Review Article | Open Access
Immunotherapy Landscape in Prostate Cancer: Sucesses, Failures and Promises
Sabeeh-ur-Rehman Butt1, 2, Muhammad S Khan2, Carmen Murias1, Maria Reyes Gonzalez-Exposito1, Hendrik-Tobias Arkenau1, 3, Anna Patrikidou1, 3
1Sarah Cannon Research Institute, 93 Harley Street, London, W1G 6AD, UK.
2Guy’s and St. Thomas’ Hospital, Great Maze Pond, London, SE1 9RT, UK.
3University College London Cancer Institute, 72 Huntley Street, London, WC1E 6AG, UK.
Correspondence: Sabeeh-ur-Rehman Butt (Sarah Cannon Research Institute 93 Harley Street, London, W1G 6AD, UK; Email: dr.s.butt01@gmail.com ).
Annals of Urologic Oncology 2019, 2(2): 71-88, https://doi.org/10.32948/auo.2019.09.04
Received: 23 Jun 2019 | Accepted: 03 Sep 2019 | Published online: 15 Sep 2019
As research focus in oncology has recently shifted to immunomodulation, the era of introduction of immunotherapeutic agents in the management of prostate cancer has just begun. With the success of checkpoint blockade drugs in certain advanced tumours, ongoing efforts are aimed at identification and validation of new actionable immune targets to consolidate and expand the initial success in other tumour types. In this paper, we review the immunotherapy research in the management of prostate cancer to date, as well as the various emerging immunotherapeutic agents and their possible use. Although monotherapy has thus far had disappointing results in prostate cancer, promising combination strategies are under evaluation.
keywords: prostate cancer; immunotherapy; immunomodulation; combination therapy
Immunotherapy has rapidly shifted the treatment paradigm for many cancers in the recent past including melanoma, renal cancer, and lung cancer [16-18]. Preclinical data suggest that prostate cancer is moderately immunogenic [19]. However, programmed cell death-ligand 1 (PD-L1), a biomarker for immune checkpoint inhibition in many cancer types, albeit non-uniformly, does not appear to be highly expressed in prostate cancer [20]. Furthermore, immune cells such as myeloid-derived suppressor cells and tumour-associated macrophages within the prostate tumour microenvironment (TME) restrict accumulation of T-cells and promote immune suppression [21]. In addition, a relative paucity of T-cells resulting from low non-synonymous mutation rate (0.3-2 mut/Mb) [22], correlating with lower number of tumour-associated antigens, leads to a restricted anti-tumour response [23]. On the other hand, resistance to second-generation hormonal therapy with enzalutamide in mCRPC seems to be associated with the expression of PD-1 and PD-L1/2 on antigen presenting cells [24].
In contrast to the marked success of checkpoint inhibition monotherapy in certain types of tumours, this strategy has thus far has limited success in prostate cancer. In this paper, we will review the role of current immunotherapeutic agents available for the treatment of prostate cancer and discuss various novel immunotherapy agents that are currently in development phase, as well as combination therapy strategies, designed to overcome the limited success immune checkpoint inhibition has thus far shown in prostate cancer.
The development of immune checkpoint inhibitors has revolutionised the field of immuno-oncology. These agents generate anti-tumour response by blocking co-inhibitory signalling pathways and promote immune-mediated killing of tumour cells by preventing tumour cells evading immunosurveillance.
Ipilimumab, a monoclonal antibody directed against cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), prevents T-cell inhibition and promotes the activation and proliferation of effector T-cells, was the first approved checkpoint inhibitor for patients with advanced melanoma [25-27]. The approval of ipilimumab, paved way for other immune checkpoint inhibitors to be evaluated.
Pembrolizumab and nivolumab, programmed cell death-1 (PD-1) inhibitors, showed promising objective response rate (ORR) of 40–45% in melanoma and non-small cell lung cancer (NSCLC) [28-30] and 24% in urothelial cancer patients [31] while in triple-negative breast cancer (TNBC) patients, the response to PD-1 inhibitors was relatively moderate (19%) [32]. In contrast, an ORR of 87% with 17% complete response (CR), was observed in relapsed or refractory Hodgkin’s lymphoma [23]. Subsequently, checkpoint inhibitors were approved by US Food and Drug Administration (FDA) for various further cancers and hence form an integral part of treatment algorithm (Table 1)
Only a subgroup of patients benefits from checkpoint inhibitors despite the success of CTLA-4 inhibitors and PD-1/PD-L1 inhibitors. Anti-tumour activity is regulated through complex factors in the TME, which is of three types based on the immune-cells infiltration: immune desert, immune excluded and immune inflamed [34]. These phenotypes exhibits specific mechanisms for preventing anti-tumour immune response [34]. Immune deserts are deprived of T-cells in the TME and lack T-cell priming and activation. The immune excluded TME signifies the presence of multiple chemokines and growth factors; however, accrued T-cells are unable to infiltrate the TME. Immune inflamed tumours exhibit infiltration of multiple immune cell subtypes [34].
Some cancer patients on checkpoint inhibitors develop severe immune-related adverse events (irAEs) [35] which are due to the inhibition of immune checkpoints that protect against autoimmunity, leading to various local and systemic immune-mediated autoimmune-like responses (Table 1). Recently long-term follow up of patients who received checkpoint inhibitors also reported cardiac toxicity and death [36].
With checkpoint inhibitors use becoming more common in treatment of different cancers, it has become imperative to understand the complex mechanism of resistance processes both primary and acquired, affecting the efficacy of these drugs. The interaction of tumour immunogenicity in TME plays an important role [37]. Poorly immunogenic tumours with low tumour mutational burden (TMB), such as prostate cancer, are primarily more resistant to treatment with checkpoint inhibition. Similarly, constant interactions between the immune system and cancer cells can result in varying heterogeneity intratumourally which in return may lead to poorly immunogenic tumour subclones within the tumour that lack expression of neoantigens, and hence develop acquired resistance to immunotherapy. Various factors within TME such as Tregs, myeloid-derived suppressor cells, tumour-associated macrophages and various chemokines can affect the response to immunotherapy by stimulating tumour-cell motility, angiogenesis and immune-evasion. Other factors that play a role in developing immune-resistance are deficiencies in antigen presentation, compensatory upregulation of alternative immune checkpoints and concomitant aberrant activation of traditional oncologic pathways [37]. More recently changes in gut-biome has been linked to development of immune-resistance as well [37].
Keeping above in mind, it is crucial to develop predictive biomarkers to differentiate between responders and non-responders (both primary and acquired), and to determine the outcome of a proposed therapy in a patient before its initiation. To date, PD-L1 expression remains the most studied potential biomarker of checkpoint inhibitor response. Immunohistochemical detection of tumour cell PD-L1 expression has been associated with clinical response in clinical trials [38-40].
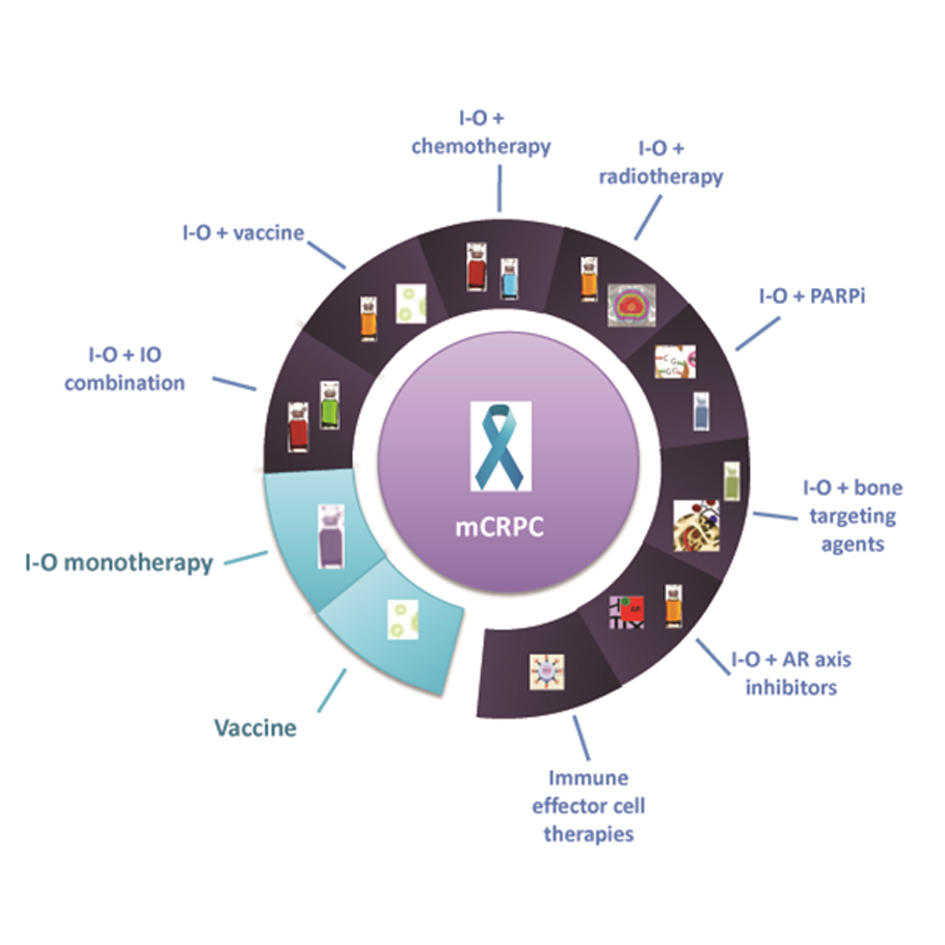 Figure 1. Treatment spectrum of immunotherapeutic agents in prostate cancer management. I-O: Checkpoint inhibitors; PARPi: poly-ADP ribose polymerase; AR: Androgen receptor; mCRPC: metastatic castration-resistant prostate cancer.
Figure 1. Treatment spectrum of immunotherapeutic agents in prostate cancer management. I-O: Checkpoint inhibitors; PARPi: poly-ADP ribose polymerase; AR: Androgen receptor; mCRPC: metastatic castration-resistant prostate cancer.
Ipilimumab
A phase-1/2 study of ipilimumab in 71 chemotherapy-naive patients with mCRPC showed durable PSA responses independent of prior chemotherapy, warranting further studies [41]. A randomised phase-3 trial reported no survival benefit in 598 men with asymptomatic or minimally symptomatic chemotherapy-na?ve mCRPC without visceral metastasis who received ipilimumab (10mg/kg) or placebo, every three weeks, up to four doses, and then maintenance ipilimumab or placebo every three months until progression [42]. Although median progression free survival (PFS) was 5.6 months in the ipilimumab arm as compared to 3.8 months in the placebo arm, the median overall survival (OS) was 28.7 months and 29.7 months, respectively. Grade-3/4 treatment-related AEs occurred in 40% (ipilimumab arm) versus 6% (placebo arm), with diarrhoea being the most common AE (43%). A phase-3 trial that randomised 799 men with mCRPC with at least one bone metastasis and had progressed after docetaxel chemotherapy, to receive bone-directed radiotherapy (8Gy in one fraction) followed by either ipilimumab or placebo. Median PFS and median OS were 4 months and 11.2 months versus 3.1 months and 10 months for ipilimumab and placebo respectively. Post-hoc analysis showed a median OS of 22.7 months versus 15.8 months in patients with favourable prognostic features such as alkaline phosphatase concentration of less than 1.5 times of upper limit of normal (ULN), a haemoglobin concentration of 110 g/L or higher, and no visceral metastases [43].
Ipilimumab was also studied in the neoadjuvant setting in a phase-2 study of 16 men with high-risk prostate cancer who pre-surgically received a single 3-month depot of androgen-deprivation therapy (ADT) plus ipilimumab 10mg/kg two doses three months apart, to identify potential immune-inhibitory mechanisms to immunotherapy [44]. Although an increase recruitment of T-cells and macrophages were observed into the prostate tumour, a higher expression of inhibitory molecules such as PDL-1 and V-domain immunoglobulin-containing suppressor of T-cell activation (VISTA) on macrophages was present, which in turn inhibited T-cell response accounting for the acquired resistance to ipilimumab.
Given the modest PFS responses along with the lack of a meaningful survival benefit, and the fact that multiple immunosuppressive mechanisms act in unison to affect anti-tumour response, the next step would be combination strategies to expand the immunotherapeutic effect. Such trials in prostate cancer include combination with chemotherapy (NCT03098160, NCT02423928), sipuleucel-T (NCT01804465) and PROSTVAC vaccine (NCT02506114, NCT00113984).
Nivolumab
In the phase-1 trial of nivolumab in 17 patients with various solid tumours, maximum tolerated dose (MTD) was not reached as no dose-limiting toxicity was observed at any dose up to 20 mg/kg [45]. Preliminary anti-tumour activity was seen in three patients who had a partial response (PR). Further development of nivolumab continued in melanoma, NSCLC, renal cancer, bladder cancer, and various other malignancies. In two phase-1 trials, nivolumab did not show encouraging ORRs in 25 heavily pretreated mCRPC patients [38, 39]. However, results of a phase-2 trial of combination immunotherapy with ipilimumab reported earlier this year were promising [46]. 78 patients with mCRPC were divided into two cohorts: asymptomatic or minimally symptomatic, who had progressed after at least one second-generation hormone therapy with no prior chemotherapy (cohort-1), and patients progressing after chemotherapy (cohort-2). ORRs were 26% and 10% in cohort-1 and-2 respectively, with four patients experiencing a CR, two in each cohort. PSA-response rate (>50% decline) was 21% in cohort-1 and 13% in cohort-2. ORR were higher in biomarker-enriched population, notably in patients with high TMB, PD-L1 expression >1%, DNA damage repair (DDR) defects and homologous recombination deficiency. These results were encouraging and several biomarker-driven clinical trials are now investigating nivolumab either alone or in combination in prostate cancer (NCT03040791, NCT03061539, NCT02601014, NCT03570619) while some trials are investigating combination of nivolumab with chemotherapy (NCT03338790), radiotherapy (NCT03543189), poly-ADP ribose polymerase inhibitors (PARPi) (NCT03572478, NCT03338790) and with peroxisome proliferator-activated receptor (PPAR)-alpha (NCT03829436). Nivolumab is also being investigated in combination with other immunomodulators such as vaccines (NCT02933255, NCT03815942, NCT03532217) and interleukin-8 inhibitor (NCT03689699).
Pembrolizumab
In the phase-1b KEYNOTE-028 study, patients with mCRPC and PD-L1 expression >1%, had a 13% (3/23) ORR and a median OS of eight months [47]. KEYNOTE-199, a phase-2 trial, investigated 258 patients with docetaxel-refractory mCRPC receiving pembrolizumab 200mg q3 weeks, dividing them in PD-L1 positive (>1%) and measurable disease (cohort-1), PD-L1 negative (<1%) and measurable disease (cohort-2), and non-measurable disease (cohort-3) patients [48]. PSA and radiologic response were noted in all three cohorts; 11% of all patients in three cohorts had a >50% PSA decline. Disease-control rate (DCR) were 27%, 42% and 57% respectively. Encouragingly, two patients in cohort-1 achieved CR. PD-L1 status did not accurately predicted response, however a higher response rate was noted in patients with BRCA1/2 or ATM mutations. These results, consistent with reports in other mismatch-repair deficient tumours [49], supported further research of pembrolizumab in mCRPC either alone or in combination, to look for predictive biomarker especially those with deleterious mutations.
Graff et al reported activity of the addition of pembrolizumab to enzalutamide in 28 patients with mCRPC who progressed on enzalutamide and had not received chemotherapy previously [50]. Pembrolizumab seemed to be able to partially reverse enzalutamide resistance. PSA-response was observed in five patients (18%), while radiologic response was at 25%. Median PSA-PFS, radiographic-PFS and median OS were 3.8, 10.8 and 22.2 months respectively. Neither microsatellite instability nor DDR accurately predicted for response to treatment.
Preliminary results of the phase-1b/2 umbrella KEYNOTE-365 study were presented earlier this year [51]. In the cohort-A, 41 mCRPC patients with post-docetaxel (and <2 lines of second-generation hormonal therapy) received pembrolizumab for up to two years plus olaparib 400mg twice daily until progression. Interestingly, 27% patients were PD-L1 positive, while none of them had DDR. PSA-response rate was 13% while radiologic-response rate was 7%. Overall DCR was 29%, while median OS was 14 months. Grade-3/4/5 AEs were seen in 51% of cases, higher than seen with pembrolizumab monotherapy in the past. Cohort-B of the KEYNOTE-365 investigated the combination of pembrolizumab with docetaxel in mCRPC patients progressing after second-generation hormonal therapy (72 patients, PD-L1 positive 29%). DCR was 57%, while median OS was not reached for a median follow-up of 10 months. Grade-3/4/5 AEs occurred in 27 (38%) patients.
Three phase-3 trials are investigating pembrolizumab against enzalutamide (docetaxel-na?ve), docetaxel and olaparib (docetaxel-refractory) (NCT03834493, NCT03834506, NCT03834519) to consolidate its position within the mCRPC treatment landscape. Another trial is investigating pembrolizumab in mCRPC with or without DDR defects (NCT03248570). Moreover, further early phase trials are being conducted using pembrolizumab in combination with various therapeutic agents such as Radium-223 (NCT03093428), 177Lu-prostate specific membrane antigen (PSMA) (NCT03658447, NCT03805594) and various novel immunomodulating agents (NCT03406858, NCT03473925, NCT03454451, NCT03910660, NCT03007732, NCT03695835) including vaccines (NCT02325557, NCT02499835).
Atezolizumab
Kim et al reported results of 15 patients in a phase-1a trial investigating atezolizumab in patients with mCRPC previously progressed on enzalutamide +/- sipuleucel-T (pre-Docetaxel) [52]. It was very well tolerated with grade-3 irAEs of 7%, while no grade-4/5 events were noted. It also demonstrated median PFS of 3.4 months and 12-month OS rate of 55.6%. Median OS was not reached. 9% had a PR while 13% had a ≥50% decrease in PSA.
IMbassador250 is a phase-3 randomised clinical trial comparing atezolizumab 1200mg q3 weeks plus enzalutamide 160mg daily with enzalutamide alone in patients with mCRPC who have previously progressed on abiraterone acetate and a taxane chemotherapy [53]. The results of this trial are awaited. Further trials are being carried out to maximise the potential of atezolizumab in combination with various therapeutic strategies such as Sipuleucel-T (NCT03024216), Rad-223 (NCT02814669), cabozanitinib (NCT03170960), ipatasertib; a PIK3CA/AKT-inhibitor (NCT03673787) and different novel immunomodulators (NCT02655822, NCT03138889, NCT02410512).
Durvalumab
A phase-1 study of combination of durvaluma band olaparib in patients with mCRPC who had previously progressed on second-generation hormonal therapy showed 47% ORR in all comers [54]. 65% of these patients also had received chemotherapy previously. Overall 12-month PFS was 51.5%, however it was 83.3% for patients with DDR defects compared with 36.4% for those without DDR defects (p?=?0.031). The combination was well tolerated with common grade-3/4 AEs of anaemia (35%), lymphopenia (24%), nausea (18%), fatigue (18%) and diarrhoea (18%). Further trials are being carried out investigating durvalumab in combination with different treatment modalities in different settings of prostate cancer i.e. with olaparib in patients with biochemically recurrent M0 prostate cancer harbouring DDR (NCT03810105, NCT02484404); with anti-CTLA (tremelimumab) in mCRPC (NCT02788773); with stereotactic ablative radiation in oligometastatic recurrent HSPC (NCT03795207); with chemotherapy along with dual immunotherapy in mCRPC (NCT03518606); and with novel immunomodulating agents such as adenosine-A2 receptor antagonist (NCT02740985) and toll-like 3 receptor agonist (NCT02643303).
Avelumab
In a phase-1 avelumab monotherapy trial in 18 patients with heavily pretreated mCRPC, 41.1% patients had stable disease (SD)at 24 weeks of treatment, while 35.2% had disease progression at 6 weeks, radiologically reconfirmed at 12 weeks. PSA-response was assessed as PSA doubling time (PSADT) at three months. 17.6% patients had a prolonged PSADT, 41.1% patients had stable PSADT and 41.1% had decreased PSADT. Only 11% patients had grade-3/4 AEs (asymptomatic amylase and lipase elevations) [55]. Further trials with avelumab are investigating their use in neuroendocrine prostate cancer (NCT03179410), and combinations with enzalutamide or abiraterone in mCRPC (NCT03770455); with PARPi (NCT03330405); with chemotherapy (NCT03409458); and a novel immunomodulator (NCT03861403).
Drugs targeting checkpoint proteins
Lymphocyte activation Gene-3 (LAG-3)
LAG-3 is expressed on cell surface of lymphocytes and has been recently recognised as an important new target in cancer immunology [56]. Preclinical studies have revealed widespread co-expression of PD-1 and LAG-3 on tumour-infiltrating T-cells in several cancers and dual (anti-LAG-3/anti-PD-1) blockade has shown good synergistic results in animal tumour models [57]. Several such drugs, such as BMS986016, REGN3767, TSR033 and LAG525, are currently under evaluation (as monotherapy or in combination) in solid organ malignancies including prostate cancer, such as the phase-1b/2 MAGIC-8 (nivolumab +/-BMS-986016 in mHSPC, NCT03689699).
Killer-cell Ig-like receptors (KIR)
KIRs are expressed on mature natural-killer (NK) cells whose ligands are HLA molecules. Binding of HLA molecules to KIR results in inhibitory signalling that decreases NK cell-mediated tumour destruction. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer [58].
Lirilumab (anti-KIR) was tested in a dose-escalation study and was deemed well tolerated [59]. A phase-1/2 trial recently reported good safety profile [60] and significant clinical activity of lirilumab (in combination with nivolumab) in patients with advanced platinum-refractory squamous cell cancer of head and neck [61]. Further data from other expansion cohorts of this trial will provide important information on future development of these agents in other malignancies, including prostate cancer. The triple combination of lirilumab, nivolumab and ipilimumab is also being evaluated (NCT03203876).
Drugs targeting CD47 and CEACAM (Carcinoembryonic antigen cell adhesion molecules)
CD47
CD47 is an inhibitory signal protein present on tumour cells to avoid phagocytosis [62]. Preliminary data also suggest that CD47 is upregulated in various cancers, including prostate cancer [63]. Several molecules including CC-90002 (NCT02367196), Hu5F9-G4 (NCT02216409) and SRF-231 (NCT03512340), as well as bispecific antibodies such as TG-1801 (anti-CD47/CD19 bispecific MAb NI-0701) are being evaluated in haematological malignancies at present (NCT03804996).
CEACAM5
CEACAMs are the members of the CEA family of immunoglobulin glycoprotein cell adhesion molecules and being increasingly recognised as playing a key role in modulation of human malignancies [65].
CEACAM5 is a tumour-associated surface antigen expressed in over 60% of small cell neuroendocrine prostate cancers. Engineered chimeric antigen receptor T-cells targeting CEACAM5 induced antigen-specific cytotoxicity in neuroendocrine prostate cancer cell lines [64].
Drugs targeting co-stimulatory receptors
CD137
CD137 is a co-stimulatory receptor present on cytotoxic and regulatory T-cells (Tregs) as well as NK cells. Its functions include activation of cytotoxic T-cells, inhibition of suppressive functions of Tregs and enhancement of antibody-dependent cytotoxicity. A phase-1 dose finding study of urelumab, a monoclonal antibody agonist of CD-137, showed transaminitis as dose-limited toxicity and determined 0.1 mg/kg (q3 weeks) recommended phase-2 dose [65]. A phase-1/2 study combining urelumab with nivolumab showed ORR of 50% in melanoma (regardless of PD-L1 status), and lung, head, and neck cancer patients [66]. This combination was well tolerated with common treatment-related AEs being fatigue, transaminitis, and anaemia.
Another similar drug, PF-05082566, was evaluated in a phase-1 study in combination with rituximab in patients with relapsed or refractory non-Hodgkin lymphoma with good response rates [67]. It is being evaluated in solid organ malignancies (NCT01307267). In haematological malignancies, CD137-Chimeric Antigen Receptor (CAR) T-cell therapy and bispecific antibodies have emerged. This latter concept has also been tested in prostate cancer models, with an anti-CD137/PSMA bispecific antibody showing promising results [68].
CD27
CD27 is a co-stimulatory receptor that belongs to the tumour necrosis factor receptor superfamily and is expressed on T-cells, B-cells, and NK cells. Varlilumab, a CD27-agonist antibody, has shown to be well-tolerated and of promising anti-tumour activity in a phase-1 trial in patients with solid tumours, including prostate cancer [69]. Varlilumab is also being investigated in various combinations in several other malignancies (NCT02543645, NCT02413827, NCT02335918, and NCT02270372). A varlilumab/stereotactic body radiation therapy in a phase-1 combination trial for prostate cancer has been terminated because of low accruals (NCT02284971).
CD40
CD40 is largely expressed on antigen presenting cells (APCs) and is associated with APC maturation and immune enhancement, resulting in tumour-specific T-cell activation [70]. CP-870893, an anti-CD40, in two separate phase-1 studies in patients with advanced solid tumours showed encouraging activity with grade-1/2 cytokine release-syndrome being the most common AE [71,72]. Lucatumumab (HCD122), ADC-1013, SEA-CD40, and APX005M are other anti-CD40 agents that are currently being investigated.
Glucocorticoid-induced tumour necrosis factor receptor (GITR) agonists and OX-40 agonists
GITR is expressed on Tregs and induce activation of CD4+ and CD8+ cells. Preclinical studies of GITR-agonistic antibodies (including combinations with checkpoint inhibitors) showed preliminary signal of activity [73]. Phase-1 studies of TRX518 (NCT01239134) and MK-4166 (NCT02132754) in solid tumours are currently underway.
OX-40 (CD134) is expressed on CD4+, CD8+, and NK cells, and potentiates T-cell receptor (TCR) signalling on the surface of T-lymphocytes, leading to their activation and enhancement of Tregs activity. In a phase-1 trial of OX40 agonist, 9B12/MEDI0562 showed limited anti-tumour activity with acceptable safety profile in patients with metastatic solid malignancies refractory to the conventional therapy [74]. A humanised version of the same drug (MEDI0562) is being tested in patients with solid organ malignancies in a phase-1 study (NCT02318394). Another trial using RG7888/MOXR0916 in combination with atezolizumab with or without bevacizumab is recruiting patients with metastatic carcinomas (NCT02410512). A combination of an anti-OX40, radiation and cyclophosphamide were studied in a phase-1/2 clinical trial in prostate cancer, achieving synergy in stimulating immune responses via tumour antigen release [75].
Drug-targeting tryptophan catabolism
Indoleamine 2,3-dioxygenase-1 (IDO1) is a tryptophan-catabolising enzyme expressed in many cancers that induces immune-tolerance by suppressing T-cell activity. IDO1 has been linked to the progression of prostate cancer with some prognostic significance [76]. The IDO1-inhibitor epacadostat, after encouraging early phase results [77], failed to reach its primary endpoint of improved PFS in a phase-3 combination study in melanoma [78], which led to halting of other phase-3 trials using IDO1-inhibitors. In another early phase study [79], epacadostat administered with ipilimumab inpatients with metastatic melanoma yielded an ORR of 30%. Combinational studies of epacadostat with nivolumab are currently in progress (NCT02327078).
A phase-1 study of single-agent indoximod involving 48 advanced cancer patients concluded this agent to be safe up to 2000mg taken twice daily [80]. Although there were no objective responses, durable SD (>6 months) was observed in five patients. Indoximod has been evaluated in combination with docetaxel in a dose-escalation study in 27 patients with metastatic cancer [81]. Investigators reported a PR rate of 18%, SD lasting less than 6 months in 36%, and SD lasting over 6 months in 4% of patients. In a phase-1b study, indoximod was combined with ipilimumab in metastatic melanoma with good tolerability [82]. A phase-2 combination study of indoximod with clinician choice of either ipilimumab, pembrolizumab, or nivolumab is ongoing (NCT02073123).
GDC-0919 is yet another experimental agent employing the same pathway. In a phase-1a study of 19 patients with recurrent/advanced solid tumours, MTD was not reached; however, 800 mg twice a day on a 21-/28-day cycle was well tolerated [83]. 44% had SD at the time of interim analysis with acceptable toxicity with an exception of one grade-4 lower gastrointestinal haemorrhage. A phase-1b study is currently recruiting patients with locally advanced or metastatic solid tumours for GDC-0919 and atezolizumab combination (NCT02471846).
Drugs targeting adenosine2A receptors (A2AR)
A2AR is activated in TME by accumulation of extracellular adenosine resulting in anti-tumour immunity suppression [84].
CPI-444, an oral selective A2AR-antagonist was investigated alone and in combination with atezolizumab in 47 patients with advanced cancers [85]. Overall DCR was 45% (mostly SD) in multiple histologies, including one patient with prostate cancer, and was equal for single agent cohort and for combination cohort. Further trials are being carried out in combination with atezolizumab (NCT02655822) and pembrolizumab (NCT03454451).
AZD4635, another A2AR-antagonist, is being investigated in advanced solid malignancies, including prostate cancer, as monotherapy and in combination with enzalutamide/abiraterone, olaparib or durvalumab (NCT02740985).
Drug-targeting chemokine signalling
The presence of immune cells in TME largely depends on chemokine ligands on these cells and their receptors on tumour cells [86]. Chemokines are structurally divided into four subgroups, namely, CXC, CC, CX3C, and C. Targeting the chemokine pathway could prove an important breakthrough in cancer treatment.
CXCR1/2
CXCR1/2-CXCL8 axis activates multiple intracellular signalling pathways that regulate proliferation and differentiation of immune cells. This axis also mediates progression of multiple cancers and hence is associated with early relapse and poor prognosis [87].
Reparixin, an inhibitor of CXCR1/2, has already shown activity in combination with paclitaxel both in hormone receptor positive and triple receptor negative breast cancer [88]. However, its impact on the prostate cancer TME remains to be studied.
AZD5069, a CXCR2 inhibitor, is being evaluated in combination with durvalumab for solid cancers including prostate cancer in an early phase study [89]. Interim results have suggested clinical benefit with manageable safety profile. The final results of this study are expected next year (NCT02499328).
CXCR4
Activation of CXCR4-CXCL12 axis activates intracellular pathways associated with cancer growth, metastasis and immune response. Recent evidence suggests that prostate cancer cells express CXCR4 and its upregulated in metastatic disease [90]. BL8040, LY2510924, and PTX9908 are currently undergoing evaluation in various solid and haematological malignancies.
Toll-like receptor (TLR) agonists
TLRs enhance immunity through recognition of microbial pathogen-associated molecular patterns and endogenous danger signals released from dying cells. It has been reported that TLR expression is reduced in prostate cancers. Disappointingly, TLR agonists as a single agent have shown poor efficacy in earlier trials, thus, necessitating further evaluation in combination with other agents to enhance their immunostimulatory effects. VTX-2337 is a TLR8 agonist that in combination with cetuximab in patients with head and neck cancer in a phase-1b clinical trial showed good tolerability and treatment response [91]. Another trial of VTX-2337 in combination with pegylated-doxorubicin involving patients with metastatic ovarian cancer is currently underway (NCT02431559).
In a double-blinded phase-2 trial, MGN1703 (TLR9 agonist) has shown promising activity as a maintenance therapy in 59 patients with metastatic colorectal cancer who had normalised tumour markers after the first-line induction therapy [92]. In a subgroup of patients with high-activated NK cell counts at baseline, there was a significant improvement in PFS. These results are encouraging but need further validation due to small study sample and immature survival data.
SD-101 (TLR9 agonist), in combination with pembrolizumab in a phase-1 study has shown good activity in 22 patients with melanoma [93]. ORR was 78% in checkpoint inhibitor-na?ve patients as compared to 15% in patients who received checkpoint inhibitors previously. The 12-month PFS was 88%, and the OS was 89%. A phase-2 study of SD-101 in combination with radiotherapy and pembrolizumab in prostate cancer is ongoing (NCT03007732).
Poly-ICLC (TLR3 agonist), is an immunostimulant being investigated in mCRPC patients in multiple trials as a vaccine adjuvant. A phase-1 study of 15 patients with mCRPC studied poly-ICLC along with dendritic vaccine and stereotactic radiotherapy [94]. The treatment was well tolerated. One heavily pretreated mCRPC patient had a mixed response while nine patients experienced SD. Further studies are ongoing combining this therapy with various treatment modalities including tremelimumab and durvalumab (NCT02643303), pembrolizumab (NCT03007732) and MUC1 vaccine (NCT00374049).
Drugs targeting the interleukin pathway
NKTR-214
NKTR-214 recombinant human interleukin-2 (IL-2), has shown good activity in preclinical tumour models [95]. Trials are being conducted for various tumours, including prostate cancer, alone and in combination with other checkpoint inhibitors (NCT03138889).
ALT-801
Recombinant human IL-2 is known to be able to induce durable CR in a small number of patients with metastatic melanoma and kidney cancer; however, it is associated with significant toxicities such as hypotension, capillary leak syndrome, and oliguria. ALT-801 is an innovative immunotherapeutic fusion protein consisting of IL-2, linked to a single-chain T-cell receptor domain that recognises a peptide epitope (aa264-272) of the human p53 antigen displayed on cancer cells in the context of HLA-A*0201 (p53+/HLA-A*0201). A phase-1 study of ALT-801 for advanced cancers showed SD in 38% of all 26 patients including one patient with mCRPC as best response [96].
ALT-803
Interleukin-15 (IL-15) is a key factor for the development, proliferation, and activation of NK cells and CD8+ memory T-cells. ALT-803 is a novel IL-15 agonist (N72D) with enhanced IL-15 biological activity and has so far been studied in animal models only. It has demonstrated durable anti-tumour activity in breast and colon murine models [97]. It is being investigated in combination with checkpoint inhibitor for mCRPC. Further clinical studies in combination with other immunotherapeutic agents in prostate cancer are warranted.
BMS986253
Interleukin-8 (IL-8) is known to promote immune escape and tumour progression and high serum IL-8 levels correlate with poor prognosis in various tumours [98]. A phase-1 study investigated BMS986253, an anti-IL-8, in 15 patients with advanced cancer [99]. PFS at 24 weeks was 73% while no grade-3/4 AEs were observed. 13.3% experienced grade-2 fatigue, hypophosphataemia and hypersomnia. It is currently being investigated in combination with nivolumab in HSPC (NCT03689699).
Vaccines
Although there are ever increasing number of emerging therapeutic agents in oncology, cancer vaccines have now become the most exciting expanding area of immunotherapeautics.
Sipuleucel-T (Provenge) is an autologous cellular immunotherapeutic vaccine, consisting of antigen-presenting cells, which have been activated ex-vivo with a recombinant fusion protein (PA2024), which in turn stimulates T-cell immune response against prostatic acid phosphatase in prostate cancer cells. IMPACT trial was a double-blind, placebo-controlled, phase-3 trial of 512 patients with mCRPC to receive either three infusions of sipuleucel-T or placebo two weeks apart. A median OS of 25.8 months in the sipuleucel-T patients versus 21.7 months in the placebo group was observed [100]. It was well-tolerated with grade-1/2 chills, fever and headache in most patients. This study elicited significant criticism regarding the observed albeit modest OS benefit without correlation with a PFS benefit or a T-cell response, and the absence of alternative mechanisms to explain the survival benefit [101]. Nevertheless, sipuleucel was approved by US FDA in 2010 for mCRPC. In a phase-2 study, 42 men with localised prostate cancer received sipuleucel-T prior to radical prostatectomy [102]. Increased incidence of T-cells was observed in the post-operative prostate gland histology compared to pre-operative biopsies. Currently, clinical trials are investigating combination of sipuleucel-T with other approved drugs, such as abiraterone acetate, enzalutamide, radium-223 and ipilimumab (NCT01487863, NCT01981122, NCT02463799, NCT01832870, NCT01804465).
rV-PSA is a recombinant vaccinia virus encoding human PSA in a phase-1 study of 33 mCRPC patients, showed a PSA response in 57.5% patients [103].
PROSTVAC-VF is a poxvirus-based vaccine that acts through genetically modified vaccinia virus and fowlpox virus encoding PSA. In a phase-1 trial of 10 patients with mCRPC who received PROSTVAC-VF, 40% patients had PSA stabilisation [104]. In a phase-2 study, 82 patients with mCRPC achieved 30% 3-year OS as compared to 17% in the control group with a median OS benefit of 9.9 months (26.2 months vs 16.3 months) [105]. A second reported phase-2 trial on 32 patients reported a median OS of 26.6 months, with patients with greater PSA-specific T-cell responses showing a trend (p = 0.055) towards enhanced survival [106]. However, the recently reported phase-3 study on 1298 asymptomatic or minimally symptomatic mCRPC patients, although confirmed its safety profile, failed to substantiate an OS benefit [107]. After these disappointing results, more trials are being run in combination with other immunotherapeutic agents, such as anti-PD-1, ipilimumab and nivolumab (NCT2506114, NCT02933255, NCT03532217). One such reported trial tested the combination of PROSTVAC-VF with ipilimumab in mCRPC in a phase-1 clinical trial. 14 of the 24 chemotherapy-naïve patients had reduction in PSA, six of them with a reduction of more than 50%. The median OS was 31.3 months, which was longer than PROSTVAC alone [108].
The granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells vaccine (GVAX) showed promising activity in a phase-1 trial [109]. A median OS of 26.2 months in patients with asymptomatic mCRPC was observed. Most common AE was grade-1/2 injection-site reaction (100%). GVAX was later studied in combination with ipilimumab in 28 mCRPC patients in a phase-1 trial [110]. 39% grade-3/4 irAEs were seen (most common: hypophysitis, alveolitis, and hepatitis). 25% had PSA < 50% decline while 53.5% had SD radiologically. Another phase-1 trial investigated the use of neoadjuvant degarelix alone or in combination with GVAX and cyclophosphamide in 28 high-risk prostate cancer patients undergoing radical prostatectomy, versus no neoadjuvant treatment. Intratumoural immune infiltrates were marginally augmented by cyclophosphamide/GVAX/degarelix versus degarelix alone, while CD8+ and Treg densities were significantly greater in both study arms versus the control group. Time-to-PSA-relapse was also improved although not statistically significant (Hazard ratio = 0.42) [111]. Further trials with immunotherapy are warranted.
Interim results of the phase-1 trial investigating the Proscavax vaccine [PSA/Interleukin-2(IL-2)/Granulocyte-macrophage colony-stimulating factor (GM-CSF)] in patients with M0 biochemical relapse (HSPC or CRPC) indicated a good safety profile with interesting activity in slowing PSADT and mounted immune responses to PSA [112]. A phase-2 trial of Proscavax versus active surveillance in localised prostate cancer is underway (NCT03579654).
A phase-1/2 clinical study evaluated 22 biochemically recurrent M0 prostate cancer patients, who received pTVG-HP/PAP DNA-based vaccine encoding prostate acid phosphatase [113]. No significant AEs were observed. 31.8% of the patient had a doubling of PSADT while 45.4% had T-lymphocyte responses correlating with increased number of vaccinations [114]. A phase-2 trial reported good efficacy and safety profile of pTVG-HP/PAP when used in combination with pembrolizumab [115]. Further trials are investigating pTVG-HP/PAP versus GM-CSF in patients with biochemically recurrent prostate cancer (NCT01341652), combination with sipuleucel-T in mCRPC (NCT01706458), and combination with nivolumab in patients with PSA-recurrent prostate cancer (NCT03600350).
AdV-tk is a new gene-mediated cytotoxic immunotherapy vaccine. Intratumoural delivery of a Herpes virus thymidine-kinase gene inserted in an adenoviral vector mediates the effect of vaccine. Based on the promising results in a phase-1/2 trial [116], a phase-3 trial is investigating vaccine immunotherapy in combination with radiation therapy for intermediate to high-risk prostate cancer patients (NCT01436968).
PAN-301-1 is a human aspartyl-asparaginyl-β-hydroxylase-directed nanoparticle vaccine. A phase-1 trial is evaluating its safety and efficacy inpatients with biochemically-relapsed prostate cancer (NCT03120832).
Modified vaccinia virus Ankara vaccine delivering the 5T4 tumour-associated antigen (Tro-Vax) has failed to show objective response in a phase-2 CRPC trial, despite 5T4-specific immune responses and delayed time to PSA-progression [117].
The novel ChAdOx1-MVA5T4 vaccine consists of two recombinant viruses designed to produce the 5T4 protein once injected into the body. It was investigated in a phase-1 study in with low and intermediate risk prostate cancer [118]. The vaccine was well tolerated. 5T4-specific CD4 and CD8 T-cells were extracted from patients’ prostate biopsies. A clinical trial is investigating its combination with nivolumab in intermediate risk and advanced prostate cancer (NCT03815942).
ADXS-PSA, an attenuated Listeria monocytogenes-based immunotherapy targeting PSA, designed to create antigen-specific T-cell effectors that kill tumour cells. A trial evaluated 51 patients with heavily pretreated mCRPC in 2 groups: A- ADXS-PSA; and B- ADXS-PSA in combination with pembrolizumab [119]. Common AEs (any grade) were cytokine release symptoms. PSA-response was 14% versus 43% in group-A and group-B respectively while PSA-response >50% was 0% versus 22%. Of the evaluable patients, SD was noted for 20% and 43% respectively.
NEO-PV-01 is a unique vaccine employing the concept of neoantigens. Tumour cell surface neoantigens are the “unique to cancer DNA sequences”, that once identified, are synthesized in the lab and mixed with an adjuvant immune enhancer. This concept is being tested in a phase-1 study along with nivolumab in advanced malignancies (NCT02897765).
Coxsackie virus A21 is a bio-selected oncolytic and immunotherapeutic strain of Coxsackie family given intratumourally to provoke an immune response. It is being tested alone in several tumours including CRPC (NCT02043665), and in combination with pembrolizumab and ipilimumab, intravenously or intratumourally.
Another phase-1b clinical trial combining oncolytic virus, Ad11/Ad3 chimeric group-B adenovirus with nivolumab is underway in metastatic cancers (NCT02636036).
Dendritic cells (DCs) are leukocytes that are spread throughout the body and have ability to present antigens to T-cells and play an important role in immunosurveillance. A DC-vaccine comprises isolated DCs loaded ex-vivo with tumour-specific antigen to activate antigen-specific T-cells and generate an immune response in-vivo against antigen-bearing cancer cells [120]. A randomised phase-2 study compared a DC vaccine plus docetaxel to docetaxel monotherapy in 43 patients with mCRPC. Although the PSA responses and PFS were comparable, a tumour-associated antigen immune-response was observed in 78% patients in the doublet arm [121].
Immune effector cell therapies
The concept of development of modified and activated T-cells with innate anti-tumour activity using CARs, TCR and tumour-infiltrating lymphocytes is still experimental in epithelial malignancies but has been successfully trialled in patients with relapsed acute lymphoblastic leukaemia resulting in a high remission rate [122]. This opens up a new immunotherapeutic possibility in prostate cancer research models. This includes prostate stem cell antigen (PSCA) targeted studies such the GEM3PSCA bispecific antibody engaging T-cells (NCT03927573), as well as PSCA-CAR T-cell studies in CRPC (NCT03873805, NCT024744287, NCT03089203). Other approaches such as PSMA CAR-T therapy are still in early phase of development showing moderate success [123].
Given the moderate immunogenicity of prostate cancer, significant progress is more likely to occur either with combinations or with newer immune effector therapy approaches, such CAR-T or TCR therapies (Figure 1). Several studies are ongoing to find the best tolerated dose of newer agents alone or in combination with other chemotherapeutic and established immunotherapeutic agents. The ultimate utility of these agents would depend on survival results from ongoing clinical trials along with finding an appropriate biomarker for efficacy.
The future successful development of immunotherapy in prostate cancer would involve overcoming many obstacles, including better understanding of tumour heterogeneity, elucidating mechanisms of primary and secondary treatment resistance, developing effective synergistic combinations (and regimens) without increased treatment-related toxicities, and tackling a high cost of new agents in the era of constrained resources. In the battle against these unique challenges, the incorporation of unparalleled genomic information, new biomarkers for efficacy in clinical trials and strong pharmacodynamic endpoints may lead the way forward.
Future clinical trials in prostate immuno-oncology should be geared towards finding the right drug for the right patient at the right time by innovative designs that are enriched for patients who would obtain the greatest survival benefit. As the prostate oncology community is ambitiously aiming to transform advanced prostate cancer into a chronic disease, clear survival benefit in the context of improved or maintained quality of life at a sustainable cost, especially for long-term treatments, is sine qua non.
Funding
None.
Ethics approval and consent to participate
This study did not require prior ethics approval or consent from human participants.
Author contributions
SB wrote and prepared the manuscript. MSK prepared the figures. MRG , CM and HTA reviewed the manuscript. AP prepared the figures, and reviewed the manuscript.
Competing interests
The authors declare no conflict of interest with the work.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68(6): 394-424.
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Saad F, et al: Abiraterone and Increased Survival in Metastatic Prostate Cancer. N Engl J Med 2011, 364: 1995-2005.
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et al: Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N Engl J Med 2012, 367: 1187-1197.
- Ryan CJ, Smith MR, Fizazi F, Saad F, Mulders PFA, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, MD et al: Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015, 16(2): 152-160.
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S,Carles J, Chowdhury S, et al: Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N Engl J Med 2014, 371: 424-433.
- de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L, et al for TROPIC Investigators: Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010, 376(9747): 1147-1154.
- Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke Met al for the ALSYMPCA Investigators: Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013, 369: 213-223.
- Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al: Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011, 377(9768): 813-822.
- Fizazi K, Tran NP, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, et al: Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017, 377: 352-360.
- James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AWS, Parker CC, Russell JM, Attard G, et al: Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387(10024): 1163-1177.
- Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, et al: Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019, https://doi.org/10.1007/s10637-016-0411-2. Epub ahead of print.
- Chi KM, Agarwal N, Bjartell A, Chung BH, Gomes AJP, Given RW, Soto AJ, Merseburger AS, Ozguroglu M, Uemuraet H et al: First results from TITAN: A phase III double-blind, randomized study of apalutamide (APA) versus placebo (PBO) in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) receiving androgen deprivation therapy (ADT). J Clin Oncol 2019, 37(15_suppl): 5006-5006.
- Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U,Ivashchenko P, Demirhan E, Modelska K, Phung D, et al: Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2018, 378: 2465-2474.
- Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, et al: Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med 2018, 378: 1408-1418.
- Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, Jievaltas M, Luz M, Alekseev B, Kuss I, et al: Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med 2019, 380: 1235-1246.
- Lugowska I, TeteryczP, and Rutkowski P: Immunotherapy of melanoma. Contemp Oncol (Pozn) 2018, 22(1A): 61–67.
- Santoni M, Massari F, Nunno VD, Conti A, Cimadamore A, Scarpelli M, Montironi R, Cheng L, Battelli N, and Lopez-Beltran A: Immunotherapy in renal cell carcinoma: latest evidence and clinical implications. Drugs Context 2018, 7: 212528.
- Corrales L, Scilla K, Caglevic C, Miller K, Oliveira J and Rolfo C: Immunotherapy in Lung Cancer: A New Age in Cancer Treatment. AdvExp Med Biol 2018, 995: 65-95.
- Drake CG: Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol 2010, 10(8): 580–593.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012, 366(26): 2443-2454.
- Garcia AJ, Ruscetti M, Arenzana TL, Tran LM, Bianci-Frias D, Sybert E, Priceman SJ, Wu L, Nelson PS, Smale ST, et al: Pten null prostate epithelium promotes localized myeloid-derived suppressor cell expansion and immune suppression during tumor initiation and progression. Mol Cell Biol 2014, 34(11): 2017-2028.
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA et al: Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499(7457): 214-218.
- Colli LM, Machiela MJ, Myers TA, Jessop L, Yu K, and Chanock SJ: Burden of Nonsynonymous Mutations among TCGA Cancers and Candidate Immune Checkpoint Inhibitor Responses. Cancer Res 2016, 76(13): 3767-3772.
- Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, and Zoubeidi A: PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6(1): 234-242.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010, 363(8): 711-723.
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C,Lebbe C, Baurain JF, Testori A, Grob JJ, et al: Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med 2011, 364: 2517-2526.
- Gibney GT, Weiner LM, and Atkins MB: Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016, 17(12): e542-e551. doi: 10.1016/S1470-2045(16)30406-5
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al: Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med 2015, 373: 1627-1639.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M,Horn L, et al: Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N Engl J Med 2015, 372: 2018-2028.
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al: Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015, 373: 23-34.
- Cheng W, Fu D, Xu F, and Zhang Z: Unwrapping the genomic characteristics of urothelial bladder cancer and successes with immune checkpoint blockade therapy. Oncogenesis 2018, 7(1): 2. doi: 10.1038/s41389-017-0013-7.
- Polk A, Svane IM, Andersson M, and Nielsen D: Checkpoint inhibitors in breast cancer - Current status.Cancer Treat Rev 2018, 63: 122-134. doi: 10.1016/j.ctrv.2017.12.008. Epub 2017 Dec 14.
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M,Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al: PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N Engl J Med 2015, 372: 311-319.
- Chen DS and Mellman, I: Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541: 321.
- Feng Y, Roy A, Masson E, Chen TT, Humphrey R and Weber JS: Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma.Clin Cancer Res 2013, 19(14): 3977-3986. https://doi.org/10.1158/1078-0432.CCR-12-3243. CCR-12-3243. Epub 2013 Jun 5.
- Bajwa R, Cheema A, Khan T, Amirpour A, Paul A, Chaughtai S, Patel S, Patel T, Bramson J, Gupta V, et al: Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J Clin Med Res 2019, 11(4): 225–236. doi: 10.14740/jocmr3750\u.
- Fares CM, Van Allen EM, Drake CG, Allison JP, and Hu-Lieskovan S: Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? American Society of Clinical Oncology Educational Book 39 (2019): 147-164.
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al: Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010, 28: 3167–3175.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012, 366: 2443–2454.
- Taube JM: Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology 2014, 3: e963413.
- Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, Scher HI, Chin K, Gagnier P, McHenry MB, et al: Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann Oncol 2013, 24: 1813-1821.
- Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, et al: Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol 2017, 35(1): 40-47.
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H, et al: Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014, 15(7): 700–712.
- Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al: Investigation of mechanisms of resistance to ipilimumab therapy with a pre-surgical trial in patients with high-risk, localized prostate cancer. J Clin Oncol 2017, 35(no. 15_suppl): 5081-5081.
- Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y,Honda K, Tanabe Y, Wakui H, and Tamura T: Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Investig N Drugs 2017, 35(2): 207–216. https://doi.org/10.1007/s10637-016-0411
- Sharma P, Pachynski RK, Narayan V, Flechon A, Gravis G, Galsky MD, Mahammedi H, Patnaik A, Subudhi SK, Ciprotti M, et al: Initial results from a phase II study of nivolumab (NIVO) plus ipilimumab (IPI) for the treatment of metastatic castration-resistant prostate cancer (mCRPC; CheckMate 650). J Clin Oncol 2019, suppl 7S: abstr 142.
- Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, Wallmark JM, Keam B, Delord JP, Aggarwal R, et al: Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol 2018, 29(8): 1807-1813.
- De Bono JS, Goh JC, Ojamaa K, Rodriguez JMP, Drake CG, Hoimes CJ, Wu H, Poehlein CH, and Antonarakis ES: KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2018, 36(no. 15_suppl): 5007-5007.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al: PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015, 372: 2509-2520.
- Graff JN, Alumkal JJ, Thompson RF, Moran A, Thomas GV, Wood MA, Drake CG, Slottke R, and Beer TM: Pembrolizumab (Pembro) plus enzalutamide (Enz) in metastatic castration resistant prostate cancer (mCRPC): Extended follow up. J Clin Oncol 2018, 36(15): 5047-5047.
- Yu EY, Massard C, Retz M, Tafreshi A, Galceran JC, Hammerer P, Fong PCC, Shore ND, Joshua A, Linch MD, et al: Keynote-365 cohort-A: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J Clinical Oncol 2019, 37(7): 145-145.
- Kim JW, Shaffer DR, Massard C, Powles T, Harshman LC, Braiteh FS, Conkling PR, Sarkar I, Kadel EE, Mariathasanet S, et al: A phase Ia study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2018, 36(6): 187-187.
- Gillessen SS, Powles T, Drake C, Rathkopf D, Narayanan S, Green M, Leng N, Schiff C, Sweeney C, and Fizazi K: IMbassador 250: A phase III trial in patients with metastatic castration-resistant prostate cancer (mCRPC) comparing atezolizumab plus enzalutamide vs enzalutamide alone. Eur Urol Suppl 2018, 17(2): e1155.
- Karzai F, VanderWeele D, Madan RA, Owens H, Cordes LM, Hankin A, Couvillon A, Nichols E, Bilusic M, Beshiri ML, et al: Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer 2018, 6: 141.
- Fakhrejahani F, Madan RA, Dahut WL, Karzai F, Cordes LM, Schlom J, and Gulley JL: Avelumab in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017, 35(6): 159-159.
- Andrews LP, Marciscano AE, Drake CG and Vignali DAA: LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 2017, 276: 80–96.
- Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, Fulton A, Tamada K, Strome SE, and Antony PA: Restoring immune function of tumour specific CD4 + T cells during recurrence of melanoma. J Immunol 2013, 190: 4899–4909.
- Pasero C, Gravis G, Granjeaud S, Guerin M, Thomassin-Piana J, Rocchi P, Salem N, Walz J, Moretta A, and Olive D: Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget 2015, 6: 14360-14373.
- Vey N, Goncalves A, Karlin L, Lebouvier-Sadot S, Broussais F, Berton-Rigaud DM, Andre P, ZerbibRA, Buffet R, Prébet T, et al: A phase 1 dose-escalation study of IPH2102 (lirilumab, BMS-986015, LIRI), a fully human anti KIR monoclonal antibody (mAB) in patients (pts) with various hematologic (HEM) or solid malignancies (SOL). J Clin Oncol 2015, 33(15): 3065.
- Segal NH, Infante JR, Sanborn RE, Gibney GT, Lawrence DP, Rizvi N, Leidner R, Gajewski TF, Bertino E, Sharfman WH, et al: Safety of the natural killer (NK) cell targeted anti-KIR antibody, lirilumab (liri), in combination with nivolumab (nivo) or ipilimumab (ipi) in two phase 1 studies in advanced refractory solid tumors. Annals Oncol 2016, 27; suppl_6: 1086P.
- Leidner R, Kang H, Haddad R,Segal NH, Wirth LJ, Ferris RL, Hodi FS, Sanborn RE, Gajewski TF, Sharfman W, et al: Preliminary efficacy from a phase 1/2 study of the natural killer cell–targeted antibody, lirilumab in combination with nivolumab in squamous cell carcinoma of the head and neck. Presented at: SITC annual meeting 2016, abstract 456: 9–13.
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, et al: The CD-47 signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumours. PNAS 2012, 109(17): 6662–6667.
- Beauchemin N and Arabzadeh A: Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 2013, 32(3–4): 643–671.
- Lee JK, Bangayan NJ, Chai T, Smith BA, Pariva TE, Yun S, Vashisht A, Zhang Q, Park JW, Corey E, et al: Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. PNAS 2018, 115 (19): E4473-E4482.
- Segal NH, Logan TF, Hodi FS, McDermott D, Melero I, Hamid O,Schmidt H, Robert C, Chiarion-Sileni V, Ascierto PA, et al: Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res 2017, 23(8): 1929–1936.
- Phase 1/2 data combining urelumab with Opdivo (nivolumab) in hematologic and solid tumors suggest increased antitumor effect in patients with melanoma. Bristol-Myers Squibb website http://news.bms.com/press-release/bmy/phase-12-data-combiningurelumab-opdivo-nivolumab-hematologic-and-solid-tumors-sug. Accessed 20th August 2019.
- Gopal A, Levy R, Houot R, Patel S, Hatake K, Popplewell L, ChenY, Davis C, Huang B,Cesari R, et al: A phase 1 study of Utomilumab (PF-05082566), a 4–1BB/CD137 agonist, in combination with Rituximab in patients with CD20+ non-Hodgkin’s lymphoma. Hematol Oncol 2017, 35(Supplement S2): 260.
- Pastor F, Kolonias D, McNamara II JO and Gilboa E: Targeting 4-1BB Costimulation to Disseminated Tumor Lesions with Bi-specific Oligonucleotide Aptamers. MolTher 2011, 19(10): 1878–1886.
- Burris HA, Infante JR, Ansell SM, Nemunaitis JJ, Weiss GR, Villalobos VM, Sikic BI, Taylor MH, Northfelt DW, Carson WE, et al: Safety and Activity of Varlilumab, a Novel and First-in-Class Agonist Anti-CD27 Antibody, in Patients with Advanced Solid Tumors. J Clin Oncol 2017, 35(18): 2028-2036.
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, and Noelle RJ: Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009, 229(1): 10.1111/j.1600-065X.2009. 00782.x.
- Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, et al: Clinical activity and immune modulation in cancer patients treated with CP-870,893, a Novel CD40 agonist monoclonal antibody. J Clin Oncol 2007, 25(7): 876–883.
- Bajor DL, Mick R, Riese MJ, Huang AC, Sullivan B, Richman LP, Torigian DA, George SM, Stelekati E, Chen F, et al: Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology 2018, 7(10): e1468956.
- Lu L, Xu X, Zhang B, Zhang R, Ji H and Wang X: Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med 2014, 12:36.
- Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, Walker J, Gonzalez I, Meeuwsen T, Fox BA, et al: OX40 is a potent immune stimulating target in late stage cancer patients. Cancer Res 2013, 73(24): 7189–7198.
- Kovacsovics-Bankowski M, Chisholm L, Vercellini J, Crittenden M, Lary S, Curti B, and Weinberg A: Phase I/II clinical trial of anti-OX40, radiation and cyclophosphamide in patients with prostate cancer: immunological analysis. J Immunother Cancer 2013, 1(1): P255.
- Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S, and Li Y: Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol 2018, 11: 100.
- Mitchell TC, Hamid O, Smith DC, Bauer TM, Wasser JS, Olszanski AJ, Luke JJ, Balmanoukian AS, Schmidt EV, Zhao Y, et al: Epacadostat Plus Pembrolizumab in Patients with Advanced Solid Tumors: Phase I Results from a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J Clin Oncol 2018, 36(32): 3223-3230.
- Long GV, Dummer R, Hamid O, Gajewski T, Caglevic C, Dalle S, Arance A, Carlino MS, Grob JJ, Kim TM, et al: Epacadostat (E) plus pembrolizumab (P) versus pembrolizumab alone in patients (pts) with unresectable or metastatic melanoma: Results of the phase 3 ECHO-301/KEYNOTE-252 study.J Clin Oncol 2018, 36(15): 108-108.
- Gibney G, Hamid O, Lutzky J, Olszanski A, Mitchell TC, Gajewski TF, Chmielowski B, Hanks BA, Zhao Y, Newton RC, et al: Updated results from a phase 1/2 study of epacadostat (INCB024360) in combination with ipilimumab in patients withresectable or metastatic melanoma.J Immunother Cancer 2019, 7(1): 80.
- Soliman HH, Minton SE, Han HS, Ismail-Khan R, Neuger A, Khambati F, Noyes D, Lush R, Chiappori AA, Roberts JD, et al: A phase I study of indoximod in patients with advanced malignancies. Oncotarget 2015, 7(16): 22928–22938.
- Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H, Ismail-Khan R, Minton S, Vahanian NN, Link C, et al: A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget 2014, 5(18): 8136–8146.
- Zakharia Y, Drabick JJ, Khleif S, McWilliams RR, Munn D, Link CJ, Vahanian NN, Kennedy E, Shaheen MF, Rixe O, et al: Updates on phase1b/2 trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus checkpoint inhibitors for the treatment of unresectable stage 3 or 4 melanoma. J Clin Oncol 2016, 34(15): 3075-3075.
- Nayak A, Hao Z, Sadek R, Dobbins R, Marshall L, Vahanian N, Ramsey J, Kennedy E, Mautino M, Link C, et al: Phase 1a study of the safety, pharmacokinetics, and pharmacodynamics of GDC-0919 in patients with recurrent/advanced solid tumors. Eur J Cancer 2015, 51(3): S69.
- Sek K, Mølck C, Stewart GD, Kats L, Darcy PK, and Beavis PA: Targeting Adenosine Receptor Signaling in Cancer Immunotherapy. Int J Mol Sci 2018, 19: 3837.
- Emens L, Powderly J, Fong L, Brody J, Forde P, Hellmann M, Hughes B, Kummar S, Loi S, Luke J, et al: Abstract CT119: CPI-444, an oral adenosine A2a receptor (A2aR) antagonist, demonstrates clinical activity in patients with advanced solid tumors. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017, 2017 Apr 1-5; Washington, DC. Philadelphia (PA): AACR; Cancer Res 2017, 77(13 Suppl): Abstract nr CT119.
- Mukaida N, Sasaki S, and Baba T: Chemokines in cancer development and progression and their potential as targeting molecules for cancer treatment. MediatInflamm 2014, 2014: 170381.
- Liu Q, Li Ab, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, and Wu K: The CXCL8-CXCR1/2 pathways in cancer. Cytokine & Growth Factor Reviews 2016, 31: 61-71.
- Schott AF, Wicha SM, Perez RP, Kato G, Avery T, Cristofanilli M, Reuben JM, Alpaugh RK, McCanna S, Ruffini PA, et al: Abstract P6-03-01: a phase Ib study of the CXCR1/2 inhibitor reparixin in combination with weekly paclitaxel in metastatic HER2 negative breast cancer—first analysis. Can Res 2015, 75(9): P6-03-01.
- Hong D, Falchook G, Cook CE, Harb W, Lyne P, McCoon P, Mehta M, Mitchell P, Mugundu GM, Scott M, et al: A phase 1b study (SCORES) assessing safety, tolerability, pharmacokinetics, and preliminary anti-tumor activity of durvalumab combined with AZD9150 or AZD5069 in patients with advanced solid malignancies and SCCHN. Ann Oncol 2016, 27(6): 1049PD.
- Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, and Taichman RS: Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem 2003, 89(3): 462-473.
- Chow LQM, Morishima C, Eaton KD, Baik CS, Goulart BH, Anderson LN, Manjarrez KL, Dietsch GN, Bryan JK, Hershberg RM, et al: Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin Cancer Res 2017, 23(10): 2442-2450.
- Schmoll HJ, Riera-Knorrenschild J, Kopp HG, Mayer F, Kroening H, Kuhlmann DN,ZiebermayrR,AndelJ, Arnold D, Schmidt M, et al: Maintenance therapy with the TLR-9 agonist MGN1703 in the phase II IMPACT study of metastatic colorectal cancer patients: A subgroup with improved overall survival. J Clin Oncol 2015, 33(3): 680-680.
- Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ, Barve M, Daniels GA, Wong DJ, Schmidt EV, et al: SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov 2018, 8(10): 1250–1257.
- Rodríguez-Ruiz ME, Perez-Gracia JL, Rodríguez I, Alfaro C, Oñate C, Pérez G, Gil-Bazo I, Benito A, Inogés S, López-Diaz de Cerio A, et al: Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann Oncol 2018, 29(5): 1312-1319.
- Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, VanderVeen LA, et al: NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin Cancer Res 2016, 22(3): 680-690.
- Fishman MN, Thompson JA, Pennock GK, Gonzalez R, Diez LM, Daud AI, Weber JS, Huang BY, Tang S, Rhode PR, et al: Phase 1 trial of ALT-801, an interleukin-2/T cell receptor fusion protein targeting p53 (aa264-272)/HLA-A*0201 complex, in patients with advanced malignancies. Clin Cancer Res 2011, 17(24): 7765–7775.
- Kim PS, Kwilas AR, Xu W, Alter S, Jeng EK, Wong HC, Schlom J, and Hodge JW: Il-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15Sa/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells and mediates potent anti-tumour activity against murine breast and colon carcinomas. Oncotarget 7: 16130–16145.
- Sanmamed MF, Carranza-Rua O, Alfaro C, Oñate C, Martín-Algarra S, Perez G, Landazuri SF, Gonzalez A, Gross S, Rodriguez I, et al: Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res 2014, 20(22): 5697-707.
- Collins JM, Heery CR, Donahue RN, Palena C, Madan RA, Strauss J, Gatti-Mays ME, Schlom J,Gulley JL, and Bilusic M: Phase I trial of BMS-986253, an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Clin Oncol 2018, 36 (no. 15_suppl): 3091-3091.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al: Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med 2010, 363: 411-422.
- Huber ML, HaynesL, ParkerC, and Iversen P: Interdisciplinary Critique of Sipuleucel-T as Immunotherapy in Castration-Resistant Prostate Cancer. JNCI: J Nat Cancer Institute 2012, 104(4): 273–279.
- Fong L, Weinberg VK, Chan SE, Corman JM, Amling CL, Stephenson RA, Formaker C, Simko J, Sims RB, Carroll P, et al: Neoadjuvant sipuleucel-T in localized prostate cancer: Effects on immune cells within the prostate tumor microenvironment. J Clin Oncol 2012, 30(no. 15_suppl) : 2564-2564.
- Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, et al: A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin. Cancer Res 2000, 6: 1632–1638.
- DiPaola RS, Plante M, Kaufman H, Petrylak DP, Israeli R, Lattime E, Manson K, and Schuetz T: A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med 2006, 4: 1.
- Kantoff PW, Gulley JL, and Pico-Navarro C: Revised Overall Survival Analysis of a Phase II, Randomized, Double-Blind, Controlled Study of PROSTVAC in Men with Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2017, 35(1): 124-125.
- Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, et al: Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010, 59(5): 663-674.
- Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J, et al: Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer.J Clin Oncol 2019, 37(13): 1051-1061.
- Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, et al: Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012, 13(5): 501-508.
- Simons JW, Carducci MA, Mikhak B, Lim M, Biedrzycki B, Borellini F, Clift SM, Hege KM, Ando DG, Piantadosi S, et al: Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clin Cancer Res, 12 (2006), pp. 3394-3401.
- van den Eertwegh AJM, Versluis J, van den Berg HP, Santegoets SJAM, van Moorselaar RJA, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, et al: Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol 2012; 13(5): 509-517.
- Antonarakis ES, Zahurak M, Schaeffer EM, Partin AW, Ross A, Allaf M, Tosoian J, Nirschl T, Chapman C,O'Neal TS, et al: Neoadjuvant randomized trial of degarelix (Deg) ± cyclophosphamide/GVAX (Cy/GVAX) in men with high-risk prostate cancer (PCa) undergoing radical prostatectomy (RP). J Clin Oncol 2017; 35(15): 5077-5077.
- https://immuno-oncologynews.com/proscavax/ Accessed 20th August 2019.
- McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, et al: Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol 2009; 27: 4047-54.
- Becker JT, Olson BM, Johnson LE, Davies JG, Dunphy EJ, and McNeel DG: DNA vaccine encoding prostatic acid phosphatase (PAP) elicits long-term T-cell responses in patients with recurrent prostate cancer. J Immunother 2010; 33: 639-47.
- McNeel DG, Eickhoff JC, Jeraj R, Staab MJ, Straus J, Rekoske B, and Liuet G: DNA vaccine with pembrolizumab to elicit antitumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017; 35(7): 168-168.
- Rojas-Martinez A, Manzanera AG, Sukin SW, Esteban-María J, González-Guerrero JF, Gomez-Guerra L, Garza-Guajardo R, Flores-Gutiérrez JP, Elizondo Riojas G, Delgado-Enciso I, et al: Intraprostatic distribution and long-term follow-up after AdV-tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther 2013, 20: 642–649.
- Amato RJ, Drury N, Naylor S, Jac J, Saxena S, Cao A, Hernandez-McClain J, and Harrop R: Vaccination of prostate cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother 2008, 31(6): 577-85.
- Redchenko I, Cappuccini F, Pollock E, Bryant R, Carter L, Verrill C, Hollidge J, Goodwin L, Harrop R, Romero PJ, et al: VANCE: first in human phase I study of a novel ChAdOx1-MVA 5T4 vaccine in low and intermediate risk prostate cancer. J Clin Oncol 2019, 36(no. 15_suppl): 3018.
- Stein MN, Fong L, Tutrone RF, Mega AE, Lobo M, Hong Q, and Haas NB: KEYNOTE-046: ADXS-PSA plus pembrolizumab (pembro) in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2018, 36(15): 5019-5019.
- Timmerman JM, and Levy R: Dendritic Cell Vaccines for Cancer Immunotherapy. Ann Rev of Medic 1999, 50: 507-529.
- Kongsted P, Borch TH, Ellebaek E, Iversen TZ, Andersen R, MetÖ, Hansen M, Lindberg H, Sengeløv L, and Svane IM: Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: A randomized phase II study. Cytotherapy 2017, 19(4): 500-513.
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al: Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014, 371(16): 1507–1517.
- Junghans RP, Ma Q, Rathore R, Gomes EM, Bais AJ, Lo AS, Abedi M, Davies RA, Cabral HJ, Al-Homsi AS, et al: Phase I Trial of Anti-PSMA Designer CAR-T Cells in Prostate Cancer: Possible Role for Interacting Interleukin 2-T Cell Pharmacodynamics as a Determinant of Clinical Response. Prostate 2016, 76(14): 1257-1270.
- Bristol-Myers Squibb. Highlights of prescribing information for YERVOY® (ipilimumab). 2018. https://packageinserts.bms.com/pi/pi_yervoy.pdf(Accessed 20th August 2019).
- Bristol-Myers Squibb. Highlights of prescribing information for OPDIVO® (nivolumab). 2018. https://packageinserts.bms.com/pi/pi_opdivo.pdf (Accessed 20th August 2019).
- Genentech, Inc. Highlights of prescribing information for TECENTRIQ® (atezolizumab). 2018. https://www.gene.com/download/pdf/tecentriq_prescribing.pdf (Accessed 20th August 2019).
- Merck KGaA. Highlights of prescribing information for BAVENCIO® (avelumab). 2017. http://www.emdserono.com/ms.country.us/en/images/Bavencio_PI_tcm115_161084.pdf?Version (Accessed 20th August 2019).
- AstraZeneca UK Limited. Highlights of prescribing information for IMFINZI? (durvalumab). 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761069s000lbl.pdf (Accessed 20th August 2019).
- Merck & Co., Inc. Highlights of prescribing information for KEYTRUDA® (pembrolizumab). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf (Accessed 20th August 2019).
Annals of urologic oncology
p-ISSN: 2617-7765, e-ISSN: 2617-7773
 Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Ann Urol Oncol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript