Research Article | Open Access
CD105 and CD34 as Markers of Angiogenesis of Urothelial Carcinoma: A Cross Sectional Study
Sheenam Azad1, Aditi Bisht1, Avneesh Malviya2, Jyotsna Bhateja1, Seema Acharya1
1Department of Pathology, Shri Guru Ram Rai Institute of Medical & Health Sciences, Dehradun, Uttarakhand, India.
2Department of Pathology, Government Doon Medical College, Dehradun, Uttarakhand, India.
Correspondence: Jyotsna Bhateja (Department of Pathology, Shri Guru Ram Rai Institute of Medical & Health Sciences, Dehradun, Uttarakhand 248001, India; Email: khushi.bhateja.91@gmail.com).
Annals of Urologic Oncology 2023, 6(3): 104-110. https://doi.org/10.32948/auo.2023.07.10
Received: 12 Jun 2023 | Accepted: Accepted 05 Jul 2023 | Published online: 10 Jul 2023
Methods 50 cases of histopathologically diagnosed UC were included. CD105 and CD34 IHC staining was performed. Microvessel density (MVD) evaluation by CD105 and CD34 was compared. The number of microvessels were counted in high power in three hot spots. The highest value obtained amongst three fields were reported as MVD. Statistical analysis was done by (SPSS 28.00 version for windows) using independent t-test.
Results There were 45 high grade cases UC 5 low grade cases of UC. Invasive papillary UC was seen in 25 cases (50%) followed by invasive UC seen in 13 cases (26%). Both Lamina propria (LP) invasion and muscularis propria invasion were noted in 16 cases of high-grade urothelial carcinoma. Only LP invasion was seen in 3 cases of low grade and 25 cases of high-grade UC respectively. Evaluation of MVD CD105 and MVD CD34 did not show any significant association of MVD with grade with a p value of 0.214 & 0.429 respectively. Aso there was no significant association of MVD CD105 (p=0.580) & CD34 (p=0.747) with muscularis propria invasion. Overall mean CD105 and CD34 were similar with no statistical difference between the two markers.
Conclusion Both CD105 and CD34 can be used as marker to assess angiogenesis. However, there is no significant association of MVD with grade or muscle invasion in UC.
Key words CD105, CD34, microvessel density, bladder cancer
Bladder cancer is thought to be a genetically controlled process affecting cell physiology and the host organism. A few studies suggest that papillary and non-papillary urothelial carcinomas may use different molecular pathways which could explain their physiologic differences [2]. Angiogenesis is one of the most essential components in tumour growth and metastasis. It facilitates progressive tumour growth by giving adequate oxygenation to the tumour through a multi-step process involving endothelial cell proliferation, motility of endothelial cells through the extracellular matrix towards angiogenic stimuli and capillary differentiation. Hence, the intensity of angiogenesis is considered to be a poor prognostic factor and has become one of the major determinants for the development of various cancers [3]. Angiogenesis is measured by microvessel density. Quantification of angiogenesis can be done by immunohistochemical staining with vessel wall specific antibodies. Endoglin, also called CD105 is a 633 aminoacid, 180-Kda homodimeric transmembrane glycoprotein that belongs to Transforming Growth Factor beta family receptor (TGF-β), type III and plays an important role in angiogenesis. It is mainly expressed on endothelial cells and overexpressed on tumor-associated vascular endothelium. It is also involved in vascular development and remodeling. Hence, it is considered as a better immunohistochemical marker in assessing microvascular density than any other endothelial marker [4, 5]. CD34, a transmembrane glycoprotein, is a hematopoietic progenitor cell antigen that is expressed in vascular endothelial cells. It is a sensitive marker of endothelial differentiation that stains endothelial cells in neoplasia more strongly than normal endothelium [6]. This study was conducted to compare CD105 & CD34 as immunohistochemical marker of angiogenesis in urothelial carcinoma by assessing microvessel density and find their relationship with grades of urothelial carcinoma and muscle invasion.
This study was an observational prospective study conducted in the department of Pathology Shri Guru Ram Rai Institute of Medical and Health sciences over a period of two years after taking approval from the institutional ethical committe.
Collection of specimens
Inclusion Criteria: A total of fifty cases of TURBT chips histopathologically diagnosed as urothelial carcinoma were evaluated. Exclusion Criteria: The inadequate biopsies and cases with marked inflammation obscuring the epithelium were excluded.
Tissue Processing
The tissue received in histopathology in neutral buffered was grossed as per the standard protocol of the histopathology section. The tissues were processed in an automated tissue processor, paraffin-embedded, sectioned and stained with Hematoxylin -Eosin.
IHC Procedure
One section each from a representative block was subjected to IHC marker Endoglin (CD105) and CD34. Details of primary antibodies used are in Supplementary Material 1. Representative sections of 2-3 microns were cut and taken on poly-L- lysine coated slides and were processed for IHC as per departmental SOP.
IHC Result Interpretation
CD105 (endoglin) and CD34 positive brown stained endothelial cell or endothelial cell cluster with or without lumen was considered as single, countable microvessel. Slides were examined at low power magnification to find high density areas of stained vessels (hot spots). Then the number of microvessels was counted in high power in three such hot spots. The highest value obtained amongst three fields were reported as microvessel density (MVD).
Statistical Analysis
Data obtained was entered into MS Excel sheet. The means and standard deviations of the measurements per group were used for statistical analysis (SPSS 28.00 version for windows). Comparison between two groups was determined using independent t-test. A p-value < 0.005 was accepted as statistically significant.
Males (86%) were more commonly affected than females (14%). The mean age and standard deviation was 60.14 ± 15.04. A Majority of the cases seen were in the age group above 50 years (74%) (Figure 1). Only one case was seen in the age group 21-30 years.
Histological analysis & case distribution
Amongst the 50 cases of UC, papillary urothelial carcinoma was the most common subtype (27/50; 54%) as shown in Table 1. It was found that the maximum numbers of cases were high-grade urothelial carcinoma 90.0% (45/50). Only 10.0% (5/50) were low grade. The cases were further categorized as invasive/non-invasive based on invasion into lamina propria and muscularis propria invasion. A Majority of the cases showed invasion (48/50; 96%). Only 4% (2/50) were non-invasive. Amongst 48 invasive cases, 28 cases (58.4%) showed only lamina propria invasion and 16 (33.3%) showed both lamina propria and muscularis propria invasion. In addition, there were 4 cases (8.3%) where muscularis propria presence/ invasion could not be determined due to marked cautery artefact. It was also observed that all high-grade cases showed lamina propria invasion (100%) whereas only 60% (3/5) low grade urothelial carcinoma showed lamina propria invasion.
IHC analysis
IHC analysis with panel of two markers CD105 and CD34 were performed. The mean MVD score of the two markers were similar and there was no statistical difference between the two markers and the p value was observed to be 0.773. The mean MVD CD105 score and CD34 score were 42.22 ± 11.45 and 41.31 ± 11.08 respectively in high-grade tumors. The mean MVD of CD105 score and CD34 in low-grade tumors were 35.40 ± 11.86 and 36.80 ± 19.32 respectively. No significant difference was observed between the two markers in different grades (Figure 2). Thus, there was no association of MVD with grades. The comparison of mean MVD of CD105 and CD34 in cases showing lamina propria invasion and muscularis propria invasion was also done and no significant difference was observed in cases wth presence or abscence of lamina propria or muscularis propria invasion (Figure 3, 4).
|
Table 1. Distribution of histological sub types of urothelial carcinoma. |
||||
|
S. No |
Histological sub types of urothelial carcinoma |
Male |
Female |
Total |
|
1 |
Papillary urothelial carcinoma, non-invasive |
2 |
00 |
2 (4%) |
|
2 |
Papillary urothelial carcinoma, invasive |
20 |
5 |
25(50%) |
|
3 |
Urothelial carcinoma, invasive |
11 |
2 |
13(26%) |
|
4 |
Urothelial carcinoma, lymphoepithelioma variant |
1 |
0 |
1(2%) |
|
5 |
Urothelial carcinoma with squamous differentiation |
6 |
0 |
6(12%) |
|
6 |
Urothelial carcinoma with glandular differentiation. |
3 |
0 |
3(6%) |
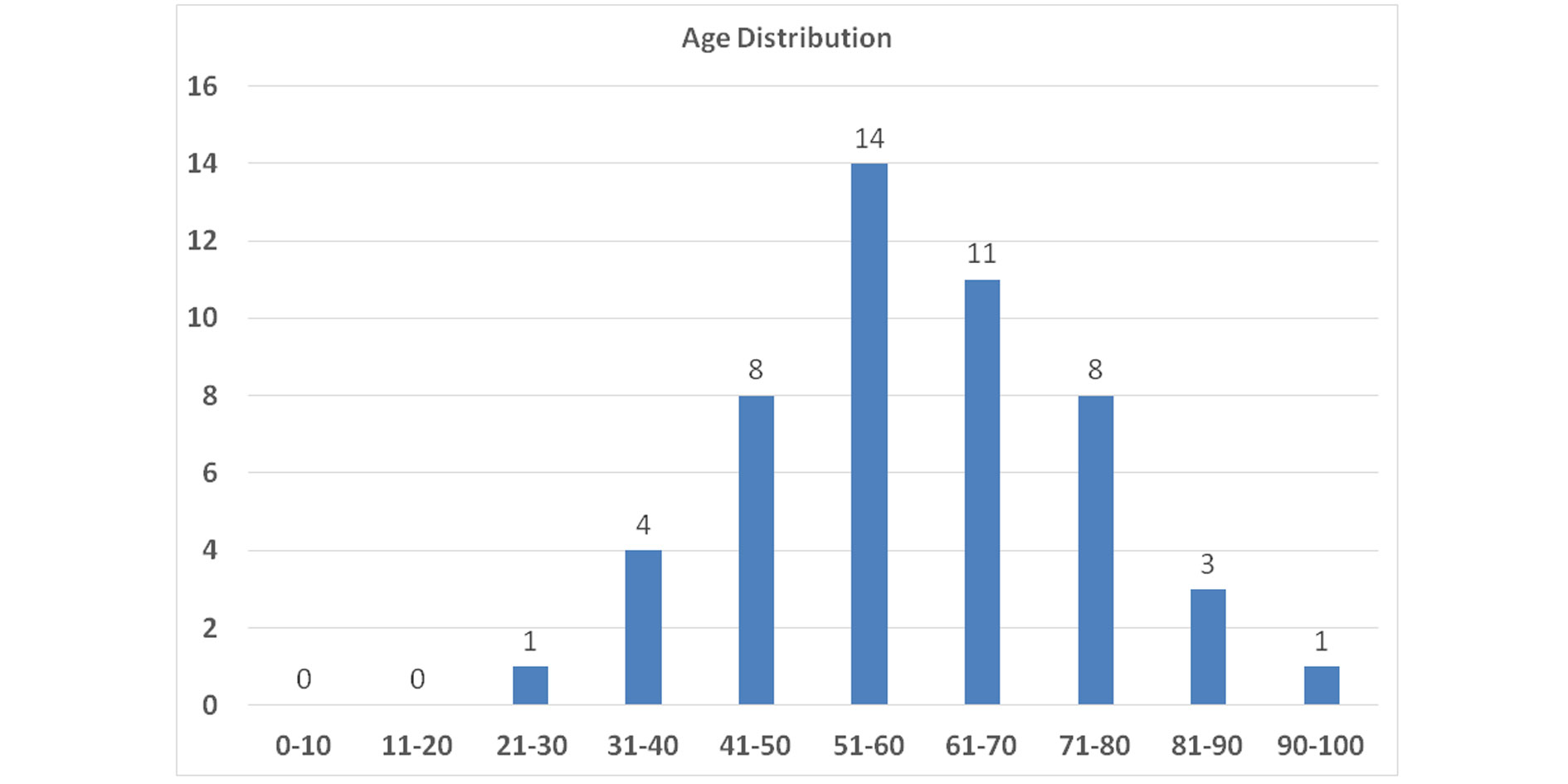 Figure 1. The peak incidence of urothelial carcinoma was in the age group 51-60 years (14/50, 28.0%) followed by age group 61-70 years (11/50, 22.0%) as shown in the above figure.
Figure 1. The peak incidence of urothelial carcinoma was in the age group 51-60 years (14/50, 28.0%) followed by age group 61-70 years (11/50, 22.0%) as shown in the above figure.
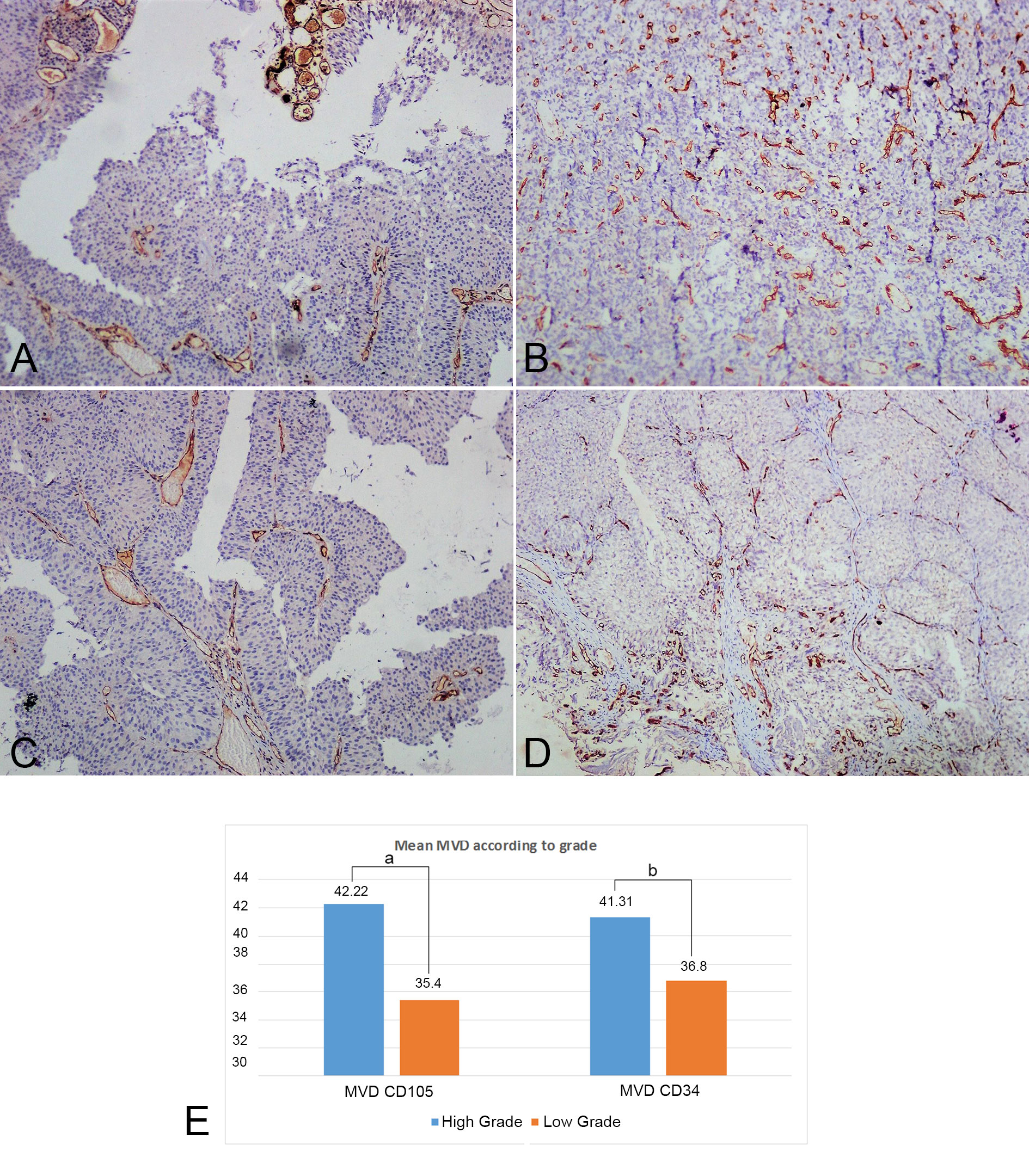 Figure 2. CD105 and CD34 tissue expression in low and high grade urothelial carcinoma. (A) CD34 stained microvessels in hotspot areas in low grade urothelial carcinoma (100X, IHC); (B) CD34 stained microvessels in hotspot areas in high grade urothelial carcinoma (100X, IHC); (C) CD105 stained microvessels in hotspot areas in low grade urothelial carcinoma (100X, IHC); (D) CD105 stained microvessels in hotspot areas in high grade urothelial carcinoma (100X, IHC); (E) Comparison between Mean MVD of both markers in different grades (a: p=0.214, b: p=0.429).
Figure 2. CD105 and CD34 tissue expression in low and high grade urothelial carcinoma. (A) CD34 stained microvessels in hotspot areas in low grade urothelial carcinoma (100X, IHC); (B) CD34 stained microvessels in hotspot areas in high grade urothelial carcinoma (100X, IHC); (C) CD105 stained microvessels in hotspot areas in low grade urothelial carcinoma (100X, IHC); (D) CD105 stained microvessels in hotspot areas in high grade urothelial carcinoma (100X, IHC); (E) Comparison between Mean MVD of both markers in different grades (a: p=0.214, b: p=0.429).
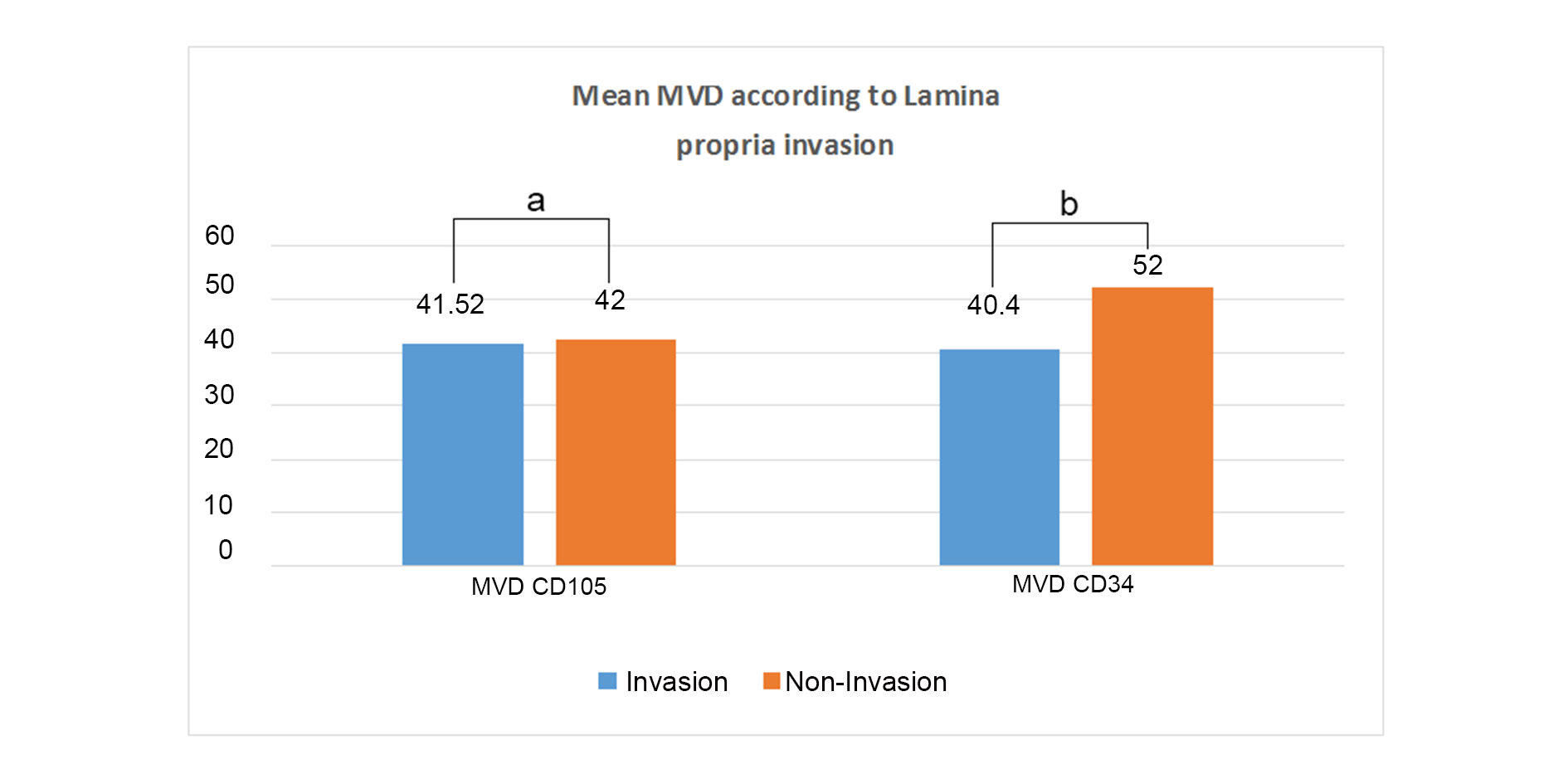 Figure 3. Comparison between both markers in Lamina propria invasive/non invasive cases (a: p=0.955, b: p=0.181).
Figure 3. Comparison between both markers in Lamina propria invasive/non invasive cases (a: p=0.955, b: p=0.181).
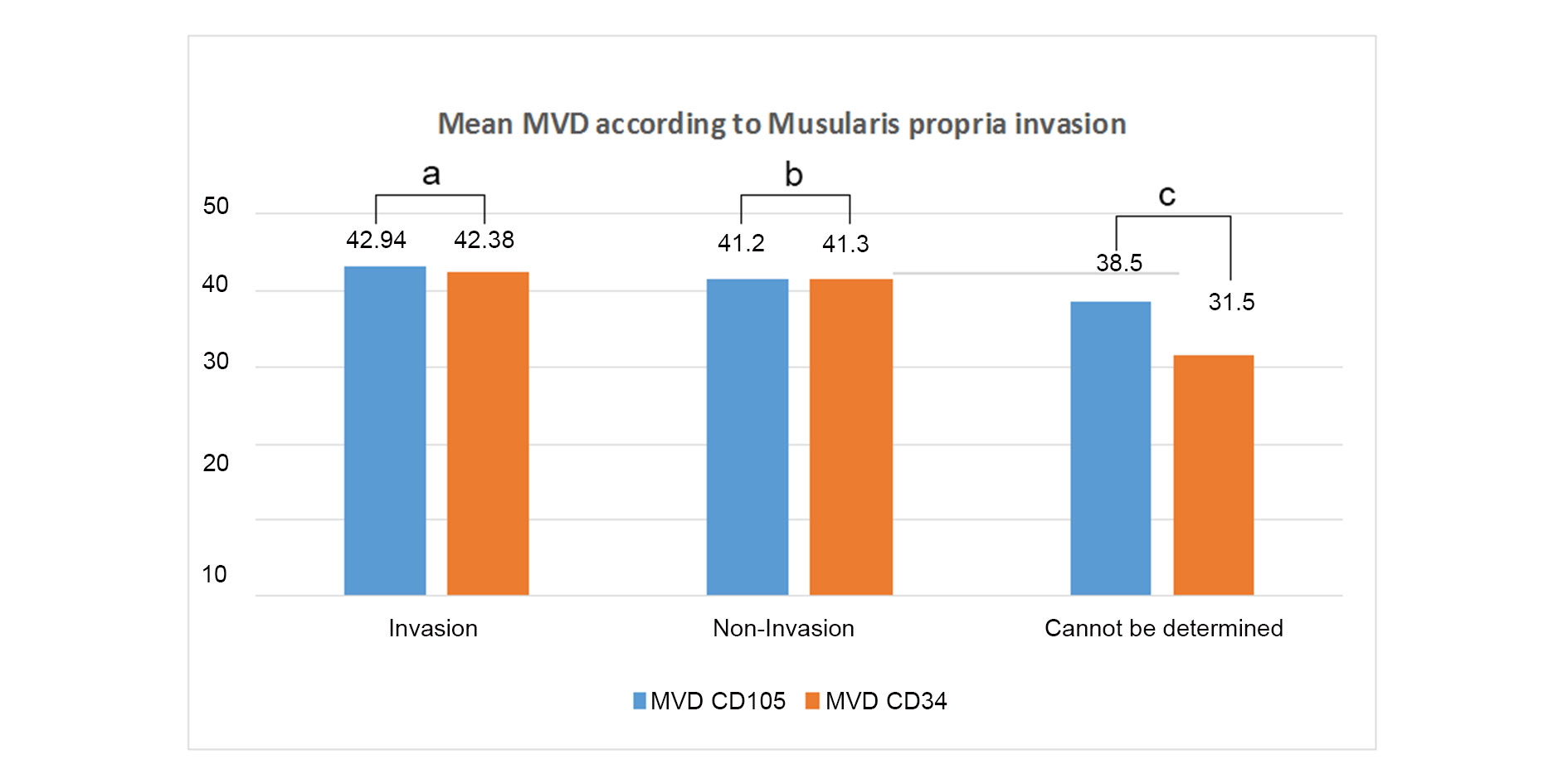 Figure 4. Comparison of Mean MVD according to invasion (a: p=0.580, b: p=0.747, c: p value was not calculated).
Figure 4. Comparison of Mean MVD according to invasion (a: p=0.580, b: p=0.747, c: p value was not calculated).
The world-wide incidence of urothelial carcinoma shows male preponderance as reported in studies by Gupta et al and Biswas et al. The present study also showed similar results. 86% of cases were males and male:female ratio was 6.1 : 1. Most of the cases of urothelial carcinoma occurs above the age of 50 years. Very rarely, it can occur in children and young adults. Gupta et al. in their study found mean age 60 years ± 4.4 years at presentation. Biswas et al. reported maximum number of cases above age group 60 years [9, 10]. The present study also showed similar results. There was wide range of age distribution of cases but maximum numbers of cases were seen in the age group above 50 years (74%). Several classification systems for urothelial carcinoma have been proposed over the past decades. The current WHO classification 2016 is recommended for reporting urothelial carcinoma [11]. In the present study, the most common histologic subtype of urothelial carcinoma was found to be invasive papillary urothelial carcinoma (25/50) followed by invasive urothelial carcinoma (13/50). Only two cases were of non-invasive papillary UC which was comparable with the studies by Quinten et al. and Jhaveri et al. [12, 13].
The grading system of urothelial neoplasms of the bladder is of great prognostic significance. On categorization of urothelial carcinoma into high grade and low-grade, high-grade cases were more frequent (90.0%) as compared to low grade cases (10.0%). Haque et al in 2018 in their study found 72.0% patients with high grade urothelial carcinoma and 28.0% with low grade urothelial carcinoma [14]. It was observed in the current study that amongst 48 invasive cases; 28 cases (58.4%) had only lamina propria invasion (non-muscle invasive) and 16 cases (33.3%) had both lamina propria invasion and muscle invasion while in the remaining 4 cases (8.3%) muscularis propria presence/invasion could not be determined. Gupta et al. found that only 26% patients had muscle invasive disease whereas 34.5% of patients lacked detrusor muscle in the biopsy specimen [9]. Chinnasamy et al. reported relatively lesser high grade urothelial carcinoma cases (63.4%) and higher number of non-muscle invasive urothelial carcinoma (86.5%) compared to muscle invasive urothelial carcinoma 13.5% cases [15]. The number of high-grade UC and invasive UC was relatively more in the present study. Uttarakhand being a hilly state, there are inadequate facilities for routine check-up in peripheral hilly areas. In addition, late arrival at the hospital due to lack of awareness, social as well as religious restrictions might be responsible for patients presenting with high grade tumors more frequently.
Although grade and stage are important prognostic markers for bladder carcinoma and additional prognostic information can be provided by certain biological markers. Microvessel density, a measurement of angiogenesis has been found to be strongly associated with tumor progression. However, its role as a prognostic marker has not been firmly established. In the present study assessment of MVD was done in 50 cases of urothelial carcinomas of bladder using two different markers CD105 and CD34 as IHC marker for angiogenesis. Mean MVD scores of CD105 and CD34 in high grade cases were found to be slightly higher in comparison to the low grade cases. However, the difference was not significant (p-value > 0.05).
The mean MVD CD105 and CD34 were compared in cases with the presence or absence of lamina propria invasion, Mean MVD of CD105 was almost similar in lamina propria invasive cases and lamina propria non-invasive cases. On the contrary, the mean MVD of CD34 was higher in cases with the absence of lamina propria invasion as compared to those with the presence of lamina propria invasion. Lesser number of non-invasive cases might be responsible for abberent results in this group. The Mean MVD CD 34 was also higher in comparison to the MVD CD105. However, the difference was not significant (p-value > 0.05). The mean MVD of CD105 and CD34 were almost similar in cases showing lamina propria invasion. A comparison of these different means revealed no significant difference between the two markers (P > 0.05). Mean MVD CD105 and CD34 were also compared in cases with presence or absence of muscularis propria invasion and were found to be similar. On comparing the mean MVD of individual markers in muscularis propria invasive versus non-invasive cases, it was found that there was not much difference in the individual mean MVD of CD105 and CD34. No statistical significance was observed (p-value > 0.05). Stavropoulos et al also showed no significant association of CD34 MVD or VEGF with clinicopathologic features, progression and tumor recurrence indicating MVD is not an independent prognostic factor in superficial bladder carcinoma cases [16]. However, Ahmed et al. observed mean MVD score of CD34 in relation to tumor grade was higher in high grade cases (79.1 ± 23.42) as compared to low grade cases (59.8 ± 20.37) with a significant p-value 0.047. They also showed that the mean MVD of CD34 (81.9 ± 23.87) was found to be higher with the pathological pT3-pT4 stage of tumor (muscle invasive) but the relation was found to be not significant (p-value > 0.05) [17]. Goddard et al. studied 180 cases of TCCs and found significant correlation between MVD with stage and progression of the tumour. In their study also, mean MVD was increased in high grade tumours, however the association was not statistically significant [18].
It has been mentioned in few studies that vessels undergoing neoangigenesis are better stained by CD105 as compared to other pan endothelial markers. In the present study, the overall mean of MVD CD105 and CD34 in 50 cases of UC were almost similar (41.54 ± 11.56 and 40.86 ± 11.94 respectively) with no significant difference between the two markers (p value = 0.773). The results were in concordance with the study done by Hai-Bo wang et al. In their study, although the mean values of CD105-MVD (139 ± 41.8) and CD34-MVD (134.9 ± 36.2) were much higher than those in the present study, but no significant difference (p-value > 0.05) was found between CD105-MVD and CD34-MVD [20]. The difference in the mean MVD score of this study and the present study could be due to different staining techniques, differences in the choice of antibody, method of unmasking, tissue characterstics or differences in the method of counting microvessels. Conflicting results between different studies could be due to different staining techniques, difference in the choice of antibody, techniques of antigen retrieval, tissue characteristic or difference in the method of counting microvessels etc.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
SA: Conception, design of study and manuscript preparation; AB: Data collection and analysis; JB: IHC images and manuscript editing; AM and SA: proofreading.
Competing interests
The authors have no competing interest.
Funding
None.
- Kaseb H, Aeddula NR: Bladder Cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2023, PMID: 30725608.
- Rosai J: Rosai and Ackerman's surgical pathology e-book. Elsevier Health Sciences 2011.
- Folkman J: What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990, 82(1): 4-6.
- Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M: Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 2003, 22(42): 6557-6563.
- Gougos A, Letarte M: Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem 1990, 265(15): 8361-8364.
- Traweek ST, Kandalaft PL, Mehta P, Battifora H: The human hematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol 1991, 96(1): 25-31.
- Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F: Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017, 71(1): 96-108.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68(6): 394-424.
- Gupta P, Jain M, Kapoor R, Muruganandham K, Srivastava A, Mandhani A: Impact of age and gender on the clinicopathological characteristics of bladder cancer. Indian J Urol 2009, 25(2): 207-210.
- Biswas RR, Mangal S, Guha D, Basu K, Karmakar D: An epidemiological study of cases of urothelial carcinoma of urinary bladder in a tertiary care centre. J Krishna Institute of Med Sci 2013, 2(1): 82.
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE: The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol 2016, 70(1): 106-119.
- Quentin T, Schlott T, Korabiowska M, Käthei N, Zöller G, Glaser F, Kunze E: Alteration of the vascular endothelial growth factor and angiopoietins-1 and -2 pathways in transitional cell carcinomas of the urinary bladder associated with tumor progression. Anticancer Res 2004, 24(5A): 2745-2756.
- Jhaveri PN, Makwana SV, Oza KK, Shah CK: A histopathological study of urinary bladder neoplasms. Indian J Pathol Oncol 2021, 8(1): 59-63.
- Haque S, Dewan RK, Saleh S, Jennah SA, Jahan F, Akter F, Sultana T, Ferdaus NJ: Histomorphological study of urinary bladder tumor and status of HER2/Neu and Ki67 expression in urothelial carcinoma. J Histopathol Cytopathol 2018, 2(2): 99-108.
- Chinnasamy R, Krishnamoorthy S, Joseph L, Kumaresan N, Ramanan V: Clinico-pathological study of bladder cancer in a tertiary care center of South India and Impact of age, gender, and tobacco in causing bladder cancer: a single center experience. Int J Sci Study 2016, 3(10): 72-77.
- Stavropoulos NE, Bouropoulos C, Ioachim IE, Michael M, Hastazeris K, Tsimaris I, Kalogeras D, Liamis Z, Stefanaki S, Agnantis NI: Prognostic significance of angiogenesis in superficial bladder cancer. Int Urol Nephrol 2004, 36(2): 163-167.
- Ahmed SM, Mohammed HE, Muhammad ES: The Value of CD34 Expression in Squamous Cell Carcinoma and Urothelial Carcinoma of the Urinary Bladder. Ain Shams medical journal 2018, 69: 573-583.
- Goddard JC, Sutton CD, Furness PN, O'Byrne KJ, Kockelbergh RC: Microvessel density at presentation predicts subsequent muscle invasion in superficial bladder cancer. Clin Cancer Res 2003, 9(7): 2583-2586.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript