Review Article | Open Access
Research Progress of Metabolic Syndrome and Renal Cancer
Yiwen Wang1, Yajun Shi1, Mengye Zhang1, Jiao Cao2
1Department of Pharmacology, Shaanxi University of Chinese Medicine, Xianyang 712046, China.
2Department of Pharmacy, Xijing Hospital, Fourth Military Medical University, Xi'an 710032, China.
Correspondence: Jiao Cao (Department of Plastic and Reconstructive Surgery, Xijing Hospital, Fourth Military Medical University, Changle West Road 169, Xi'an, Shaanxi Province, P.R. China, 710000; Email: caojiao1986@163.com)
Annals of Urologic Oncology 2024, 7(1): 26-32. https://doi.org/10.32948/auo.2024.03.24
Received: 24 Feb 2024 | Accepted: 14 Mar 2024 | Published online: 30 Mar 2024
Key words metabolic syndrome, kidney cancer, risk, invasive, prognosis
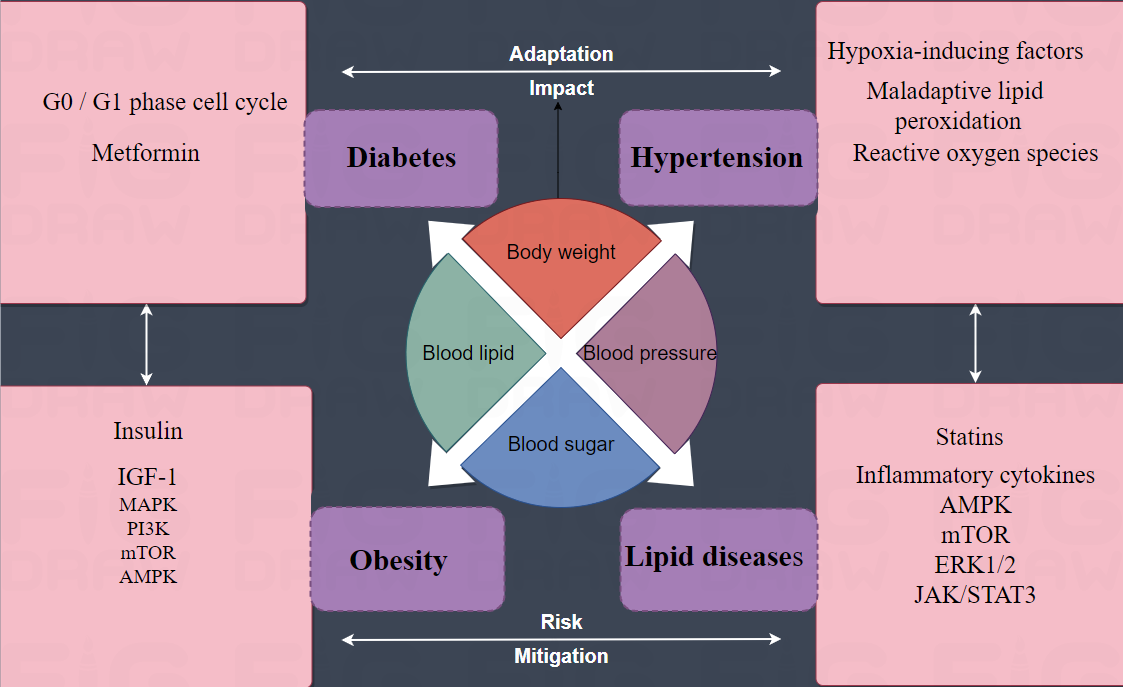 Figure 1. Metabolic syndrome and renal cancer. By inducing apoptosis and G0 / G1 phase cell cycle arrest, the antisugar metformin can inhibit the growth of kidney cancer cells in vivo and in vitro. Glyceryl triester was the only lipid component statistically associated with renal cancer. In addition, statins used for the treatment of lipid diseases, especially hypercholesterolemia, have shown significant inhibitory effects on renal cancer cells in vitro, suggesting that abnormal lipid metabolism may be related to the growth, invasion, angiogenesis and metastasis of renal cancer cells. Only one component of hypertension was associated with tumor pathology, patients with hypertension were more likely to have renal cancer with non-clear cell histology. Hyperinsulinemia/insulin resistance. The effect of insulin on cancer cell proliferation is thought to be related to IGF-1 stimulation. IGF-1 plays a role in promoting mitosis, cell migration, angiogenesis and inhibition of cytoplasmic death by activating mitogen-activated protein kinase (MAPK) and phosphoinositol 3 kinase (PI3K) signaling pathways. The combination of anti-IGF antibody and mammalian target of rapamycin (mTOR) inhibitor may be effective in renal cancer. Down-regulation of AMP-activated kinases and increased acetyl-CoA carboxylase are another common metabolic change that leads to increased fatty acid synthesis. Maladaptive lipid peroxidation and increased reactive oxygen species are thought to be pathogenic factors. In hypertensive states, upregulation of vasogenesis and hypoxia-inducing factors, including HIF-1α, is also thought to contribute to an increased risk of kidney cancer. The pro-inflammatory cytokines IL-6 and IL-10 are strongly expressed in both renal cancer cells and stroma, and IL-10 levels are higher in more advanced tumors (pT3). In vitro experiments have shown that adiponectin secreted by white adipose tissue can inhibit tumor growth by activating AMP-activated egg white kinase (AMPK) and regulate mTOR from below. Leptin can mediate the proliferation of kidney cancer AKI-2 cells by activating extracellular signal-regulated kinase (ERK1/2) and janus kinase/signal transduction and transcriptional activator 3 (JAK/STAT3) signaling pathways, and elevated serum leptin levels and leptin receptor overexpression are associated with renal cancer invasion and progression. AMPK: Adenosine 5‘-monophosphate (AMP)-activated protein kinase; mTOR: mammalian target of rapamycin; ERK1/2: extracellular regulated protein kinases; JAK/STAT3: janus kinase/signal transduction and transcriptional activator 3; MAPK: mitogen-activated protein kinase; IGF-1: Insulin-like growth factor 1; PI3K: An intracellular phosphatidylinositol kinase that is related to the products of cancer genes such as V.SC and V.R.AS, and which itself has serine/threonine (Ser/Thr) kinase activity.
Figure 1. Metabolic syndrome and renal cancer. By inducing apoptosis and G0 / G1 phase cell cycle arrest, the antisugar metformin can inhibit the growth of kidney cancer cells in vivo and in vitro. Glyceryl triester was the only lipid component statistically associated with renal cancer. In addition, statins used for the treatment of lipid diseases, especially hypercholesterolemia, have shown significant inhibitory effects on renal cancer cells in vitro, suggesting that abnormal lipid metabolism may be related to the growth, invasion, angiogenesis and metastasis of renal cancer cells. Only one component of hypertension was associated with tumor pathology, patients with hypertension were more likely to have renal cancer with non-clear cell histology. Hyperinsulinemia/insulin resistance. The effect of insulin on cancer cell proliferation is thought to be related to IGF-1 stimulation. IGF-1 plays a role in promoting mitosis, cell migration, angiogenesis and inhibition of cytoplasmic death by activating mitogen-activated protein kinase (MAPK) and phosphoinositol 3 kinase (PI3K) signaling pathways. The combination of anti-IGF antibody and mammalian target of rapamycin (mTOR) inhibitor may be effective in renal cancer. Down-regulation of AMP-activated kinases and increased acetyl-CoA carboxylase are another common metabolic change that leads to increased fatty acid synthesis. Maladaptive lipid peroxidation and increased reactive oxygen species are thought to be pathogenic factors. In hypertensive states, upregulation of vasogenesis and hypoxia-inducing factors, including HIF-1α, is also thought to contribute to an increased risk of kidney cancer. The pro-inflammatory cytokines IL-6 and IL-10 are strongly expressed in both renal cancer cells and stroma, and IL-10 levels are higher in more advanced tumors (pT3). In vitro experiments have shown that adiponectin secreted by white adipose tissue can inhibit tumor growth by activating AMP-activated egg white kinase (AMPK) and regulate mTOR from below. Leptin can mediate the proliferation of kidney cancer AKI-2 cells by activating extracellular signal-regulated kinase (ERK1/2) and janus kinase/signal transduction and transcriptional activator 3 (JAK/STAT3) signaling pathways, and elevated serum leptin levels and leptin receptor overexpression are associated with renal cancer invasion and progression. AMPK: Adenosine 5‘-monophosphate (AMP)-activated protein kinase; mTOR: mammalian target of rapamycin; ERK1/2: extracellular regulated protein kinases; JAK/STAT3: janus kinase/signal transduction and transcriptional activator 3; MAPK: mitogen-activated protein kinase; IGF-1: Insulin-like growth factor 1; PI3K: An intracellular phosphatidylinositol kinase that is related to the products of cancer genes such as V.SC and V.R.AS, and which itself has serine/threonine (Ser/Thr) kinase activity.
None.
Ethical policy
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Approval from institutional ethical committee was taken.
Availability of data and materials
All data generated or analysed during this study are included in this publication.
Author contributions
YWW, YJS and MYZ: Conception, design of study, literature search and review, figure production, manuscript writting; JC: Supervision and approval for the final version of the manuscript.
Competing interests
The authors have no competing interest.
Funding
None.
- Reaven GM: Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37(12): 1595-1607.
- Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ: Prevalence of the metabolic syndrome in the United States, 2003-2012. Jama 2015, 313(19): 1973-1974.
- Minhas S, Kayes O, Hegarty P, Kumar P, Freeman A, Ralph D: What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int 2005, 96(7): 1040-1043.
- Wu Q, Chen G, Wu WM, Zhou L, You L, Zhang TP, Zhao YP: Metabolic syndrome components and risk factors for pancreatic adenocarcinoma: a case-control study in China. Digestion 2012, 86(4): 294-301.
- Lindkvist B, Almquist M, Bjørge T, Stocks T, Borena W, Johansen D, Hallmans G, Engeland A, Nagel G, Jonsson H et al: Prospective cohort study of metabolic risk factors and gastric adenocarcinoma risk in the Metabolic Syndrome and Cancer Project (Me-Can). Cancer Causes Control 2013, 24(1): 107-116.
- Forootan M, Tabatabaeefar M, Yahyaei M, Maghsoodi N: Metabolic syndrome and colorectal cancer: a cross-sectional survey. Asian Pac J Cancer Prev 2012, 13(10): 4999-5002.
- Häggström C, Stocks T, Rapp K, Bjørge T, Lindkvist B, Concin H, Engeland A, Manjer J, Ulmer H, Selmer R et al: Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can). Int J Cancer 2011, 128(8): 1890-1898.
- Häggström C, Rapp K, Stocks T, Manjer J, Bjørge T, Ulmer H, Engeland A, Almqvist M, Concin H, Selmer R et al: Metabolic factors associated with risk of renal cell carcinoma. PLoS One 2013, 8(2):e57475.
- Siegel RL, Miller KD: Cancer statistics, 2023. CA Cancer J Clin 2023, 73(1): 17-48.
- Siegel R, Ma J, Zou Z, Jemal A: Cancer statistics, 2014. CA Cancer J Clin 2014, 64(1): 9-29.
- Stewart SB, Thompson RH, Psutka SP, Cheville JC, Lohse CM, Boorjian SA, Leibovich BC: Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol 2014, 32(36): 4059-4065.
- Printz C: American cancer society reports on 25-year cancer mortality rate goals. Cancer 2016, 122(15): 2289-2291.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J: Cancer statistics in China, 2015. CA Cancer J Clin 2016, 66(2): 115-132.
- Alexander MP, Patel TV, Farag YM, Florez A, Rennke HG, Singh AK: Kidney pathological changes in metabolic syndrome: a cross-sectional study. Am J Kidney Dis 2009, 53(5): 751-759.
- Zhang Y, Wu T, Xie J, Yan L, Guo X, Xu W, Wang L: Effects of metabolic syndrome on renal function after radical nephrectomy in patients with renal cell carcinoma. Int Urol Nephrol 2021, 53(10): 2127-2135.
- Bulut S, Aktas BK, Erkmen AE, Ozden C, Gokkaya CS, Baykam MM, Memis A: Metabolic syndrome prevalence in renal cell cancer patients. Asian Pac J Cancer Prev 2014, 15(18): 7925-7928.
- Graff RE, Sanchez A: Type 2 Diabetes in Relation to the Risk of Renal Cell Carcinoma Among Men and Women in Two Large Prospective Cohort Studies. Diabetes Care 2018, 41(7): 1432-1437.
- Liu J, Li M, Song B, Jia C, Zhang L, Bai X, Hu W: Metformin inhibits renal cell carcinoma in vitro and in vivo xenograft. Urol Oncol 2013, 31(2): 264-270.
- Colt JS, Schwartz K, Graubard BI, Davis F, Ruterbusch J, DiGaetano R, Purdue M, Rothman N, Wacholder S, Chow WH: Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011, 22(6): 797-804.
- Colt JS, Hofmann JN, Schwartz K, Chow WH, Graubard BI, Davis F, Ruterbusch J, Berndt S, Purdue MP: Antihypertensive medication use and risk of renal cell carcinoma. Cancer Causes Control 2017, 28(4): 289-297.
- Turco F, Tucci M, Di Stefano RF, Samuelly A, Bungaro M, Audisio M, Pisano C, Di Maio M, Scagliotti GV, Buttigliero C: Renal cell carcinoma (RCC): fatter is better? A review on the role of obesity in RCC. Endocr Relat Cancer 2021, 28(7): R207-r216.
- Sanchez A, Furberg H, Kuo F, Vuong L, Ged Y, Patil S, Ostrovnaya I, Petruzella S, Reising A, Patel P et al: Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol 2020, 21(2): 283-293.
- Van Hemelrijck M, Garmo H, Hammar N, Jungner I, Walldius G, Lambe M, Holmberg L: The interplay between lipid profiles, glucose, BMI and risk of kidney cancer in the Swedish AMORIS study. Int J Cancer 2012, 130(9): 2118-2128.
- Horiguchi A, Ito K, Sumitomo M, Kimura F, Asano T, Hayakawa M: Decreased serum adiponectin levels in patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2008, 38(2): 106-111.
- Kocher NJ, Rjepaj C, Robyak H, Lehman E, Raman JD: Hypertension is the primary component of metabolic syndrome associated with pathologic features of kidney cancer. World J Urol 2017, 35(1): 67-72.
- Ozbek E, Otunctemur A, Sahin S, Dursun M, Besiroglu H, Koklu I, Polat EC, Erkoc M, Danis E, Bozkurt M: Renal cell carcinoma is more aggressive in Turkish patients with the metabolic syndrome. Asian Pac J Cancer Prev 2013, 14(12): 7351-7354.
- Zhu Y, Wang HK, Zhang HL, Yao XD, Zhang SL, Dai B, Shen YJ, Liu XH, Zhou LP, Ye DW: Visceral obesity and risk of high grade disease in clinical t1a renal cell carcinoma. J Urol 2013, 189(2): 447-453.
- Steffens S, Grünwald V, Ringe KI, Seidel C, Eggers H, Schrader M, Wacker F, Kuczyk MA, Schrader AJ: Does obesity influence the prognosis of metastatic renal cell carcinoma in patients treated with vascular endothelial growth factor-targeted therapy? Oncologist 2011, 16(11): 1565-1571.
- Eskelinen TJ, Kotsar A, Tammela TLJ, Murtola TJ: Components of metabolic syndrome and prognosis of renal cell cancer. Scand J Urol 2017, 51(6): 435-441.
- Kriegmair MC, Mandel P, Porubsky S, Dürr J, Huck N, Nuhn P, Pfalzgraf D, Michel MS, Wagener N: Metabolic Syndrome Negatively Impacts the Outcome of Localized Renal Cell Carcinoma. Horm Cancer 2017, 8(2): 127-134.
- Liu Z, Wang H, Zhang L, Li S, Fan Y, Meng Y, Hu S, Zhang Q, He Z, Zhou L et al: Metabolic syndrome is associated with improved cancer-specific survival in patients with localized clear cell renal cell carcinoma. Transl Androl Urol 2019, 8(5): 507-518.
- Luzzago S, Palumbo C, Rosiello G, Pecoraro A, Deuker M, Stolzenbach F, Mistretta FA, Tian Z, Musi G, Montanari E et al: Metabolic Syndrome Predicts Worse Perioperative Outcomes in Patients Treated With Partial Nephrectomy for Renal Cell Carcinoma. Urology 2020, 140: 91-97.
- Chen L, Li H, Gu L, Ma X, Li X, Gao Y, Zhang Y, Shen D, Fan Y, Wang B et al: The Impact of Diabetes Mellitus on Renal Cell Carcinoma Prognosis: A Meta-Analysis of Cohort Studies. Medicine (Baltimore) 2015, 94(26): e1055.
- Antonelli A, Arrighi N, Corti S, Zanotelli T, Cozzoli A, Cosciani Cunico S, Simeone C: Pre-existing type-2 diabetes is not an adverse prognostic factor in patients with renal cell carcinoma: a single-center retrospective study. Urol Oncol 2013, 31(7): 1310-1315.
- Höfner T, Zeier M, Hatiboglu G, Eisen C, Schönberg G, Hadaschik B, Teber D, Duensing S, Trumpp A, Hohenfellner M et al: The impact of type 2 diabetes on the outcome of localized renal cell carcinoma. World J Urol 2014, 32(6): 1537-1542.
- Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM: Influence of body mass index, smoking, and blood pressure on survival of patients with surgically-treated, low stage renal cell carcinoma: a 14-year retrospective cohort study. J Korean Med Sci 2013, 28(2): 227-236.
- Parker A, Freeman LB, Cantor K, Lynch C: Self-report of smoking, obesity and hypertension history and survival among a cohort of iowa renal cell carcinoma cases. Ann Epidemiol 2000, 10(7): 467-468.
- Haddad AQ, Jiang L, Cadeddu JA, Lotan Y, Gahan JC, Hynan LS, Gupta N, Raj GV, Sagalowsky AI, Margulis V: Statin Use and Serum Lipid Levels Are Associated With Survival Outcomes After Surgery for Renal Cell Carcinoma. Urology 2015, 86(6): 1146-1152.
- Ohno Y, Nakashima J, Nakagami Y, Gondo T, Ohori M, Hatano T, Tachibana M: Clinical implications of preoperative serum total cholesterol in patients with clear cell renal cell carcinoma. Urology 2014, 83(1): 154-158.
- Kaffenberger SD, Lin-Tsai O, Stratton KL, Morgan TM, Barocas DA, Chang SS, Cookson MS, Herrell SD, Smith JA, Jr., Clark PE: Statin use is associated with improved survival in patients undergoing surgery for renal cell carcinoma. Urol Oncol 2015, 33(1): 21.e11-21.e17.
- Choi Y, Park B, Jeong BC, Seo SI, Jeon SS, Choi HY, Adami HO, Lee JE, Lee HM: Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer 2013, 132(3): 625-634.
- Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, Ciriello G, Mikklineni N, Fiegoli B, Kim PH, Voss MH et al: An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 2013, 105(24): 1862-1870.
- Lee HW, Jeong BC, Seo SI, Jeon SS, Lee HM, Choi HY, Jeon HG: Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. Int J Urol 2015, 22(5): 455-461.
- Persky S, de Heer HD, McBride CM, Reid RJ: The role of weight, race, and health care experiences in care use among young men and women. Obesity (Silver Spring) 2014, 22(4): 1194-1200.
- Labochka D, Moszczuk B, Kukwa W, Szczylik C, Czarnecka AM: Mechanisms through which diabetes mellitus influences renal cell carcinoma development and treatment: A review of the literature. Int J Mol Med 2016, 38(6): 1887-1894.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr.: Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120(16): 1640-1645.
- Bellastella G, Scappaticcio L, Esposito K, Giugliano D, Maiorino MI: Metabolic syndrome and cancer: "The common soil hypothesis". Diabetes Res Clin Pract 2018, 143: 389-397.
- Uzunlulu M, Telci Caklili O, Oguz A: Association between Metabolic Syndrome and Cancer. Ann Nutr Metab 2016, 68(3): 173-179.
- Simone S, Gorin Y, Velagapudi C, Abboud HE, Habib SL: Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2'-deoxyguanosine-DNA glycosylase. Diabetes 2008, 57(10): 2626-2636.
- Ibrahim YH, Yee D: Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res 2004, 14(4): 261-269.
- Kasprzak A: Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int J Mol Sci 2021, 22(12): 6434.
- Tracz AF, Szczylik C, Porta C, Czarnecka AM: Insulin-like growth factor-1 signaling in renal cell carcinoma. BMC Cancer 2016, 16: 453.
- Sudarshan S, Karam JA, Brugarolas J, Thompson RH, Uzzo R, Rini B, Margulis V, Patard JJ, Escudier B, Linehan WM: Metabolism of kidney cancer: from the lab to clinical practice. Eur Urol 2013, 63(2): 244-251.
- Cancer Genome Atlas Research Network: Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013, 499(7456): 43-49.
- Tong WH, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, Romero VV, Sougrat R, Vaulont S, Viollet B et al: The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell 2011, 20(3): 315-327.
- van der Mijn JC, Panka DJ, Geissler AK, Verheul HM, Mier JW: Novel drugs that target the metabolic reprogramming in renal cell cancer. Cancer Metab 2016, 4: 14.
- Matsuda M, Shimomura I: Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract 2013, 7(5): e330-341.
- Zhang GM, Zhu Y, Ye DW: Metabolic syndrome and renal cell carcinoma. World J Surg Oncol 2014, 12: 236.
- Schödel J, Ratcliffe PJ: Mechanisms of hypoxia signalling: new implications for nephrology. Nat Rev Nephrol 2019, 15(10): 641-659.
- Micucci C, Valli D, Matacchione G, Catalano A: Current perspectives between metabolic syndrome and cancer. Oncotarget 2016, 7(25): 38959-38972.
- Harvey AE, Lashinger LM, Hursting SD: The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci 2011, 1229: 45-52.
- Wang Y, Zhang Y: Prognostic role of interleukin-6 in renal cell carcinoma: a meta-analysis. Clin Transl Oncol 2020, 22(6): 835-843.
- Sugiyama M, Takahashi H, Hosono K, Endo H, Kato S, Yoneda K, Nozaki Y, Fujita K, Yoneda M, Wada K et al: Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol 2009, 34(2): 339-344.
- Wang H, Wu J, Gu W, Wang B, Wan F, Dai B, Zhang H, Shi G, Shen Y, Zhu Y et al: Serum Adiponectin Level May be an Independent Predictor of Clear Cell Renal Cell Carcinoma. J Cancer 2016, 7(10): 1340-1346.
- Li L, Gao Y, Zhang LL, He DL: Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biol Ther 2008, 7(11): 1787-1792.
- Perumal K, Mun KS, Yap NY, Razack AHA, Gobe GC: A Study on the Immunohistochemical Expressions of Leptin and Leptin Receptor in Clear Cell Renal Cell Carcinoma. Biomed Res Int 2020, 2020: 3682086.
- Horiguchi A, Sumitomo M, Asakuma J, Asano T, Zheng R, Asano T, Nanus DM, Hayakawa M: Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol 2006, 176(4 Pt 1): 1631-1635.
- Ciccarese C, Brunelli M, Montironi R, Fiorentino M, Iacovelli R, Heng D, Tortora G, Massari F: The prospect of precision therapy for renal cell carcinoma. Cancer Treat Rev 2016, 49: 37-44.
- Psutka SP, Boorjian SA, Lohse CM, Stewart SB, Tollefson MK, Cheville JC, Leibovich BC, Thompson RH: The association between metformin use and oncologic outcomes among surgically treated diabetic patients with localized renal cell carcinoma. Urol Oncol 2015, 33(2): 67.e15-23.
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript