Research Article | Open Access
Long Term Results of Elective Nodal Salvage Radiotherapy in Oligometastatic Prostate Cancer: A Mono-institutional Series
Riccardo Vigna-Taglianti1, Alberto Boriano2, Anna Maria Merlotti1, Stefania Martini1, Gianello Luca1, Salvatore Solla1, Spinelli Lavinia1, Francesco Olivero2, Fabrizio Bergesio2, Adriano De Maggi2, Alessia Reali3 and Elvio Grazioso Russi1
1Radiation Oncology Department, Santa Croce and Carle Hospital, 12100, Cuneo, Italy.
2Medical Physics Department, Santa Croce and Carle Hospital, 12100, Cuneo, Italy.
3Radiation Oncology S.S., Michele and Pietro Ferrero Hospital, Verduno (CN), Italy.
Correspondence: Riccardo Vigna-Taglianti (A.O. S.Croce e Carle, Radiation Oncology Department, Via M. Coppino 26 - 12100 Cuneo, Italy; Email: vigna.r@ospedale.cuneo.it).
Annals of Urologic Oncology 2022; 5(1): 34-41. https://doi.org/10.32948/auo.2022.09.30
Received: 22 Sep 2022 | Accepted: 30 Sep 2022 | Published online: 06 Oct 2022
Methods We retrospectively assessed a series of 50 patients treated from 2015 to 2021 at our center who presented with recurrent pelvic lymph node pCa disease with 1-3 lymph-nodes lateralized to one side of the pelvis. Patients were treated with intensity modulated Rapid Arc radiotherapy (IMRT), limiting the treatment volume to the chain of the affected side only. During the follow-up, the patients who presented a biochemical recurrence of the disease were evaluated by PET.
Results The biochemical progression-free survival and the metastatic progression-free survival were respectively 36% and 49% at 5 years. 22/50 patients presented a documented recurrence on PET. Only one patient presented a relapse within the irradiated volume and no patient presented a relapse on the contralateral pelvic lymph node chain. No patient had gastrointestinal toxicity > grade 1 RTOG.
Conclusion Treatment of patients with oligorecurrent (1-3 lesions) pelvic pCa, limiting the volume of irradiation to only one side of the affected lymph node chain, results in good biochemical disease control and presents a low risk of neoplastic contralateral progression.
Key words prostate cancer, ENRT, SBRT, salvage, oligometastatic
The free interval (time to biochemical progression of the disease after the first treatment), the PSA doubling time (PSADT) and the absolute value reached by the PSA at the time of relapse are known as predictive factors on the survival of these pts [2].
Similarly, the number and location of metastatic lesions have an impact on survival [3].
The modern metabolic radiological investigations (choline PET, PSMA-PET, FLUcivolone PET) are able to discriminate pts who have a relapsed disease in a limited number of sites (metastatic oligorecurrent ) from those who have a situation of disseminated disease (polymetastatic) [4, 5].
The main sites of pCa oligometastases are located in the bone and lymph node, particularly in the pelvis [6, 7].
Hormone therapy (HT) has been a cornerstone in the treatment of metastatic pCa for many years [8] but in the situation of oligometastatic disease it has recently been shown that the treatment of secondary lesions with surgery or radiation therapy is able to provide an advantage on biochemical disease-free survival (bDFS) and overall survival (OS), in particular in the situation of pelvic lymph node oligorecurrence [9, 10].
The radiotherapy (RT) treatment schedules used for pelvic oligorecurrent disease differ in the size of the irradiated volume, the dose delivered and the number of fractions, and are defined as elective nodal radiotherapy (ENRT) and stereotactic body radiotherapy (SBRT).
In the last decade several retrospective studies of SBRT and ENRT have been published, identifying at least five types of treatment covering progressively increasing lymph node volumes (Involved node SBRT, Involved site SBRT, Involved field RT, ENRT, super extended ENRT) [11].
To date, no prospective studies have compared the results obtained with these different RT modalities, nor is there a consensus on doses and treatment volumes to use.
In the case of nodal progression limited to one side of the pelvic lymphatic chain, it is not clear whether the reduction in the treatment volume to the hemi-pelvis affected by the disease may be burdened by a significant rate of contralateral metastatic lymph node progression.
At our center we started treating pts who presented with a lateralized nodal oligorecurrent disease in the pelvis from 2015 using a treatment schedule and volume similar to that subsequently described by Soldatov in 2018 and currently defined as Involved field RT [12].
In our series of pts, treated uniformly for doses and volumes and with long follow-up, we have retrospectively evaluated the sites of nodal recurrence identified during the follow-up, using the currently available biomolecular investigations, with the primary objective of assessing the recurrence rate on the contralateral pelvic volume compared to the irradiated one.
Secondly, we have studied bDFS and free from metastatic progression (DMFS) and their correlation with known risk factors such as the Gleason score, the PSADT at the beginning of RT and the value reached by PSA at the time of radiotherapy.
Diagnostic imaging before treatment
Metastatic lesions were identified by choline PET or 68 Ga PSMA-PET. Until 2018, the only diagnostic modality available was choline PET (cPET); in the following period both methods were used. The minimum PSA threshold value used for carrying out the cPET was 1.5ng / mL; the minimum PSA threshold value for performing PSMA PET was 0.5ng / mL.
Treatment volumes and doses
The lymphatic regions treated included the obturator, internal, external and common iliac (up to including the iliac bifurcation) ipsilateral lymph nodes with respect to the site of the lesions; the presacral lymph nodes were included only if radiologically involved. In pts not previously irradiated to the prostatic fossa and with nodal recurrence below the bifurcation of the common iliac chain, the treatment volume was extended to include the prostatic fossa as well. The delivered dose was 45Gy in 25 fractions with Rx 6Mv and IMRT-Rapid arc technique on the precautionary volume (lymph nodes chains with or without prostatic bed); the metabolically active and evident nodal lesions were irradiated with simultaneous Integrated Boost (SIB) up to the dose of 56.25Gy.
PSA testing and HT
All pts underwent a PSA test almost one month before starting radiation treatment. PSADT was calculated using the dosages performed in the last 6 months before salvage radiation. HT with monthly LHRh analogue administration concomitant with RT and for a duration of 1 year was associated in all pts who had reached a PSA value > 1.5ng / mL. After the end of RT the pts were followed quarterly for the first 2 years, subsequently every 6 months. In HT treated pts in which the primary treatment was surgery, the state of biochemical relapse (BR) of the disease was defined, after the suspension of hormonal treatment, by two dosages of PSA increasing beyond the threshold of 0.2ng / ml. In HT treated pts in which the primary treatment was radiotherapy, the threshold for defining the BR state was defined by two increases above the value of 2ng/ml. In non HT treated pts, the state of BR was defined by two increases in the PSA value beyond the value measured at the beginning of RT, regardless of the type of primary treatment performed (surgery or RT).
Diagnostic imaging after treatment
In case of BR during follow-up, pts were re-evaluated with a new cPET / PSMA PET performed at least 4 months after the end of the treatment. In the case of metabolic progression of the disease, the patient was considered to be in clinical relapse (CR). Pts diagnosed with CR were divided into four groups (Figure 2):
(a) Ipsilateral progression out-field: appearance of one or more metastatic lymph nodes at the upper or lower levels respect to the irradiated chains, including the lombo-aortic chain.
(b) Ipsilateral progression in-field: appearance of one or more metastatic lymph node lesions within the irradiated volume.
(c) Contralateral progression: appearance of one or more lesions along the pelvic chains contralateral to those irradiated, including presacral lymph nodes when not irradiated.
(d) Distant progression: appearance of one or more lesions outside the pelvic and lombo-aortic lymph node chains.
Statistical analysis
Variables were preliminarily tested for normal distribution with the Shapiro-Wilk’s W test and data were expressed as mean ± standard deviation (SD) when normally distributed, as median and interquartile range (IQR) when not normally distributed. Continuous variables with non-normal and normal distribution were analyzed by Mann-Whitney U test and t-test for unpaired samples, respectively, as appropriate. Differences in categorical variables were analyzed by 2 or Fisher’s test, as appropriate. Dichotomous variables were analyzed by non parametric binomial test.
Survival statistics (progression-free survival, distant metastasis-free survival and overall survival) were acquired in relation to the number of patients and calculated by the method proposed by Kaplan and Meier. For comparison of survival distribution, the log-rank test was used.
The level of statistical significance was set at p ≤0.05. The calculations were performed using SPSS (IBM SPSS Statistics – Version 21).
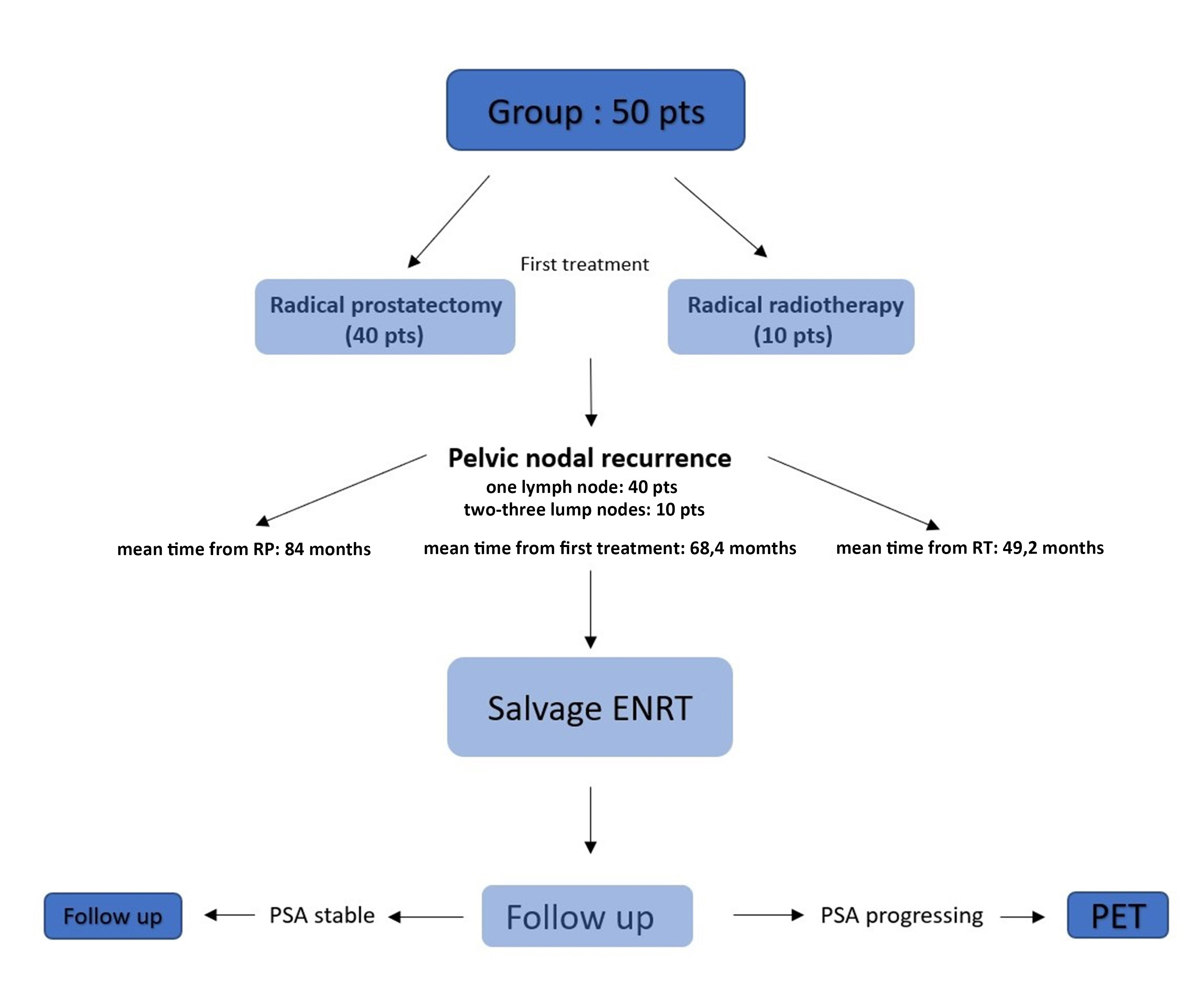 Figure 1. Progressing Flow-chart of the study. Pts who had pelvic lymph node recurrence (1-3 lateralized lymph nodes) post RT or post surgery were treated with salvage involved field ENRT. Pts who showed a biochemical progression of the disease during the follow-up underwent re-evaluation PET in order to identify the site of disease progression.
Figure 1. Progressing Flow-chart of the study. Pts who had pelvic lymph node recurrence (1-3 lateralized lymph nodes) post RT or post surgery were treated with salvage involved field ENRT. Pts who showed a biochemical progression of the disease during the follow-up underwent re-evaluation PET in order to identify the site of disease progression.
|
Table 1. Clinical and biochemical feature of the analyzed group. PSA pre-RT : PSA value before starting salvage radiotherapy. PSADT : PSA doublig time at the diagnosis of pelvic lymph node relapse. LHRh : triptorelin or leuprorelin for the duration of one year from the beginning of the RT. |
||||
|
Items |
Min |
Max |
Mean |
|
|
Age (years) |
55 |
82 |
68.4±7 |
|
|
PSA pre-RT (ng/mL) |
5.4 |
10.4 |
3±4 |
|
|
PSADT (months) |
2 |
24.5 |
8±5 |
|
|
Gleason score |
6 |
9 |
8±1 |
|
|
LHRh : yes |
30 pts |
|||
|
LHRh : no |
20 pts |
|||
|
First treatment |
Surgery : 40 pts |
Radiotherapy : 10 pts |
||
|
Number lymph nodes |
One : 40 pts |
Two-Three : 10 pts |
||
|
PSA pre-RT: PSA value before starting salvage radiotherapy; PSADT: PSA doublig time at the diagnosis of pelvic lymph node relapse; LHRh: triptorelin or leuprorelin for the duration of one year from the beginning of the RT. |
||||
Survivals and recurrence
bDFS and DMFS after 60 months were 36% and 49% respectively (Figure 3 and 4); OS after 60 months was 86%. A total of 22/50 (44%) patients had a CR assessed by PET during follow-up time. Among these patients, 10 showed an omolateral pelvic or lombo aortic nodal recurrence and 12 were classified as distant metastatic (outside the pelvic and lombo aortic nodes). One patient presented a solitary nodal relapse inside the irradiated volume. None of the pts showed a secondary recurrence in the contralateral not irradiated pelvis (p=0.000).
Correlations with risk factors
Pts with CR showed a greater value of PSA at the start of ENRT than those without (4.63.8 ng/ml vs 1.91.7 ng/ml, p=0.02). Fixing a PSA doubling time (PSA_DT) threshold of 6 months, patients were then divided in two groups : above and until the 6 months threshold. The comparison of the bDFS among the two groups showed a significant difference in favor of lower PSADT (log rank p=0.039, Figure 5). There was no significant difference in terms of bDFS and DMFS in function of the number of metastatic lymph nodes (one lymph node vs two-three lymph nodes: log-rank p=0.56 and 0,39 respectively) and in function of Gleason score (GS 6-7 versus GS 8-10 : log rank p=0,77 and 0,96 respectively).
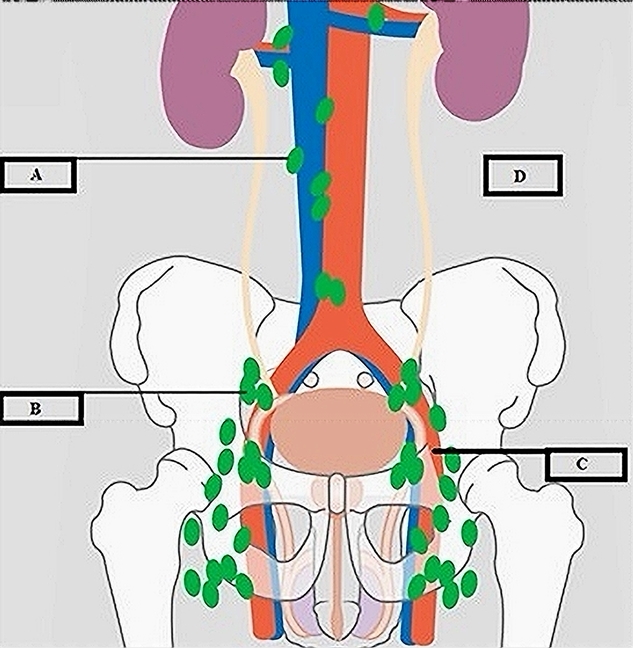
Figure 2. Sites of recurrence assessed by PET during follow-up. A : ipsilateral progression out the irradiated nodal chain. B : ipsilateral progression in the irradiated nodal chain. C : contralateral progression compared to irradiated nodal chain. D : progression outside pelvic or lumboaortic nodal volumes (distant metastases).
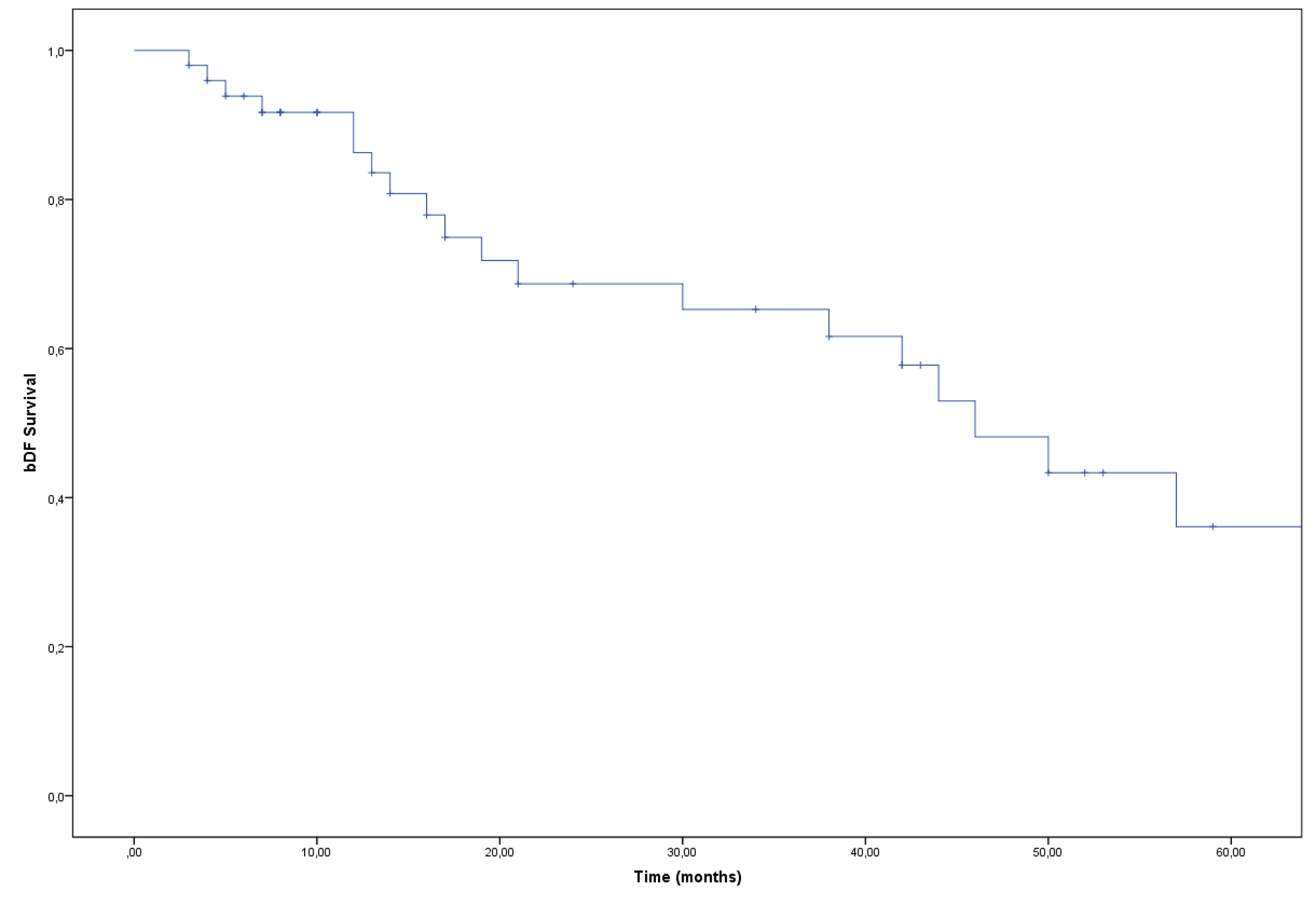 Figure 3. Kaplan Meier curve. bDFS at five years : 36%.
Figure 3. Kaplan Meier curve. bDFS at five years : 36%.
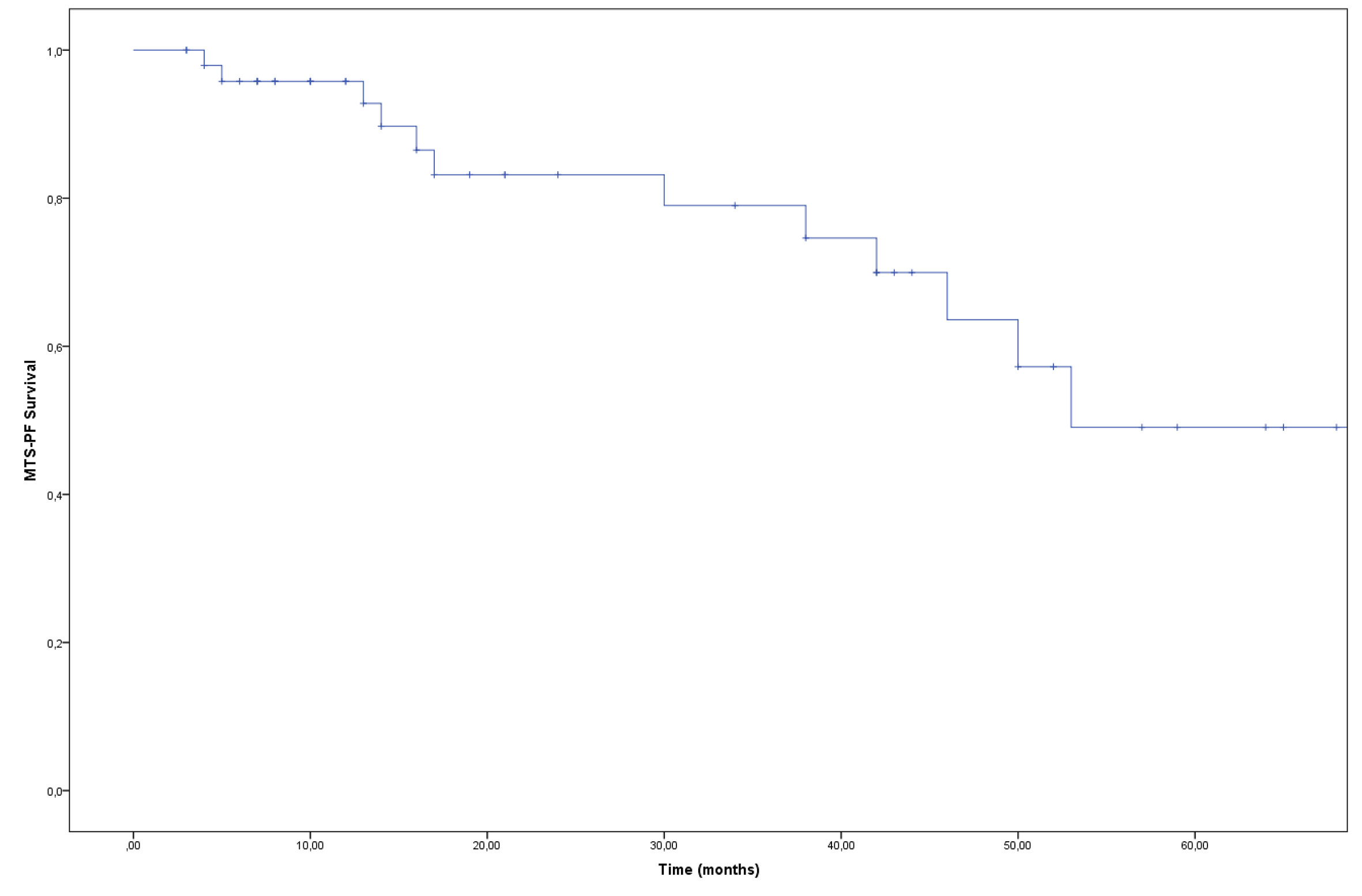 Figure 4. Kaplan Meier curve. DMFS at five years : 49%.
Figure 4. Kaplan Meier curve. DMFS at five years : 49%.
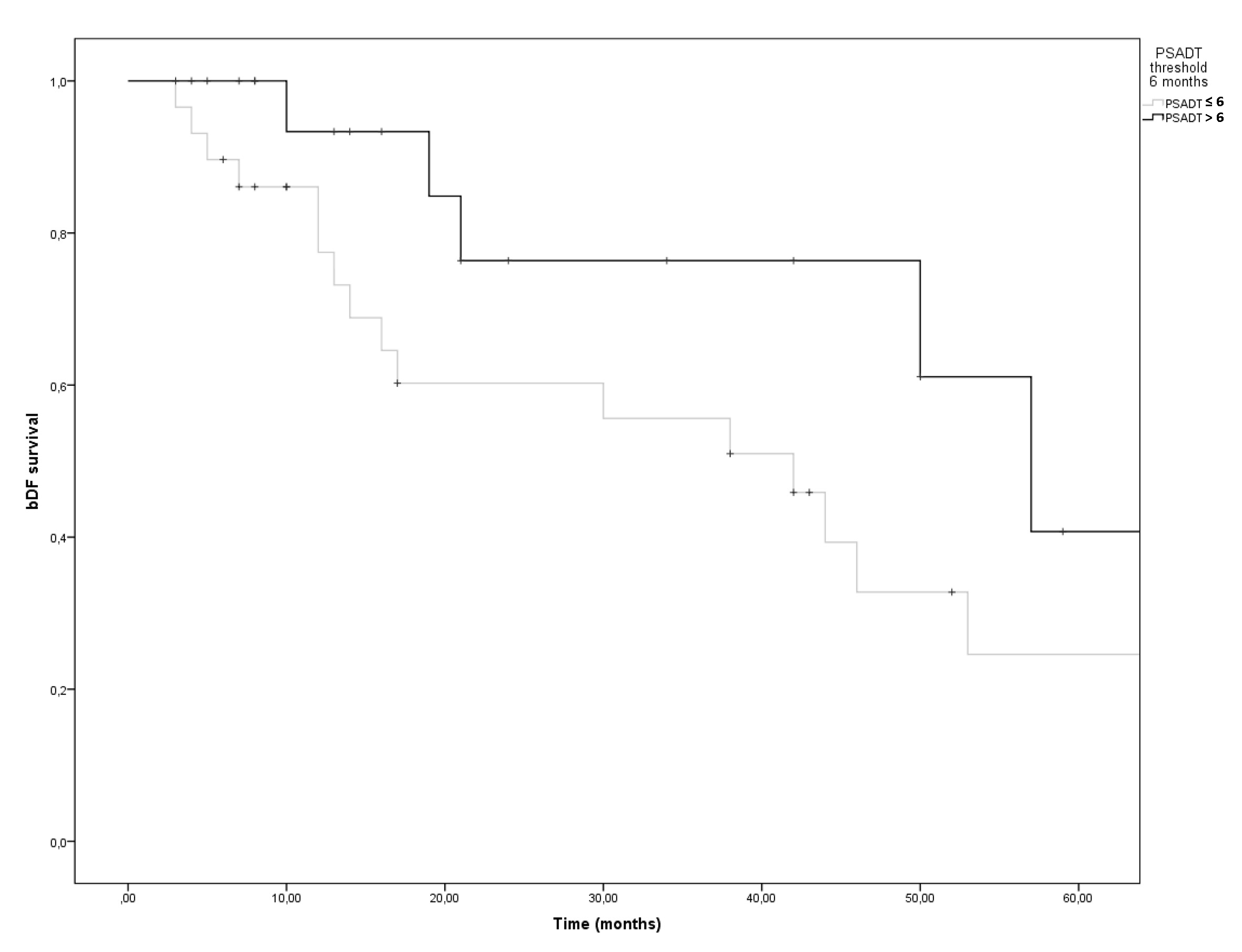 Figure 5. Kaplan Meier curve. bDFS at five years as a function of PSADT above and until the 6 months threshold. Log rank p= 0.039.
Figure 5. Kaplan Meier curve. bDFS at five years as a function of PSADT above and until the 6 months threshold. Log rank p= 0.039.
The development of the ultrasensitive method for the PSA assay and a more sophisticated metabolic diagnostic imaging have allowed the early detection of metastatic localizations of the disease.
To date, the criteria used to define an oligometastatic state in pCa are not very congruent with each other, in particular in the numerical and location definition of secondary localizations [14, 15]; more homogeneous are criteria for the subdivision into different groups of disease (de novo oligometastatic, oligorecurrent, oligoprogressive, oligopersistent), depending on the time factor and the treatments already received by the patient [16, 17].
The numerical increase in cases of pCa detected in the oligometastatic phase, expression of a situation with a lower neoplastic load and a better prognosis, has led to a series of studies aimed at evaluating the potential effectiveness of treatments targeted not only at the site of the primary cancer (when present) [18], but also to the sites identified as oligometastatic (Metastasis Directed Therapy, MTD), obtaining positive results on survival compared to hormone therapy alone [19-21].
In the case of oligorecurrent pelvic disease, two different therapeutic oncological philosophies can be identified: only the treatment of metabolically active lesions with SBRT or the precautionary treatment of lymphatic regions considered to be at greater risk of further neoplastic progression with ENRT.
In the case of SBRT the treatment is short, between 1-6 sessions, with total doses with doses normally between 24-48Gy [22-23]. In the case of ENRT the treatment is extended to cover also the pelvic nodal stations and lasts at least 5 weeks with doses ranging from 45-50Gy on the precautionary volume and 56-66Gy on metastatic sites [24, 25].
The few retrospective comparisons between these treatment modalities seem to demonstrate a superiority of the ENRT, at the expense of its greater toxicity [26, 27].
In order to identify which patients can benefit from this type of RT, adequate selection criteria are important, currently based on the number and location of the secondary lesions [28, 29].
In 2017 Jereczek-Fossa et al. [22] treated a series of 94 patients with isolated nodal recurrence at a dose of 24Gy in 3 fractions on a Planning Target Volume (PTV) generated by an expansion margin of 2-3 mm in addition to the Gross Tumor Volume (GTV), obtaining a PFS rate of 22.6% at 2 years.
Kneebone et al. [30] in 2018 reported a series of 57 patients irradiated with a SBRT focused on a double PTV treated with Simultaneous Integrated Boost (SIB): 30Gy in 3fraz or 50Gy in 5 fractions on the PET positive nodal sites (high dose Clinical Target Volume-CTV) and 24Gy in 3 fractions or 30Gy in 5 fractions on a low-dose CTV obtained from a previous volume expansion for 1 cm along the vascular axis, resulting in a 75% rate of biochemical recovery after a median follow-up of 16 months.
In consideration of the high relapse rates found in treatments with volumes limited only to the positive PET sites, probably due to an underestimation of the neoplastic load by biomolecular investigations, were also proposed treatments aimed at covering a more extensive lymph nodal volume.
Soldatov et al. [12] in 2018 treated a series of 108 patients with standard fractionation up to about 50Gy on a CTV including the pelvic lymphatic chain ipsilateral with respect to the site of the nodal recurrence up to the upper iliac bifurcation with a boost on the PET positive sites up to a dose of about 60Gy, obtaining a rate of biochemical recurrence of 43.5% at 18 months.
Kirste et al. [31] in 2021 on a multi-institutional series of 394 patients compared those treated only in the site of positive PET (204 cases) with the remaining 190 treated on a precautionary volume extended to cover the prostate bed, the bilateral pelvic and or paraortic lymph nodes, obtaining a bDFS at 3 years significantly longer in the second group.
Supiot et al. [32] in the Oligopelvis - GETUG P07 trial treated the pelvic lymph node stations, contoured according to the RTOG guidelines [33], and modified according to the indications of the GETUG group [34], including the common iliac, external and internal iliac, obturator and presacral pelvic chains in addition to the prostate fossa in cases not previously irradiated, at the dose of 54Gy in 30 fractions on the pelvic volume with SIB at 66Gy on evident PET nodal lesions, obtaining a BFS rate of 46% at 3 years.
Lepinoy et al. [35] in 2019 used a super-extended volume (including nodal stations above the iliac bifurcation) on a series of 27 patients reporting good treatment tolerance as well as a significantly better 3-year time to failure compared to a group treated with only pelvic ENRT.
From the results of a 2021 review, Pinkawa et al. [27] suggested preferring ENRT treatment over SBRT in situations of oligorecurrent lymph node disease in consideration of the best rates of metastatic progression-free survival.
De Bleser et al. [26] in 2019, on a large retrospective multi-institutional study comparing ENRT and SBRT in patients with up to 5 nodal lesions, found a significant reduction in the rate of loco-regional relapses in favour of ENRT.
The main criticisms against the ENRT techniques concern their potential toxicity due to the greater volume irradiated, the longer duration of treatment and the possibility for SBRT to be easily repeated on any new metastatic localizations that appeared during the follow-up [23].
Some decision-making criteria used today to select which patients can benefit most from an ENRT treatment are based on the number and location of metastatic lesions (1-3 lesions) associated with a "favorable" asset of the disease evaluated with PSA DT, risk class of the disease onset, PSA value at relapse [36].
Since in the experiences of SBRT the pattern of disease relapse appears to involve more frequently the regions adjacent to the irradiated sites [37, 38], unlike ENRT treatments [39], and in order to minimize the risk of GI and GU side effects, a treatment method similar to that described by Soldatov in 2018 was adopted at our center from 2015 [12].
In our experience, a consecutive series of 50 patients who presented a metabolically documented recurrence of primary operated or irradiated prostate cancer and limited to 1-3 lateralized nodal lesions in the pelvic nodes, were treated with the ENRT technique limited to the ipsilateral lymphatic chains with respect to the site of the secondary adenopathies.
The maximum threshold of three secondary locations for proposing treatment was defined on the retrospective experiences available for the treatment of oligometastatic disease [40, 41], and on prospective randomized phase 2 trials already started [18-20].
The choice of associating OT only to patients with PSA> 1.5ng / ml, was evaluated considering that at the time of implementation of the treatment (2015) it did not yet exist a unanimous attitude in the hormonal manipulation in association with salvage radiation.
In 2020 Spratt et al. [42] demonstrated that prolonged hormonal treatment can be detrimental to survival in patients who have a PSA <1.5ng / ml at the time of relapse, since it has comforted us in our therapeutic choice.
The treatment compliance was excellent (no patient experienced acute and late GU and GI toxicities > 1 according to RTOG) [43].
The bDFS e DMFS at five years were 36% and 49% respectively, aligned to literature data.
In our series a greater PSA value at the start of RT correlate significantly with the probability to develop a clinical disease progression during time (p=0.02).
Similarly, the PSDAT was found to correlate with the probability to develop clinical disease recurrence (p=0.039).
None of the patients showed during follow-up a cPET / PSMA-PET progression localized only in the contralateral pelvic level not irradiated.
We are aware that the retrospective nature and the limited number of cases however limit the results of this study.
We thank our colleagues in nuclear medicine for their support.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Author contributions
RVT: Conceptualization and methodology. AB and FB: used the software and performed data analysis. AMM, LG and AR: performed validation of results. AB and ADM: performed formal analysis. SM, SS, OF and LS: performed data collection and curation. EGR: supervisor of the manuscript.
All authors read and approved the final manuscript.
Competing interests
All authors declare no competing interests.
Funding
All authors declare that they do not receive grants or financial support for this study.
- Tewari A, Raman JD, Chang P, Rao S, Divine G, Menon M: Long-term survival probability in men with clinically localized prostate cancer treated either conservatively or with definitive treatment (radiotherapy or radical prostatectomy). Urology 2006, 68(6): 1268-1274.
- Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Partin AW: Time to prostate specific antigen recurrence after radical prostatectomy and risk of prostate cancer specific mortality. J Urol 2006, 176(4 Pt 1): 1404-1408.
- Singh D, Yi WS, Brasacchio RA, Muhs AG, Smudzin T, Williams JP, Messing E, Okunieff P: Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys 2004, 58(1): 3-10.
- Artigas C, Diamand R, Shagera QA, Plouznikoff N, Fokoue F, Otte FX, Gil T, Peltier A, Van Gestel D, Flamen P: Oligometastatic Disease Detection with 68Ga-PSMA-11 PET/CT in Hormone-Sensitive Prostate Cancer Patients (HSPC) with Biochemical Recurrence after Radical Prostatectomy: Predictive Factors and Clinical Impact. Cancers (Basel) 2021, 13(19): 4982.
- deSouza NM, Liu Y, Chiti A, Oprea-Lager D, Gebhart G, Van Beers BE, Herrmann K, Lecouvet FE: Strategies and technical challenges for imaging oligometastatic disease: Recommendations from the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer 2018, 91: 153-163.
- De Bruycker A, Lambert B, Claeys T, Delrue L, Mbah C, De Meerleer G, Villeirs G, De Vos F, De Man K, Decaestecker K, et al: Prevalence and prognosis of low-volume, oligorecurrent, hormone-sensitive prostate cancer amenable to lesion ablative therapy. BJU Int 2017, 120(6): 815-821.
- Koerber SA, Sprute K, Clemens Kratochwil C, Erik Winter, Matthias F Haefner, Sonja Katayama, Ingmar Schlampp, Klaus Herfarth, Klaus Kopka, Ali Afshar-Oromieh, et al: Clinical outcome of PSMA-guided radiotherapy for patients with oligorecurrent prostate cancer. Eur J Nucl Med Mol Imaging 2021, 48(1): 143-151.
- Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD et al: EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017, 71: 630-642.
- Knipper S, Mehdi Irai M, Simon R, Koehler D, Rauscher I, Eiber M, van Leeuwen FWB, van Leeuwen P, de Barros H, van der Poel H, et al: Cohort Study of Oligorecurrent Prostate Cancer Patients: Oncological Outcomes of Patients Treated with Salvage Lymph Node Dissection via Prostate-specific Membrane Antigen-radioguided Surgery. Eur Urol 2022, S0302-2838(22)02408-3.
- Rogowski P, Roach M 3rd, Schmidt-Hegemann NS, Trapp C, von Bestenbostel R, Shi R, Buchner A, Stief C, Belka C, Li M: Radiotherapy of oligometastatic prostate cancer: a systematic review. Radiat Oncol 2021, 16(1): 50.
- Achard V, Bottero M, Rouzaud M, Lancia A, Scorsetti M, Filippi AR, Franzese C, Jereczek-Fossa BA, Ingrosso G, Ost P, et al: Radiotherapy treatment volumes for oligorecurrent nodal prostate cancer: a systematic review. Acta Oncol 2020, 59(10): 1224-1234.
- Soldatov A, von Klot CAJ, Walacides D, Derlin T, Bengel FM, Ross TL, Wester HJ, Derlin K, Kuczyk MA, Christiansen H, et al: Patterns of Progression After 68Ga-PSMA-Ligand PET/CT-Guided Radiation Therapy for Recurrent Prostate Cancer. Int J Radiat Oncol Biol Phys 2019, 103(1): 95-104.
- Hellman S, Weichselbaum RR: Oligometastases. J Clin Oncol 1995, 13(1): 8-10.
- Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et al: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015, 373(8): 737-746.
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, et al: Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017, 377(4): 352-360.
- Kucharczyk MJ, So J, Gravis G, Sweeney C, Saad F, Niazi T: A combined biological and clinical rationale for evaluating metastasis directed therapy in the management of oligometastatic prostate cancer. Radiother Oncol 2020, 152: 80-88.
- Palacios-Eito A, Béjar-Luque A, Rodríguez-Liñán M, García-Cabezas S: Oligometastases in prostate cancer: Ablative treatment. World J Clin Oncol 2019, 10(2): 38-51.
- Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, Ritchie AWS, Attard G, Chowdhury S, Cross W, et al: Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018, 392(10162): 2353-2366.
- Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, Rowe SP, Ross AE, Gorin MA, Deville C, et al: Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020, 6(5): 650-659.
- Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, Tai KH, Udovicich C, Lim A, Selbie L, et al: Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur Urol 2018, 74(4): 455-462.
- Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, Lambert B, Delrue L, Bultijnck R, Claeys T, et al: Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018, 36(5): 446-453.
- Jereczek-Fossa BA, Fanetti G, Fodor C, Ciardo D, Santoro L, Francia CM, Muto M, Surgo A, Zerini D, Marvaso G, et al: Salvage Stereotactic Body Radiotherapy for Isolated Lymph Node Recurrent Prostate Cancer: Single Institution Series of 94 Consecutive Patients and 124 Lymph Nodes. Clin Genitourin Cancer 2017, 15(4): e623-e632.
- Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, De Vos F, Huysse W, Hautekiet A, Maes G, et al: Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014, 9: 135.
- Jethwa KR, Hellekson CD, Evans JD, Harmsen WS, Wilhite TJ, Whitaker TJ, Park SS, Choo CR, Stish BJ, Olivier KR et al: 11C-Choline PET Guided Salvage Radiation Therapy for Isolated Pelvic and Paraortic Nodal Recurrence of Prostate Cancer After Radical Prostatectomy: Rationale and Early Genitourinary or Gastrointestinal Toxicities. Adv Radiat Oncol 2019, 4(4): 659-667.
- Tran S, Jorcano S, Falco T, Lamanna G, Miralbell R, Zilli T: Oligorecurrent nodal prostate cancer: long-term results of an elective nodal irradiation approach. Am J Clin Oncol 2018, 41: 960-962.
- De Bleser E, Jereczek-Fossa BA, Zilli T, Van As N, Siva S, Fodor A, Dirix P, Gomez-Iturriaga A, Trippa F, et al: Metastasis-directed Therapy in Treating Nodal Oligorecurrent Prostate Cancer: A Multi-institutional Analysis Comparing the Outcome and Toxicity of Stereotactic Body Radiotherapy and Elective Nodal Radiotherapy. Eur Urol 2019, 76(6): 732-739.
- Pinkawa M, Aebersold DM, Böhmer D, Flentje M, Ghadjar P, Schmidt-Hegemann NS, Höcht S, Hölscher T, Müller AC, Niehoff P, et al: Radiotherapy in nodal oligorecurrent prostate cancer. Strahlenther Onkol 2021, 197(7): 575-580
- Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, Carver B, Castellano D, Chung BH, Clarke N, et al: Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol 2018, 73(2): 178-211.
- Aluwini SS, Mehra N, Lolkema MP, Oprea-Lager DE, Yakar D, Stoevelaar H, van der Poel H: Oligometastatic Prostate Cancer: Results of a Dutch Multidisciplinary Consensus Meeting. Eur Urol Oncol 2020, 3(2): 231-238.
- Kneebone A, Hruby G, Ainsworth H, Byrne K, Brown C, Guo L, Guminski A, Eade T: Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Detected via Prostate-specific Membrane Antigen Positron Emission Tomography. Eur Urol Oncol 2018, 1(6): 531-537.
- Kirste S, Kroeze SGC, Henkenberens C, Schmidt-Hegemann NS, Vogel MME, Becker J, Zamboglou C, Burger I, Derlin T, Bartenstein P et al: Combining 68Ga-PSMA-PET/CT-Directed and Elective Radiation Therapy Improves Outcome in Oligorecurrent Prostate Cancer: A Retrospective Multicenter Study. Front Oncol 2021, 11: 640467.
- Supiot S, Vaugier L, Pasquier D, Buthaud X, Magné N, Peiffert D, Sargos P, Crehange G, Pommier P, Loos G, et al: OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur Urol 2021, 80(4): 405-414.
- Lawton CA, Michalski J, El-Naqa I, Buyyounouski MK, Lee WR, Menard C, O'Meara E, Rosenthal SA, Ritter M, Seider M: RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2009, 74(2): 383-387.
- Sargos P, Guerif S, Latorzeff I, Hennequin C, Pommier P, Lagrange JL, Créhange G, Chapet O, de Crevoisier R, Azria D et al: Definition of lymph node areas for radiotherapy of prostate cancer: A critical literature review by the French Genito-Urinary Group and the French Association of Urology (GETUG-AFU). Cancer Treat Rev 2015, 41(10): 814-820.
- Lépinoy A, Silva YE, Martin E, Bertaut A, Quivrin M, Aubignac L, Cochet A, Créhange G: Salvage extended field or involved field nodal irradiation in 18F-fluorocholine PET/CT oligorecurrent nodal failures from prostate cancer. Eur J Nucl Med Mol Imaging 2019, 46(1): 40-48.
- Panje C, Zilli T, Dal Pra A, Arnold W, Brouwer K, Garcia Schüler HI, Gomez S, Herrera F, Khanfir K, Papachristofilou A, et al: Radiotherapy for pelvic nodal recurrences after radical prostatectomy: patient selection in clinical practice. Radiat Oncol 2019, 14(1): 177.
- Pasqualetti F, Panichi M, Sainato A, Matteucci F, Galli L, Cocuzza P, Ferrazza P, Coraggio G, Pasqualetti G, Derosa L, Sollini M, et al: [(18)F]Choline PET/CT and stereotactic body radiotherapy on treatment decision making of oligometastatic prostate cancer patients: preliminary results. Radiat Oncol 2016, 11: 9.
- Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, Orecchia R, Casamassima F, Surgo A, Miralbell R, et al: Pattern of Progression after Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Nodal Recurrences. Clin Oncol (R Coll Radiol) 2016, 28(9): e115-20.
- De Bruycker A, De Bleser E, Decaestecker K, Fonteyne V, Lumen N, De Visschere P, De Man K, Delrue L, Lambert B, Ost P: Nodal Oligorecurrent Prostate Cancer: Anatomic Pattern of Possible Treatment Failure in Relation to Elective Surgical and Radiotherapy Treatment Templates. Eur Urol 2019, 75(5): 826-833.
- Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, Decaestecker K, Villeirs G, Vuye P, Ost P: Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer 2013, 11(1): 27-32.
- Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, Henderson D, Casamassima F, Orecchia R, Surgo A, et al: Progression-free Survival Following Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Treatment-naive Recurrence: A Multi-institutional Analysis. Eur Urol 2016, 69(1): 9-12.
- Spratt DE, Dess RT, Efstathiou JA, Zietman AL, Wallington DG, Jairath NK, Jackson WC, Den RB, Stish BJ, Morganet TM et al: Two years of anti-androgen treatment increases other-cause mortality in men receiving early salvage radiotherapy: a secondary analysis of the NRG Oncology/RTOG9601 randomized phase III trial. Int J Radiat Oncol Biol Phys 2019, 105(3): 680.
- Cox JD, Stetz J, Pajak TF: Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995, 31(5): 1341-1346.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript