 |
.44115 |
|||
|
POST OP Sr. CR (mg/dl) Post Op 3 Months Sr.Cr (mg/dl) |
1.1975 |
40 |
.48277 |
.000 |
1.000 |
|
1.1975 |
40 |
.44115 |
|||
|
Table 6. Outcome in robotic NSS, as evaluated by Trifecta of NSS. |
||||
|
Warm ischaemia time
|
Complications (Clavien-Dindo) |
Surgical Margins (Positive) |
||
|
≤ 25 min |
32 (80%) |
< Grade 2 |
1 |
Nil |
|
> 25min |
8(20%) |
≥ Grade 2 |
Nil |
Nil |
|
Table 7. Comparison of intra-operative outcomes in the complexity groups of the different scoring groups. |
|||
|
Variables |
Low (4-6) |
Middle (7-9) |
High (10-12) |
|
Renal score |
24(60%) |
14 (27.5%) |
2(5%) |
|
DAP score |
8(20%) |
22(55%) |
10(25%) |
|
WIT (min) |
26.38 |
30.25 |
33.12 |
|
Total operative time (min) |
140.5 |
171.66 |
193.2 |
|
Estimated blood loss (ml) |
180 |
250 |
330 |
|
Complications (Clavien-Dindo) |
Nil |
Nil |
1 |
|
Surgical Margins (Positive) |
Nil |
Nil |
Nil |
Laparoscopic partial nephrectomy is gaining acceptance as an alternative to open surgery for small renal tumours, although the technical difficulty of intracorporeal suturing and concerns over warm ischemia time are limitations. The technical difficulty associated with laparoscopic intracorporeal suturing and pressure to minimize warm ischemia time render this procedure within the range of experience of a few laparoscopically adept surgeons. The advent of the da Vinci robot, with multi-jointed endo-wristed instruments and stereoscopic vision, decreases the technical difficulty of intracorporeal suturing and improves the reconstructive steps [14].
The age of the patients ranged from a minimum of 26 years to a maximum of 77 years with a mean age of 52.3 ± 11.9 years. In Castillo et al [15] studies, the mean age was 55.8 years (range 26- 77), the age range is similar but the median age was 3.5 years more than our study. In Rogers et al [16] study, the mean patient age was 60 (range 25-83) which is higher than our study.
Out of the 40 patients, 28 (70%) patients were males. The remaining 12 (30%) patients were females. Renal cell carcinoma (RCC) is more common in men than women. The study conducted by Masson-Lecomte et al [17] included 220 patients out of which 142 (64%) were male and 78 (36%) were women. In the Woldrich et al [18] study out of 236,930 patients, 147687 (62.3%) were male and 89, 243 (37.7%) were female.
Out of the total number of the 40 patients, 13 (32.5%) were diabetic. 22 (45%) were hypertensive. 5 (12.5%) had Coronary Artery Disease and 8 (20%) patients had other comorbidities like COPD and hypothyroidism. In the retrospective International Cancer Study, a 5- to 10-year history of diabetes, increased the relative risk of cancer by 40% in both men and women [19]. A study conducted by Chow et al [20] stated hypertension has a positive association with renal cell carcinoma and control of hypertension might reduce RCC risk effect independent of body weight. In our study, 45% of patients were hypertensive which is closer to the study conducted by Choi et al [21].
Out of the total number of 40 patients, 6 patients (15%) were presented with pain, 3 patients (7.5%) were presented with hematuria, 7 patients (17.5%) presented with lower urinary tract symptoms, 21 patients (52.5%) were incidentally presented and 3 patients presented with other symptoms (fatigue, urinary tract infection). Similar to a study conducted by Escudier et al [22], >50% of RCCs were detected incidentally, making the classical triad of flank pain, gross hematuria and palpable abdominal mass less frequent than in the past.
In our study, the mean size of the tumour was 4.70 ± 0.847 cm with the maximum size of the tumour being 6.7 cm & a minimum of 2.8 cm. In Gettman et al6 study, the mean tumour size was 3.5 cm and the tumour ranged from 2.0-6.0 cm, in Castillo et al15 study, the mean tumour size was 3.25 cm and the tumour ranged from 1.0-5.3 cm, which was lesser to our study.
In our study, the distribution of the side of the tumour was fairly well distributed with 22 (55%) tumours being right-sided & the remaining 18 (45%) being left-sided, which is similar to Rogers et al16 study in which out of total 157 tumours, 89 (54%) tumours were situated in the right side and 68 (45%) tumours were in the left side. In our study, 11 (27.5%) tumours were situated in the anterior upper pole. 9 (22.5%) tumours were located in the anterior lower pole, 8 (20.0%) were in the posterior upper pole, 7 (17.5%) were in the posterior lower pole and 5 (12.5%) tumours were interpolar tumours. The majority of the tumours were situated in the anterior plane, which was similar to the Pierorazio study [23].
In our study, 24 (60%) tumors were ≥ 50% exophytic, 11 (27.5%) tumors were <50% exophytic and 5 (12.5%) tumors were purely endophytic in nature. Similar to our study was the study conducted by Png et al, [24] where the majority of the cases 59% tumours were ≥ 50% exophytic, 37.4% were < 50% exophytic and 3.6% were purely endophytic.
Tumours with a 5a score were the most common amounting to 10 (25%). 5p tumours were the second most common amounting to 7 (17.5%). 24 patients (60%) had low RENAL score (4 - 6), 14 patients (35%) had moderate RENAL score (7-9) and remaining 2 patients (5%) had high RENAL score (10-12). Whereas in the study conducted by Gupta et al [25], 5.3% of tumours had a low RENAL score, 63.1% had a moderate RENAL score and the remaining 31.6% had a high RENAL score. The RENAL nephrometry scoring system represents the first method introduced to attempt to standardize the reporting of salient anatomy of an enhancing renal mass as well as provide a platform to objectify treatment decision making, minimizing the individual subjectivity and judgement [26].
All the cases that underwent surgery preoperatively had contrast-enhanced CT scan (CECT) with 3-D vascular reconstruction done. Out of 40 tumours, 33 (82.5%) had a single renal artery, whereas the other 7 (17.5%) had two or more arteries supplying the affected kidney. Hilar clamping was done for all the cases. Only the artery was clamped in 32 cases (80%). Both vein & artery were clamped in the remaining 8 (20%) cases. Hilar clamping was done with robotic bulldog clamps. In cases with multiple arteries, all the arteries were clamped.
In our study, the mean console time was 84.40± 12.05 mins. The minimum console time was 65 minutes & the maximum was 110 minutes. In the study conducted by Benway et al [27] and Kallingal et al [28] mean console time was 141.5 and 101.3, with the time range being 45-253 minutes and 44-176 minutes respectively, which was much higher than our study.
In our study, the mean total operative time was noted to be 167.00 ± 21.116 minutes. The minimum time was 130 minutes & the maximum was 240 minutes. This was much lesser than the study conducted by Gettman et al [6] and Benway et al [27] in which the mean total operative time was 215 and 210 with a time range of 130-262 and 86-370 minutes respectively.
In our study, mean warm ischemia time was recorded to be 27.28 ± 5.923 minutes. The minimum warm ischemia time was 25 minutes and the maximum time was 40 minutes. There is ample evidence, consistent across multiple human kidney models, supporting the potentially deleterious renal effects of warm ischemia during partial nephrectomy. There is no known safe threshold of warm ischemia time since each minute sequentially contributes to the risk of developing acute kidney injury and renal function decline. Ultimate renal function following partial nephrectomy is dependent on "3 Qs". Quality (renal function before surgery), quantity (renal parenchyma preserved during surgery), and quickness (ischemia time) [29]. Mean warm ischemia time was recorded 27.8 minutes (12-60 min) in Rogers et al16 study which was similar to our study. Studies conducted by Rogers et al [30] reported a mean warm ischemia time of 31 minutes with a range being 24-45 minutes which was slightly higher than our study.
In our study, 32 (80%) cases had blood loss in the range of 100-200 ml. 5 cases (12.5%) had blood loss greater than 200 ml. 3 (7.5%) cases had blood loss <100 ml. The mean blood loss was 145.75±61.075 ml, minimum blood loss was 75 ml and maximum blood loss was 460 ml. This was much lesser than the study conducted by Castillo et al [15], where the mean estimated blood loss was 440 ml with the range being 20-2000 ml. In the studies conducted by Rogers et al [16], Gettman et al [6], kaouk et al [31] also reported higher mean estimated blood loss which was 183 ml,170 ml and 260 ml with the range being 15- 1000 ml, 50-300ml, and 100-300 ml respectively.
Opening of PC system was considered an intraoperative event. In our study, 12 (30%) of the cases had an opening of PCS, all of which were repaired with vicryl 3-0. In the remaining 28 (70%) cases PCS was intact. All the cases with PC system opening and deeply placed tumours had DJ stent placed. Double J stent (DJ stent) was placed in 17 (42.5%) cases and 23 (57.5%) cases were without DJ stents. In Benway et al [27] study PC system opening was done in 52.1% of cases and in Mottrie et al [32] study PC system was done in 53% of cases which was higher than our study.
In our study, none of the cases was converted to open partial/radical nephrectomy. A similar finding was reported in a study conducted by Kallingal et al [28] and Castillo et al15 where no conversion was reported. The conversion was reported in 2% cases in Wang et al [33] study and 1% cases in Benway et al [27] study.
In our study, there were no reported cases of major vessels injury during surgery. The study conducted by Rogers et al16, reported injury to a major vessel in 1 case (0.67%) resultant bleeding which required drainage and 1 case in a study conducted by Vitorri [34] and Wang et al [3]. In our study, no visceral injury was noted in any case. Bowel injury during RAPN can be a serious complication, particularly if unrecognized. The degree of bowel injury varies from superficial abrasion to small enterotomies to frank perforation. For extensive injuries and contamination, bowel diversion may be necessary [35].
In our study, none of the cases was reported with a technical fault with the robot. In the study conducted by Aron et al [36] technical faults were reported in form of robotic camera malfunction which requires conversion to traditional laparoscopy.
In our study, none of the patients was anaemic preoperatively. Haemoglobin levels were less in the immediate postoperative period and 3 months postoperative period due to blood loss intraoperatively. The mean preoperative haemoglobin was 13.26 g/dl whereas immediate postoperative mean haemoglobin was 12.56 and 3 months postoperative mean haemoglobin was 12.78 g/dl. Only one patient had a fall in haemoglobin postoperatively from 12.7 gm/dl to 9.7 gm/dl and underwent a single unit of blood transfusion. Similar to our study was the study conducted by Masson-Lecomte et al [37] in which they reported postoperative blood transfusion in 6% of the cases (13 patients out of 220 patients).
In our study, 36 (90%) patients had normal serum creatinine values at the time of diagnosis. 4 (10%) patients were found to be azotemic. All the patients had azotemia secondary to medical renal disease with no evidence of obstructive uropathy. All of the azotemic patients had stable chronic kidney disease, with none of the patients being on dialysis however patients were taken up for surgery after nephrological clearance & also having explained the possibility of postoperative dialysis. The mean preoperative serum creatinine value was 1.01 mg/dl. Immediate postoperative and 3 months postoperative mean serum creatinine value was the same which was 1.2 mg/dl. Preoperative serum creatinine level is lower than postoperative immediate level (t: -6.231) & 3 months level (t: -6.302), but the immediate postoperative and 3 months serum creatinine levels were almost the same (t: 0.00). None of the patients needed dialysis pre and postoperatively.
In our study, the mean length of hospital stay was 4.27 ± 0.78 days. The minimum stay recorded was 3 days & the maximum stay was 7 days. In the study conducted by Masson-Lecomte et al [37], mean length of stay was 5.5 days which was higher than ours. In Gettman et al,6 mean length of hospital stay was 4.3 days which was nearly similar to our study.
In our study, out of 40 patients, 1 patient (2.5%) developed fever due to wound infection which was managed by i.v. antibiotics and conservative management. 39 (97.5%) patients had no infection. 27 (67.5%) tumours had histopathology reports as clear cell carcinoma. Next common was papillary cell carcinoma 6 (15%), 3 (7.5%) tumours as chromophobe cell carcinoma, 2 (5.0%) as oncocytoma and 2 (5.0%) as other histology as angiomyolipoma and adenoma. In the study conducted by Ener et al [11], 52.3% cases were reported as clear cell carcinoma histologically, whereas the remaining 47.6% cases were divided between chromophobe cell carcinoma, papillary cell carcinoma, oncocytoma and other histology which was nearly similar to our study.
In our study, out of 40 cases, none of the cases had positive surgical margins in histopathology reports. In Benway et al [27] studies, Mottrie et al [32] study and Gettman et al [6] study, positive surgical margins were found in 2.7%, 2%, and 7.6% cases respectively.
In our study, 24 patients had a low renal score, 14 patients had a moderate renal score and 2 patients had a high renal score. Mean warm ischemia time for low renal score patients was 23.75 ± 4.4 minutes, whereas moderate renal score patients had mean warm ischemia time of 31.64 ± 2.3 minutes and high renal score patients had a mean warm ischemia time of ± 1.4 minutes. In the study of Mayer et al [38], R- scored, and N-score were independent predictors of warm ischemia time on multivariable analyses (p < .001, p = .001, p=.026 and p<.01, respectively) and concluded that total RENAL Nephrometry score, as well as the N-and R- scores, can help predict longer WIT during LPN and RAPN. The RENAL Nephrometry score and its components may be useful in the preoperative planning and counselling of the patients undergoing LPN or RAPN. In the preoperative evaluation of partial nephrectomy, we typically use scores to predict the difficulty of the operation, the warm ischemia time and the possibility of high-level complications. Since most studies include patients with Clavien–Dindo complications ≥ grade 2, a warm ischemia time (WIT) ≤ 25 min was used as the criterion for Trifecta outcomes [39]. Therefore, we compared the predictive ability of the different scores for these factors (Table 7). In a study, RENAL and DAP were compared for laparoscopic partial nephrectomy, and DAP was found to be better correlated than RENAL with warm ischemic time and estimated blood loss [40]. Another important aspect of scoring is standardization, which increases comparability and communication. DAP scoring integrates the R.E.N.A.L. and C-index systems. Similar to the R.E.N.A.L. system, DAP provides an itemized summary of 3 relevant, specific tumor features, followed by a sum score. Similar to the C-index, axial and polar scores provide an indication of tumor centrality. For example, a 1 + 1 (axial = 1 and polar =1) tumor is located away from the axis and the equator, ie it is superficial and polar. A 3 + 3 (axial = 3, polar = 3) tumor is located at the axis and at the equator, ie it is central. A 1 + 3 (axial =1, polar = 3) tumor is located away from the axis and at the equator, ie it is superficial and interpolar, and so on.
There were no cases of urinary leak postoperative period of the cases that were performed in the study period. Urine leak is one of the most common complications of RAPN and LPN. Excision into the collecting system can be performed with increasing experience, although there is a higher risk of bleeding and urinary leakage [41].
No secondary haemorrhage was noted in any of the cases that were performed in the study period. In Patel at el [29], only 1 case was reported and in Masson-Lecomte et al [37] study, 2 cases were reported for secondary haemorrhage from pseudoaneurysm.
One patient (2.5%) had Grade 1 complications in the form of mild fever in the postoperative period, whereas the remaining 39 (97.5%) patients had no complications in the post-operative period according to clavien Dindo grade. A review of a select series reveals an overall 7.4% complication rate associated with RAPN, most of which appears to be minor. Lee et al [42] reported port site infection in 1/9 patients. Michli et al [43] reported the detection of renal abscess in 1/20 of their patients. Lee et al [42] also reported the occurrence of a urinoma in 1/9 of their patients. Michli [41] and Caruso et al [8] reported a conversion to open / hand-assisted laparoscopic partial nephrectomy in 3/20 and 2/10 patients undergoing a planned RAPN procedure. Excessive troublesome bleeding associated with RAPN was reported by Rogers et al [16] and Kaul et al [44] in 1/148, and 1/9 of their patients, respectively. In Masso-Lecomte et al [37] study, in RAPN group out of 220 patients, 45 (20.45%) patients [Clavien 1 and 2 - 24 (53%), Clavien 3 - 20(46%), Clavien 4 - 1(2%)] developed complication.
Limitations of the study: Single-centre/institutional nature of the analysis. The number of cases in our study was less compared to certain other studies; although the power of the study was proven to be significant by statistical derivation of the number of cases required to arrive at logical conclusions. Being a strictly observational single-arm study, there were no interventions made during the course. There were also no comparisons made between Robotic Assisted Partial Nephrectomy (RAPN) & Open/ Laparoscopic Partial Nephrectomy (OPN / LPN), which probably would have cast a better light on the standing of RAPN concerning the accepted norms at present. Follow up was restricted to 3 months only, which may not be a sufficient period to assess outcomes of surgery.
We extend our sincere thanks to all the patients who participated in this study.
Ethical policy
Approval was taken from institutional ethical committee. The study was performed in accordance with the Declaration of Helsinki. Patients gave their informed consent for their participation.
Author contributions
DC, Conception and design of study; STAA, Acquisition of data, Analysis and/or interpretation of data; EH, Acquisition of data, Analysis and/or interpretation of data, Drafting the manuscript; SS, Drafting the manuscript, Revising the manuscript critically for important intellectual content.
Competing interests
No conflict of interest.
Funding
None.
Informed consent
Written informed consent was obtained from the patients for publication.
Ethical Approval
Approved by the institutional ethical and research committee.
- Gautam G, Benway B, Bhayani S, Zorn K: Robot-assisted partial nephrectomy: current perspectives and prospects. Urology 2009, 74(4): 735-740.
- Khosla A, Wagner AA: Robotic surgery of kidney, bladder and prostate. Surg Clin N Am 2016, 96(3): 615-636.
- Wang AJ, Bhayani SB: Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of >100 consecutive procedures. Urology 2009, 73(2): 306-310.
- Cha EK, Lee DJ, Del Pizzo JJ: Current status of robotic partial nephrectomy (RPN). BJU Int 2011, 108(6 Pt 2): 935-941.
- Kural AR, Atug F, Tufek I, Akpinar H: Robot-assisted partial nephrectomy versus laparoscopic partial nephrectomy: comparison of outcomes. J. Endourol 2009, 23(9): 1491-1497.
- Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R: Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology 2004, 64: 914-918.
- Phillips CK, Taneja SS, Stifelman MD: Robot-assisted laparoscopic partial nephrectomy: The NYU technique. J Endourol 2005, 19: 441-445.
- Caruso RP, Phillips CK, Kau E, Taneja SS, Stifelman MD: Robot-assisted laparoscopic partial nephrectomy: initial experience. J Urol 2006, 176(1): 36-39.
- Khalifeh A, Autorino R, Hillyer SP, Laydner H, Eyraud R, Panumatrassamee K, Long JA, Kaouk JH: Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomy cases: a single surgeon experience. J Urol 2013, 189(4): 1236-1242.
- Ghani KR, Sukumar S, Sammon JD, Rogers CG, Trinh QD, Menon M: Practice patterns and outcomes of open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: results from the nationwide inpatient sample. J Urol 2014, 191(4): 907-913.
- Ener K, Canda AE, Altinova S, Atmaca AF, Alkan E, Asil E, Ozcan MF, Akbulut Z, Balbay MD: Robotic partial nephrectomy for clinical stage T1 tumours: Experience in 42 cases. Kaohsiung J Med Sci 2016, 32(1): 16-21.
- Chen DY, Uzzo RG: Evaluation and management of the renal mass. Med Clin N Am 2011, 95(1): 179-189.
- Castillo OA, Rodriguez-Carlin A, Lopez-Fontana G, Vidal-Mora I, Gomez IR: Robotic partial nephrectomy: An initial experience in 25 consecutive cases. Actas Urol Esp 2012, 36(1): 15-20.
- Volpe A, Cadeddu JA, Cestari A, Gill IS, Jewett MA, Joniau S, Kirkali Z, Marberger M, Patard JJ, Staehler M, et al: Contemporary management of small renal masses. Eur Urol 2011, 60(3): 501-515.
- Castillo OA, Rodriguez-Carlin A, Lopez-Fontana G, Vidal-Mora I, Gomez IR: Robotic partial nephrectomy: An initial experience in 25 consecutive cases. Actas Urol Esp 2012, 36(1): 15-20.
- Rogers CG, Menon M, Weise ES, Gettman MT, Frank I, Shephard DL, Abrahams HM, Green JM, Savatta DJ, Bhayani SB, et al: Robotic partial nephrectomy: a multi-institutional analysis. J Robotic Surg 2008, 2(3): 141-143.
- Masson-Lecomte A, Yates DR, Bensalah K, Vaessen C, Taille ADL, Roumiguie M, Doumerc N, Bruyère F, Soustelle L, Droupy S, et al: Robot-assisted laparoscopic nephron-sparing surgery for tumours over 4 cm: operative results and preliminary oncologic outcomes from a multicentre French study. Eur J Surg Oncol 2013, 39(7): 799-803.
- Woldrich JM, Mallin K, Ritchey J, Carroll PR, Kane CJ: Sex differences in renal cell cancer presentation and survival: an analysis of the National Cancer Database, 1993-2004. J Urol 2008, 179(5): 1709-1713.
- Czyzyk A, Szczepanik Z: Diabetes mellitus and cancer. Eur J Intern Med 2000, 11(5): 245-52.
- Chow WH, Dong LM, Devesa SS: Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010, 7(5): 245-257.
- Choi MY, Jee SH, Sull JW, Nam CM: The effect of hypertension on the risk for kidney cancer in Korean men. Kidney Int 2005, 67(2): 647-652.
- Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, Grünwald V, Gillessen S, Horwich A: Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2019, 30(5): 706-720.
- Pierorazio PM, Patel HD, Feng T, Yohannan J, Hyams ES, Allaf ME: Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology 2011, 78(4): 813-819.
- Png KS, Bahler CD, Milgrom DP, Lucas SM, Sundaram CP: The role of R.E.N.A.L. nephrometry score in the era of robot-assisted partial nephrectomy. J Endourol 2013, 27(3): 304-308.
- Gupta GN, Boris R, Chung P, Linehan WM, Pinto PA, Bratslavsky G: Robot-assisted laparoscopic partial nephrectomy for tumours greater than 4 cm and high nephrometry score: feasibility, renal functional, and oncological outcomes with minimum 1-year follow-up. In Urol Oncol-Semin Ori 2013, 31(1): 51-56.
- Canter D, Kutikov A, Manley B, Egleston B, Simhan J, Smaldone M, Teper E, Viterbo R, Chen DY, Greenberg RE, et al: Utility of the RENAL nephrometry scoring system in objectifying treatment decision-making of the enhancing renal mass. Urology 2011, 78(5): 1089-1094.
- Benway BM, Bhayani SB, Rogers CG, Porter JR, Buffi NM, Figrnshau RS, Mottrie A: Robot-assisted partial nephrectomy: An international experience. Eur Urol 2010, 57: 815-820.
- Kallingal GJS, Swain S, Darwiche F, Punnen S, Manoharan M, Gonzalgo ML, Parekh DJ: Robotic Partial Nephrectomy with the Da Vinci Xi. Adv Urol 2016: 9675095.
- Patel MN, Krane LS, Bhandari A, Laungani RG, Shrivastava A, Siddiqui SA, Menon M, Rogers CG: Robotic partial nephrectomy for renal tumours larger than 4 cm. Eur Urol 2010, 57: 310-316.
- Rogers CG, Singh A, Blatt AM, Linehan WM, Pinto PA: Robotic partial nephrectomy for complex renal tumours: surgical technique. Eur Urol 2008, 53: 514-523.
- Kaouk JH, Hillyer SP, Autorino R, Haber GP, Gao T, Altunrende F, Khanna R, Spana G, White MA, Laydner H, et al: 252 robotic partial nephrectomies: evolving renorrhaphy technique and surgical outcomes at a single institution. Urology 2011, 78: 1338-1344.
- Mottrie A, Naeyer GD, Schatteman P, Carpentier P, Sangalli M, Ficarra V: Impact of the Learning Curve on Perioperative Outcomes in Patients Who Underwent Robotic Partial Nephrectomy for Parenchymal Renal Tumors. Eur Urol 2010, 58: 127-133.
- Wang L and Lee BR: Robotic partial nephrectomy: Current technique and outcomes. Int J Urol 2013, 20: 848-859.
- Vittori G: Open versus robotic-assisted partial nephrectomy: a multicenter comparison study of perioperative results and complications. World J Urol 2014, 32: 287-293.
- Bishoff JT, Allaf ME, Kirkels W, Moore RG, Kavoussi LR, Schroder F: Laparoscopic bowel injury: incidence and clinical presentation. J Urol 1999, 161: 887-890.
- Aron M, Koenig P, Kaouk JH, Nguyen MM, Desai MM, Gill IS: Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume centre. BJU Int 2008, 102(1): 86-92.
- Masson-Lecomte A, Yates DR, Bensalah K, Vaessen C, de la Taille A, Roumiguié M, Doumerc N, Bruyère F, Soustelle L, Droupy S: Robot-assisted laparoscopic nephron-sparing surgery for tumours over 4 cm: operative results and preliminary oncologic outcomes from a multicentre French study. Eur J Surg Oncol 2013, 39(7): 799-803.
- Mayer WA, Godoy G, Choi JM, Goh AC, Bian SX, Link RE: Higher RENAL Nephrometry Score is predictive of longer warm ischemia time and collecting system entry during laparoscopic and robotic-assisted partial nephrectomy. Urology 2012, 79(5): 1052-1056.
- Bianchi L, Schiavina R, Borghesi M, Chessa F, Casablanca C, Angiolini A, Ercolino A, Pultrone CV, Mineo Bianchi F, Barbaresi U: Which patients with clinical localized renal mass would achieve the trifecta after partial nephrectomy? The impact of surgical technique. Minerva Urol Nefrol 2019, 72(3): 339-349.
- Yoshida K, Kinoshita H, Yoshida T, Takayasu K, Mishima T, Yanishi M, Komai Y, Sugi M, Kawa G, Matsuda T: Comparison of diameteraxial-polar nephrometry score and RENAL nephrometry score for surgical outcomes following laparoscopic partial nephrectomy. Int J Urol 2016, 23(2): 148-152.
- Desai MM, Gill IS, Kaouk JH, Matin SF, Novick AC: Laparoscopic partial nephrectomy with suture repair of the pelvicalyceal system. Urology 2003, 61(1): 99-104.
- Lee RS, Sethi AS, Passerotti CC, Retik AB, Borer JG, Nguyen HT, Peters CA: Robot-assisted laparoscopic partial nephrectomy: a viable and safe option in children. J Urol 2009, 181(2): 823-828.
- Michli EE, Parra RO: Robotic-assisted laparoscopic partial nephrectomy: initial clinical experience. Urology 2009, 73(2): 302-305.
- Kaul S, Laungani R, Sarle R, Stricker H, Peabody J, Littleton R, Menon M: Da Vinci-assisted robotic partial nephrectomy: technique and results at a mean of 15 months of follow-up. Eur Urol 2007, 51(1): 186-191.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript