Research Article | Open Access
Recent Advances in Advance Prostate Cancer
Nishant Lohia1
1Department of Radiation Oncology, Command Hospital Air Force, Bangalore, India.
Correspondence: Nishant Lohia (Department of Radiation Oncology, Command Hospital Air Force, Bangalore, 560008, India; Email: chikslohia@gmail.com).
Annals of Urologic Oncology 2022; 5(2): 81-88. https://doi.org/10.32948/auo.2022.12.29
Received: 28 Dec 2022 | Accepted: 29 Dec 2022 | Published online: 30 Dec 2022
Key words prostate cancer, survival, androgen deprivation therapy, immunotherapy, targeted therapy
Prostate being a slow goring tumor, therapeutic modalities in localized and organ confined prostate cancer are- either surveillance or localized treatment in form of surgery or radiotherapy depending on expected patient survival and risk stratification grouping as seen in Table 1. Risk stratification is based on varied parameters such as clinical Tumor (T) stage as in Table 2, laboratory features like pre-treatment Prostate Specific Antigen (PSA) levels and PSA density and pathological features such as prostatic biopsy and histological grade groups respectively. Histologic grade is determined form Gleason score and Gleason pattern (Table 3).
Moreover, with advent of newer molecular classification, it may soon supplant the older risk stratification in prostate cancers as the newer genomic method would seem to be a much better approach in guiding surveillance versus insinuation of early treatment.
In patients with localized disease with intermediate and high risk features aim is eradication of tumor locally and at the same time elimination of micro metastases if any. However, for patient with advance disease where disease has spread to distant organs or nodes (regional or non-regional) and also for CRPC, the aim is to improve quality of life and also prolong life by eliminating debilitating symptoms and prevention of symptoms. Over the last decade numerous newer agents have developed for management of advanced and metastatic prostate cancer and we will be focusing on these newer therapeutic modalities here.
|
Table 1. Risk stratification in prostate cancers. |
|||||
|
Risk Group |
Clinical |
Laboratory |
Pathological |
Remarks |
|
|
Very Low |
T1c |
PSA<10ng/mL PSA density <0.15ng/mL/g |
Grade Group 1 Fewer than 3 prostate biopsy fragments/cores positive and ≤ 50% cancer in each fragment/core |
Should have all the following |
|
|
Low |
T1-2a |
PSA <10ng/mL |
Grade Group 1 |
Should have all of the following |
|
|
Intermediate |
T2b-T2c |
PSA 10-20ng/mL |
Grade Group 2 or 3 |
Favorable |
Has all of the following- · 1 intermediate risk factor (IRF) · Grade Group 1 or 2 · <50% biopsy cores positive |
|
Unfavourable |
Has 1 or more of following- · 2 or 3 IRFs · Grade Group 3 · ≥50% biopsy cores positive |
||||
|
High |
T3a |
PSA >20 ng/mL |
Grade Group 4 or 5 |
Has at least one of the following |
|
|
Very High |
T3b-4 |
- |
Primary Gleason pattern 5;>4 cores with Grade Group 4 or 5 |
Has at least one of the following or has 2-3 high risk features |
|
|
Table 2. Clinical TNM staging in carcinoma prostate. |
|
|
TX |
Primary tumor cannot be assessed |
|
T0 |
No evidence of primary tumor |
|
T1 |
Clinically inapparent tumor that is not palpable |
|
T1a |
Tumor incidental histologic finding in 5% or less of tissue resected |
|
T1b |
Tumor incidental histologic finding in more than 5% of tissue resected |
|
T1c |
Tumor identified by needle biopsy found in one or both sides but not palpable |
|
T2 |
Tumor is palpable and confined within prostate |
|
T2a |
Tumor involves one-half of one side or less |
|
T2b |
Tumor involves more than one-half of one side but not both sides |
|
T2c |
Tumor involves both sides |
|
T3 |
Extraprostatic tumor that is not fixed or does not invade adjacent structures |
|
T3a |
Extraprostatic extension(unilateral or bilateral) |
|
T3b |
Tumor invades seminal vesicle(s) |
|
T4 |
Tumor is fixed or invades adjacent structures other than seminal vesicles such as external sphincter,rectum,bladder,levator muscles,and/or pelvic wall |
|
NX |
Regional lymph nodes cannot be assessed |
|
N0 |
No positive regional lymph nodes |
|
N1 |
Metastases in regional node(s) |
|
M0 |
No distant metastases |
|
M1 |
Distant metastases |
|
M1a |
Distant metastases to nonregional lymph node(s) |
|
M1b |
Distant metastases to bone(s) |
|
M1c |
Distant metastases to other site(s0 with or without bone disease |
|
Table 3. Definition of histologic grade. |
||
|
Grade Group |
Gleason Score |
Gleason Pattern |
|
1 |
≤6 |
≤3+3 |
|
2 |
7 |
3+4 |
|
3 |
7 |
4+3 |
|
4 |
8 |
4+4 |
|
5 |
9 or 10 |
4+5; 5+4; 5+5 |
In advanced prostate carcinoma where disease has progressed outside prostate, androgen deprivation therapy (ADT) plays a major role. Unfortunately, in advanced prostate cancers, most patients develop resistance to ADT and progress towards castration-resistant prostate cancer (CRPC) after 18 to 36 months [6]. CRPC is prostate cancer with clinical, radiographical and biochemical progression despite castrate levels of serum testosterone (<50ng/dl; 1.7nmol/L) [7]. This level was determined based on methodological considerations and the sensitivity of assays that were available during the early 2000s [8]. However several studies since the early 1990s have challenged the outdated benchmark of 50ng/dl and recommended revisiting the definition, with many suggesting a new benchmark of 20ng/dl;0.7 nmol/L [9]. Though these recommendations have been made, but are not yet clinically confirmed by the National Comprehensive Cancer Network (NCCN) and the castrate level considered by the regulatory authorities is still < 50 ng/dL (1.7 mmol/L).
Better understanding of the biology of disease has led to the accelerated development of newer treatments such as- biological agent ( Sipuleucel-T), cytotoxic agent(Cabazitaxel), hormonal agents(CYP17 inhibitor abiraterone acetate and and a next-generation antiandrogen, enzalutamide), bone-seeking α- emitting radionuclide (radium-223) and denosumab, a monoclonal antibody that binds the cytokine RANKL (receptor activator of nuclear factor kappa B ligand).
Despite these advances, disease still relapses due to different resistant mechanism. Recent data implicate the continued activation of the androgen axis as a stimulus for growth for CRPC. Moreover, xenograft studies have too confirmed the central role of increased AR expression in CRPC development [10]. Hence different survival and growth promoting pathways which interact with AR signaling needs to be targeted.
Hence, in this study we are focusing on newer agents that have been approved since 2010 to treat CRPC along with other ongoing newer developments in advanced prostate cancer.
(a) Androgen Biosynthesis Inhibition: Observation by Tilki and Evans that androgen axis remains active in patients even with CRPC and that metastatic prostate cancers can generate its own androgens has led to the development of agent that can impact androgen production in tumor as well as other sites in the body. The first such agent approved for mCRPC is abiraterone [11]. Prostate cancer is a testosterone dependent disease with pulsatile release of luteinizing hormone releasing hormone (LHRH) after hypothalamus receives a signal. LHRH subsequently releases luteinizing hormone (LH) which then activates leydig cells in testes to produce testosterone. The alternate steroid pathway also exists and it is via the adrenal gland. Gonadotropin-releasing hormone (GnRH) and LH regulates testicular androgen synthesis whereas corticotrophin-releasing hormone (CRH)-adrenocorticotropic hormone(ACTH) axis regulates adrenal androgen synthesis. The androgen signalling axis pathway and the drugs and enzymes inhibiting both testicular and adrenal steroid production is depicted in Figure 1 [12]. Abiraterone inhibits Cytochrome P450 17α-hydroxylase(CYP17), the enzyme involved in androgen synthesis. Other non-steroidal antiandrogens like biclutamide, flutamide, and nilutamide competitively inhibit the binding of androgens to androgen receptors and enzalutamide, another non-steroidal antiandrogen blocks the translocation of the ligand bound AR complex to the nucleus and from binding to DNA.
Abiraterone is an irreversible inhibitor of CYP17A1, a 17–20 lyase and 17- α hydroxylase of the cytochrome P450 family, that blocks androgen production in the testis, adrenal glands and prostate, thus preventing prostate cancer growth. It has antitumor effects on both chemotherapy treated and chemotherapy na?ve patients with CRPC. In April 2011 Food and Drug Administration (FDA) approved abiraterone in combination with low dose prednisone for metastatic CRPC patients who have received docetaxel based on the results of phase III trial COU-AA-301 [13]. FDA in 2012 also approved it for pre-docetaxel setting based on the results of phase III trial COU-AA-302 [14]. Moreover, very recently in February 2018, FDA approved abiraterone in combination with low dose prednisone in metastatic hormone sensitive prostate cancer(mHSPC) based on the results of two phase 3 clinical trials(STAMPEDE AND LATITUDE) that demonstrated improved overall survival (OS) over ADT alone [15, 16]. Other CYP17A1 inhibitors like orteronel and galeterone were also studied but they failed to show any substantial benefit [17].
(b) Androgen Receptor(AR) Antagonist: Enzalutamide, formerly known as MDV 3100 is a second generation pure AR antagonist as unlike first generation AR antagonist such as biclutamide or flutamide it has no known agonist activity [10]. It got FDA approval in 2012 and 2014 for mCRPC with prior docetaxel therapy and for chemotherapy na?ve mCRPC respectively based on phase 3 trials(AFFIRM and PREVAIL respectively [18, 19]. Moreover two other randomized clinical trials, TERRAIN study and STRIVE trial have demonstrated superiority of enzalutamide over biclutamide for cancer control in mCRPC [20, 21]. In addition, it also improves OS as well as PFS (MFS) in metastatic CRPC (mCRPC) patients and is category 1 option for patients with mCRPC as per national comprehensive cancer network(NCCN) guidelines. FDA also approved it in 2018 for non-metastatic CRPC(nmCRPC) and is category 1 option as per NCCN guidelines for nmCRPC if PSA doubling time(PSADT) is less than or equal to 10 months based on results of phase 3 PROSPER trial which showed improved metastasis free survival(MFS) [22].
Apalutamide is another oral AR antagonist resembling enzalutamide structurally and got FDA approval in 2018 for nm CRPC based on phase 3 SPARTAN trial which had improved MFS [23]. Darulotamide, another AR antagonist with low blood brain barrier penetration and thereby better safety profile got FDA approval in 2019 in nmCRPC based on phase 3 ARAMIS study which also pointed towards improved primary end point of MFS compared to placebo [24].
Traditional secondary hormonal therapy used before the introduction of above mentioned newer hormonal agents are first generation antiandrogen, antiandrogen withdrawal, steroids, ketoconazole, or estrogen such as diethylstilbestrol(DES). However, none has shown to increase survival in randomized clinical trials.
Chemotherapy (cytotoxic therapy)
(a) Docetaxel: Mitoxantrone was the first cytotoxic agent to get approved way back in 1996 and was indicated only in palliative setting when used in combination with prednisone. Thus it was the first cytotoxic agent which became standard to which other treatments would be compared.
Subsequently two pivotal trials (TAX 327 and SWOG 9916) which showed better palliation and delayed progression with docetaxel and prednisone combination over mitoxantrone and prednisone led to the approval of docetaxel with prednisone in mCRPC in 2004 [25]. Docetaxel also became standard of care for mHSPC based on the results from two phase 3 trials (ECOG 3805/CHAARTED and STAMPEDE) [26, 15].
(b) Cabazitaxel: Cabazitaxel, a microtubule inhibitor just like docetaxel prevents tubulin depolymerization and thereby mitotic cell division, eventually leading to cell death [6]. It has recently been approved by FDA in June 2010 in docetaxel resistant cancers based on randomized phase 3 TROPIC trial [27]. The NCCN Guidelines panel has thus included cabazitaxel as second line therapy in patients with symptomatic mCRPC who has progressed on docetaxel [28].
Immunotherapy
The autologous active cellular immunotherapy, sipuleucel-T became the first in a new class of cancer immunotherapeutic agents to be approved by FDA for mCRPC in 2010 based on the 4.1 months’ survival in IMPACT trial demonstrating its superiority in mCRPC [29]. However it failed to show significant improvement in time to progression or PSA decline. This discordance between progression free and OS may be observed in immunotherapy trials for prostate cancer as similar trend was noted when PROSTVAC, a PSA directed vaccine therapy, was compared to placebo in men with CRPC [10]. Prostvac is a prostate cancer vaccine regimen consisting of a recombinant vaccinia vector as a primary vaccination, followed by multiple booster vaccinations employing a recombinant fowl pox vector. Both vectors contain the transgenes for prostate-specific antigen (PSA) and multiple T-cell costimulatory molecules (TRICOM) [30].
Another recent focus of immunotherapy in CRPC is prostate specific membrane antigen (PSMA). The initial clinical study with a PSMA-targeting ADC gave positive results but no phase 3 study has been carried out till date. However only phase II studies have been completed in docetaxel refractory patients [6, 10].
Some of the trials leading to approval of these newer agents are summarized in Table 4.
Targeted therapy
(a) Pembrolizumab: FDA approved the use of anti PD1 antibody, pembrolizumab in May 2017 based on a study with 149 patients for patient with unresectable or metastatic microsatellite instability high(MSI-H) or mismatch repair (MMR)-deficient (dMMR) solid tumors who have progressed on prior treatment and are left with no suitable alternatives. Based on outcomes of other smaller studies and KEYNOTE-199 phase II study, NCCN panel supported the use of pembrolizumab as category 2B recommendation in patients with MSI-H or dMMR metastatic CRPC whose disease has progressed on at least one-line systemic therapy for mCRPC [28].
(b) Bone directed therapy: Most patients with CRPC have painful bone metastases. The high propensity for prostate cancers to metastasize to the bone results in significant morbidity from skeletal related events (SREs) and thereby can impact duration and Quality of Life of patients (QoL). This is further complicated by the bone loss associated with ADT and frequent use of corticosteroids. Hence, by targeting bone microenvironment, SREs can be delayed resulting in prolong and better QoL.
Zoledronic acid is the only bisphosphonate which is FDA approved for CRPC with bone metastases.
Denosumab is a fully human monoclonal antibody directed against RANKL (receptor activator of nuclear factor kappa-B ligand) and inhibits osteoclast function. Though in a phase 3 trial of 1432 patients with nmCRPC, denosumab delayed bone metastases by 4 months compared to placebo and was also statistically significant it failed to receive FDA approval for bone metastases [28].
Alpha Emitting agent, Radium 223 dichloride is a targeted alpha therapy which is administered intravenously. In CRPC patients, it significantly improved OS irrespective of prior docetaxel use and also decreased pain related to bony metastases and demonstrated a favorable safety profile. So, it was originally approved by the FDA in May 2013 for CRPC patients with bone metastases and no known visceral metastatic disease based on clinical data from phase 3 randomised trial (ALSYMPCA) [6, 28].
Beta Emitters, strontium-89(89Sr) or samarium-153(153Sm) unlike the alpha emitter radium 223 had no survival advantage and are only used in palliative setting for treatment of painful of bone metastases [28].
Targeted agents in patients with DNA repair gene mutations
There is a high incidence of DNA damage response (DDR) defects in advanced prostate cancer patients and include mainly mutations in the homologous recombination and DNA mismatch repair pathways [31]. Early studies suggest germline and somatic mutations in homologous recombination repair (HRR) genes and may be predictive of clinical benefit with poly ADP ribose polymerase (PARP) inhibitors. Presently two PARP inhibitors approved by FDA are Olaparib and Rucaparib. Both got recent FDA approval in May 2020. Olaparib got FDA approval based on favorable efficacy data from phase 3 PROfound trial for use in patients with mCRPC and deleterious or suspected deleterious germline or somatic HRR gene mutations in at least one of 14 genes (BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51c, RAD51D or RAD54L) and who had previously received treatment with enzalutamide or abiraterone. Rucaparib got accelerated FDA approval based on preliminary favorable data from TRITON 2 clinical study. It is however awaiting full FDA approval as results of phase 3 TRITON 3 study is still awaited [28].
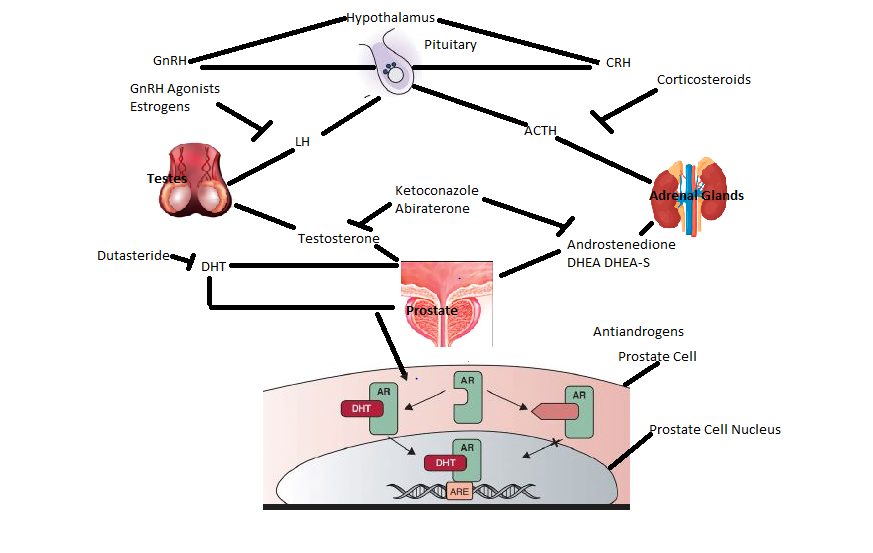 Figure 1. The Androgen Signaling axis with its inhibitors. GnRH: Gonadotropin-releasing hormone; LH: Luteinizing hormone; CRH: Corticotrophin releasing hormone; ACTH: Adrenocorticotropic hormone; DHEA: Dehydroepiandrosterone; DHEA-S: Dehydroepiandrosterone sulfate; DHT: Dihydrotestosterone; AR: Androgen receptor; ARE: Androgen response element.
Figure 1. The Androgen Signaling axis with its inhibitors. GnRH: Gonadotropin-releasing hormone; LH: Luteinizing hormone; CRH: Corticotrophin releasing hormone; ACTH: Adrenocorticotropic hormone; DHEA: Dehydroepiandrosterone; DHEA-S: Dehydroepiandrosterone sulfate; DHT: Dihydrotestosterone; AR: Androgen receptor; ARE: Androgen response element.
|
Table 4. Newer agents showing definite os benefit in castrate resistant prostate cancer and getting regulatory approval. |
|||||
|
Drug Name |
Trial Name |
Control Arm |
HR |
OS/MFS in months |
P Value |
|
Abiraterone + Prednisone |
Phase 3 COU-AA-302 Study [13] |
Placebo +Prednisone |
0.81 |
34.7 Vs 30.3 |
0.0033 |
|
Enzalutamide(post docetaxel) |
AFFIRM [17] |
Placebo |
0.631 |
18.4 vs 13.6 |
<0.0001 |
|
Enzalutamide |
PREVAIL[18] |
Placebo |
0.71 |
32.4Vs 30.2 |
<0.001 |
|
Apulatamide |
SPARTAN [22] |
Placebo |
0.28 |
Median MFS-40.5Vs 16.2 |
<0.001 |
|
Darulotamide |
ARAMIS [23] |
Palcebo |
0.41 |
Median MFS-40.4Vs 18.4 |
<0.001 |
|
Docetaxel(every 3 weekly)+Prednisone |
TAX327[24] |
Mitoxantrone+Prednisone |
0.76 |
18.9Vs 16.5 |
0.009 |
|
Cabazitaxel(post docetaxel) |
TROPIC[26] |
Mitoxantrone+Prednisone |
0.70 |
15.1 vs 12.7 |
<0.0001 |
|
Sipuleucel-T |
IMPACT[11] |
Palcebo |
0.78 |
25.8Vs 21.7 |
0.03 |
|
Abiraterone+ADT +prednisone |
STAMPEDE [14] |
ADT |
0.60 |
NRP(5 year survival 60% Vs 41%) |
NRP |
|
Abiraterone+ADT +prednisone |
LATITUDE [15] |
ADT+Placebo |
0.62 |
NRP |
<0.001 |
|
Docetaxel |
CHAARTED [25] |
ADT |
0.61 |
57.6 Vs 44 |
<0.001 |
|
OS-Overall Survival; MFS- Metastases Free Survival; NRP-Not reported. |
|||||
The androgen receptor splice variant 7 (AR-V7) variant data in patients has now been published, confirming the importance of preclinical studies. Lack of response to enzalutamide and abiraterone in mCRPC has been attributed to detection of AR-V7 mRNA in circulating tumor cells(CTCs) using an RNA based polymerase chain reaction (PCR) assay. This AR variant does not, however, predict resistance to docetaxel in the same setting. Men with AR V7 positive CTCs exhibited superior PFS with taxanes as compared to abiraterone and enzalutamide [28]. Taken together, these data indicate that AR-V7 can function as a predictive biomarker. Larger studies are needed to confirm these initial observations.
Finally, with development of varied newer agents comes the dilemma of optimal timing and sequencing and combining one modality especially newer modalities with conventional anti androgen and cytotoxic therapies. However gradually with better understanding of the rationale of these newer agents, clinicians will eventually reap more benefit from this newer and varied arsenal of therapeutic agents.
Command Hospital Air Force Bangalore, India.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
Dr. Nishant Lohia carried out review of various articles and texts and them compiling, proof reading, editing and final drafting of manuscript.
Competing interests
None identified.
Funding
None.
- H Sung, J Ferlay, RL Siegel, M Laversanne, I Soerjomataram, A Jemal: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021, 71(3): 209-249.
- Patel A, Tannock IF, Srivastava P, Biswas B, Gupta VG, Batra A, Bhethanabhotla S, Pramanik R, Mahindru S, Tilak T et al: Low-Dose Abiraterone in Metastatic Prostate Cancer: Is It Practice Changing? Facts and Facets. JCO Glob Oncol 2020, 6: 382-386.
- Huggins C, Hodges CV: Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941, 1(4): 293-297.
- Labrie, F, Dupont A, Belanger A, Cusan L, Lacourciere Y, Monfette G, Laberge JG, Emond JP, Fazekas AT, Raynaud JP et al: New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med 1982, 5: 267-275.
- Prostate Cancer Trialists’ Collaborative Group: Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Lancet 1995, 346(8970): 265-269.
- Nevedomskaya E, Baumgart SJ, Haendler B: Recent Advances in Prostate Cancer Treatment and Drug Discovery. Int J Mol Sci 2018, 19(5): 1359.
- Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R et al: Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008, 26(7): 1148-1159.
- Tombal B, Berges R: How good do current LHRH agonists control testosterone? Can this be improved with Eligard? Eur Urol 2005, 4: 30-36
- Shayegan B, Pouliot F, So A, Fernandes J, Macri J: Testosterone monitoring for men with advanced prostate cancer: Review of current practices and a survey of Canadian physicians. Can Urol Assoc J 2017, 11(6): 204-209.
- Wadia R, Petrylak DP: New developments in the treatment of castration resistant prostate cancer. Asian J Androl 2014, 16(4): 555-560.
- Gomella LG, Petrylak DP, Shayegan B: Current management of advanced and castration resistant prostate cancer. Can J Urol 2014, 21(2 Supp 1): 1-6.
- Zelefsky MJ, Morris MJ, Eastham JA: Chapter 70: Cancer of the Prostate. Principles and Practice of Oncology, 11th edition 2019.
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F et al: Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012, 13: 983-992
- Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S et al: Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013, 368: 138-148.
- James ND, Clarke NW, Cook A, Ali A, Hoyle AP, Attard G, Brawley CD, Chowdhury S, Cross WR, Dearnaley DP et al: STAMPEDE Trials Collaborative Group. Abiraterone acetate plus prednisolone for metastatic patients starting hormone therapy: 5-year follow-up results from the STAMPEDE randomised trial (NCT00268476). Int J Cancer 2022, 151(3): 422-434.
- Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Ozguroglu M, Ye D, Feyerabend S, Protheroe A et al: Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019, 20(5): 686-700.
- Alex AB, Pal SK, Agarwal N: CYP17 inhibitors in prostate cancer: Latest evidence and clinical potential. Ther Adv Med Oncol 2016, 8: 267-275.
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND et al: Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012, 367(13): 1187-1197.
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S et al: Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014, 371(5): 424-433.
- Shore ND, Chowdhury S, Villers A, Klotz L, Siemens DR, Phung D, van Os S, Hasabou N, Wang F, Bhattacharya S et al: Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol 2016, 17(2): 153-163.
- Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, Dunshee C, Wang F, Wu K, Krivoshik A et al: Enzalutamide Versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J Clin Oncol 2016, 34(18): 2098-2106.
- Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, Ivashchenko P, Demirhan E, Modelska K, Phung D et al: PROSPER: A phase 3 study of enzalutamide in nonmetastatic (M0) castration resistant prostate cancer (CRPC) patients. J Clin Oncol 2014, 32(15 Suppl): TPS5094.
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H et al: Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 2018, 378: 1408-1418.
- Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, Jievaltas M, Luz M, Alekseev B, Kuss I et al: Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2019, 380: 1235-1246.
- Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008, 26(2): 242-245.
- Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM et al: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015, 373(8): 737-746.
- Oudard S. TROPIC: Phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer. Future Oncol 2011, 7(4): 497-506.
- National Comprehensive Cancer Network. Prostate Cancer (Version 3.2020). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed December 29, 2020.
- Kawalec P, Paszulewicz A, Holko P, Pilc A: Sipuleucel-T immunotherapy for castration-resistant prostate cancer. A systematic review and meta-analysis. Arch Med Sci 2012, 8(5): 767-775.
- Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs 2009, 18(7): 1001-1011.
- Schweizer MT, Antonarakis ES: Prognostic and therapeutic implications of DNA repair gene mutations in advanced prostate cancer. Clin Adv Hematol Oncol 2017, 15(10): 785-795.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript